- Synthesis of Silicone-Acrylic Emulsions by ARGET ATRP Polymerization and Its Polymerization Kinetics
Xingbing Yang*, **, ***,†
 , Xinye Wang*, Pingting Du*, Shuang Huang*, and Xin Liu*
, Xinye Wang*, Pingting Du*, Shuang Huang*, and Xin Liu**College of Chemical and Chemical Engineering, Sichuan Institute of Arts and Science, Dazhou, 635000, China.
**Special Polymer Materials for Automobile Key Laboratory of Sichuan Province, Sichuan Institute of Arts and Science, Dazhou, 635000, China.
***Key Laboratory of Low-cost Rural Environmental Treatment Technology, Sichuan Institute of Arts and Science, Dazhou, 635000, China.- ARGET ATRP를 이용한 Silicone-Acrylic Emulsion 합성 및 반응속도 연구
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
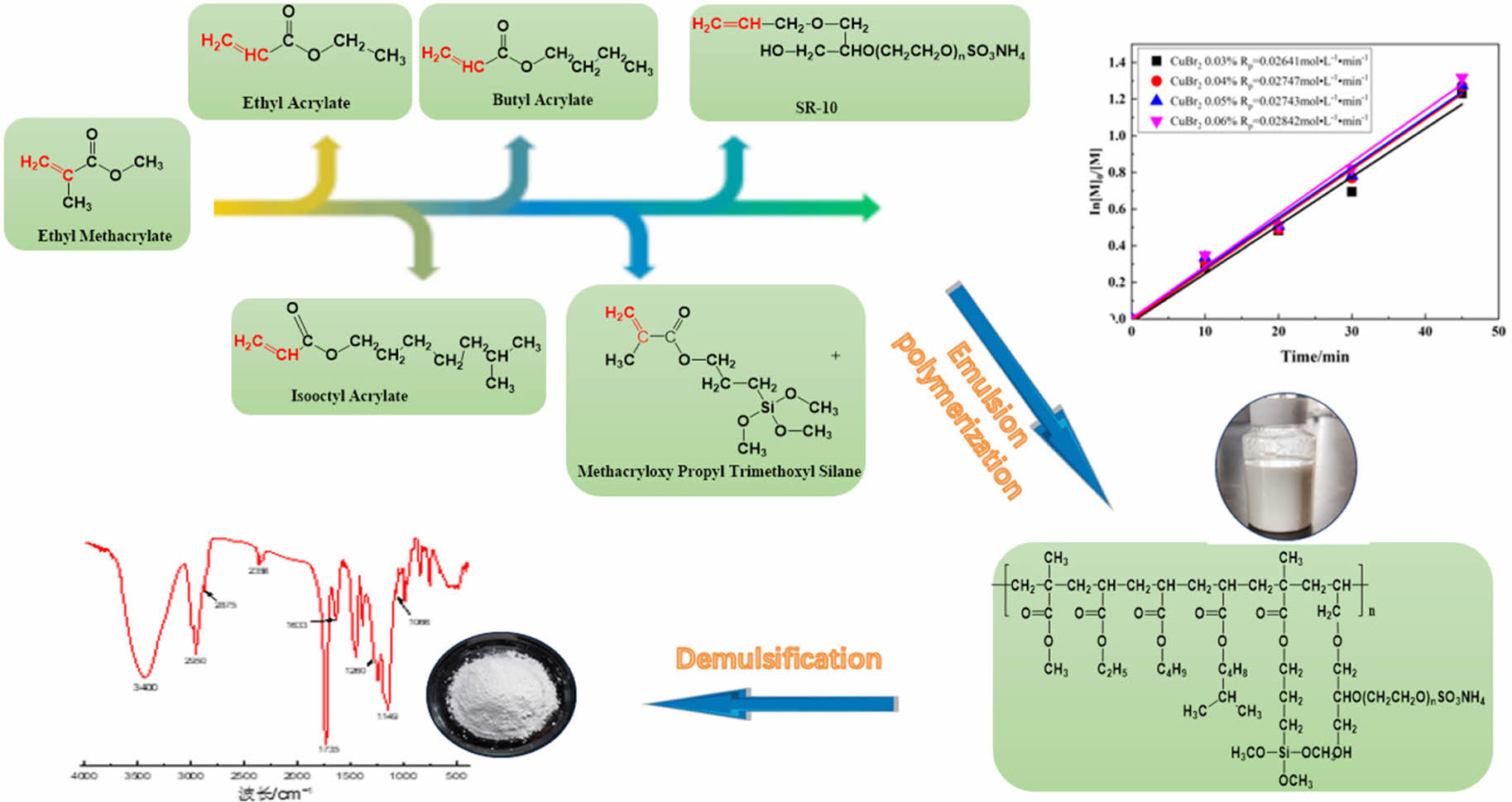
Methyl methacrylate (MMA), ethyl acrylate (EA), butyl acrylate (BA) and isooctyl acrylate (2-EHA) were used as the main monomers; γ-methacryloxypropyl trimethoxysilane (KH-570) was used as the silicone functional monomer; a reactive emulsifier and sodium dodecyl sulfate were used as the composite emulsification system; ethyl 2-bromoisobutyrate (EBIB) was used as the initiator; and copper bromide was used as the catalyst. A silicone-acrylic emulsion was prepared by the activators regenerated by electron transfer-atom transfer radical polymerization (ARGET-ATRP) with 2-bipyridine as the ligand and ascorbic acid as the reducing agent. The effects of temperature, initiator, organosilicon and bipyridine on the reaction kinetics were studied, and the polymers were characterized by infrared spectroscopy, gel permeation chromatography (GPC) and nuclear magnetic resonance spectroscopy. When the temperature increased, the conversion and the polymerization rate increased, and the apparent activation energy Ea was 79.65 kJ·mol-1. A greater initiator amount correlated to a higher conversion, a higher polymerization rate and a lower molecular weight. An increase in the amount of silicone increased the conversion and the reaction rate to 0.5% and 1%, respectively. With increasing silicone content, the gel content of the polymer, the molecular weight and PDI increased; when the silicone content was 4%, the polymer could not be dissolved by tetrahydrofuran. The characterization showed that MMA, EA, BA, 2-EHA, KH-570 and the reactive emulsifier SR-10 were all involved in the polymerization. ARGET-ATRP had a controllable molecular weight and narrow molecular weight distribution. Due to the addition amount and cost, few industrial applications exist. Therefore, in this study, the application of ARGET-ATRP on wood paint and the control of the molecular weight of the polymer to meet large-scale industrial application needs were examined.
To study the polymerization mechanism of ARGET-ATRP in complex silico-acrylic emulsion, the silico-acrylic emulsion was prepared by one-step method with methyl methacrylate, ethyl acrylate, butyl acrylate and isooctyl acrylate as raw materials, and the products were characterized. When the tempeaturer increased, the conversion and the polymerization rate increased, and the apparent activation energy Ea was 79.65 kJ·mol-1. A greater initiator amount correlated to a higher conversion, a higher polymerization rate and a lower molecular weight. An increase in the amount of silicone increased the conversion and the reaction rate to 0.5% and 1%, respectively. With increasing silicone content, the gel content of the polymer, the molecular weight and PDI increased; when the silicone content was 4%, the polymer could not be dissolved by tetrahydrofuran. The characterization showed that MMA, EA, BA, 2-EHA, KH-570 and the reactive emulsifier SR-10 were all involved in the polymerization.
Keywords: silicone-acrylic emulsion, activators regenerated by electron transfer-atom transfer radical polymerization, polymerization kinetics, emulsion polymerization, activation energy, conversion.
Special Polymer Materials for Automobile Key Laboratory of Sichuan Province at Sichuan Institute of Arts and Science (TZGC2023ZB-04-TZGC2024ZB-05); 2022 Dazhou Science and Technology Plan Project (22ZDFY0051).
The authors declare that there is no conflict of interest.
Atom transfer radical polymerization (ATRP) uses organic halides as initiators and transition metal compounds and ligands to form complexes as catalyst systems to achieve controlled polymerization.1 Due to its advantages of wide monomer coverage, easily available raw materials, mild polymerization conditions and simple operation, ATRP technology provides an effective method for the synthesis of functional polymers.2 However, because halides are highly toxic and transition metal oxides are easily oxidized by air, the application range of this method is limited. Therefore, the difficulty in this study is the need to add other co-initiators in addition to initiators (such as ligands and chlorides) in the reaction process of ATRP because leads to more impurities in the system and increases the cost of the raw materials; thus, commercial promotion is relatively slow. To improve this dilemma, industrial emulsion polymerization has made a breakthrough, and the activators regenerated by electron transfer-ATRP (ARGET-ATRP) technology with a small amount of initiator has begun to gain increase research attention. Based on the findings of Matyjaszewski3 and ATRP in 2006, ARGET-ATRP method of electron transfer4-6 of activated regenerated catalysts was proposed. This method introduces a reducing agent, which greatly decreases the effect of the catalyst, and does not require a strict oxygen-free environment. Different forms of polymers can be prepared by the ARGET-ATRP technology, such as star polymerization,7 block copolymers
8-10 and hyperbranched copolymers. Theoretically, the molecular weight distribution of ARGET-ATRP is narrow; however, in this study, the polymerization kinetics of ARGET-ATRP in a complex formulation system based on the wood paint formula is examined. The results of this experiment show that the wide molecular weight distribution is due to the reaction in a complex system and the introduction of silicone.
Acrylate emulsions are widely used in coatings, inks, adhesives and other fields because of their good mechanical strength, weather resistance, chemical agent resistance, high film gloss and permeability.11 Silicone contains Si-O bonds with high bond energies and a very special molecular structure and has the advantages of good heat resistance, weather resistance and relatively good film-forming properties with acrylic resin. The combination of silicone and the acrylate emulsions can be used to prepare silicone resin with high weather resistance, water resistance, pollution resistance, ultraviolet radiation resistance, acrylic resin bonding strength, flexibility and other characteristics of composites.12 In addition, acrylate emulsions can be used in the preparation of coatings, rubber and adhesives.13
To expand the application range of ARGET-ATRP, the acrylic emulsion ratio of wood paint for the free radical polymerization was adjusted, and a five-component co-polyacrylate emulsion with silicone was prepared by the ARGET-ATRP method. The polymer was characterized and analyzed by infrared (IR) spectroscopy, nuclear magnetic resonance (NMR) spectroscopy and gel permeation chromatography (GPC). At the same time, the effects of temperature, time and initiator on the polymerization kinetics were studied.14-15 The innovation of this study is the preparation of the acrylate emulsions by using a complex emulsion system of the reactive emulsifiers of R1, K12 and sodium dodecyl benzene sulfonate instead of the traditional small molecule single emulsion system. Silicone KH-570-modified acrylate emulsions are new and popular emulsions that are highly useful for practical production. The production technology of silicone acrylic emulsions is green, nontoxic and saves energy; thus, this technology is very beneficial for environmental protection.
Main Raw Materials. Methyl methacrylate (MMA), ethyl acrylate (EA), butyl acrylate (BA), and isooctyl acrylate (2-EHA) (AR, Chengdu Cologne Chemicals Co., Ltd., China) were used after vacuum distillation. γ-methacryloxypropyl trimethoxysilane (KH-570), sodium dodecyl benzene sulfonate, sodium dodecyl sulfate, copper bromide, and ascorbic acid were purchased from AR, Chengdu Cologne Chemicals Co., Ltd. (China) 2-Ethyl bromoisobutyrate and bipyridine were purchased from AR, Shanghai McLean Biochemical Technology Co., Ltd. (China) Reactive anionic emulsifier (SR-10) was obtained from AR, Japan Adiko Co., Ltd.
Main Equipment and Instruments. The equipment and instruments used throughout this study are as follows: a Bruker Avance 500 M NMR spectrometer (Bruker, Switzerland); a Nicolet Magna-IR550 FTIR instrument (Nicolet, USA); a high-performance gel chromatography (GPC) instrument (Waters Company); a circulating water vacuum pump (SHZ-III, Zhengzhou Yuhua Instrument Manufacturing Co., Ltd., China); an electric blast drying box (101-2AB, Tianjin Tester Instrument Co., Ltd., China); a precision timing electric mixer (JJ-1, Changzhou Yineng Experimental Instrument Factory, China); and an electronic analytical balance (AR2140, Shanghai Puchun Metrology Instrument Co., Ltd., China).
Preparation of the Silicone-acrylic Emulsions by the ARGET-ATRP Method. An emulsifier containing 0.20 g of the reactive anionic emulsifier SR-10, 0.12 g of sodium dodecyl sulfate (SDS), and 0.28 g of sodium dodecyl benzene sulfonate (SDBS) and water were added to a 500 mL four-neck flask with a reflux condensing tube, a thermometer, and mechanical stirring. The reaction was carried out at 60 ℃ at a stirring rate of 350 r/min for 30 min such that the emulsifier and water fully formed a dispersed emulsion. The reaction temperature was increased to 80 ℃ (n=300 r/min), and the monomers (methyl methacrylate (MMA), 18.81 g; butyl acrylate (BA), 4.14 g; ethyl acrylate (EA), 4.02 g; isooctyl (EHA), 0.79 g; γ-methacryloyl trimethoxysilane (KH570), 0.14 g), catalysts [bipyridyl bpy, CuBr2, ascorbic acid (AA)] and N2 were reacted for 20 min.16 The mechanical stirring speed was reduced to 240 r/min, the initiator EBIB was added, and the emulsion was obtained after 4 h of reaction.17 The reaction equation of polymerization is expressed as follows:
Analytical Methods. Determination of the conversion rate: The final monomer conversion of the polymerization system was determined by the mass method. A 1 g sample emulsion was placed in a tin foil container, a small amount of phenol ethanol solution was added to prevent the reaction, and the sample was baked in a blast oven at 105 ℃ to a constant weight.
The monomer conversion can be calculated according to the following formula:
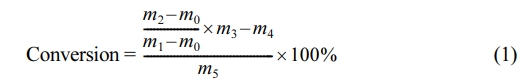
where m0 represents the mass of the tin foil container; m1 represents the total mass of the tin foil container and sample; m2 represents the total mass of the dry sample and tin foil container; m3 represents the total mass of the emulsion; m4 represents the mass of the nonvolatile components of the emulsion; and m5 represents the total mass of the monomer, unit g.
GPC analysis: gel permeation chromatography. The molecular weight and molecular weight distribution of the dried powder samples were detected by GPC after they were dissolved in tetrahydrofuran.
Infrared spectrum analysis: The sample was dried at 27 ℃ in a blast drying box, the water was fully volatilized, and the remaining sample was ground into a powder. The sample was mixing with 5 mg of KBr, and the sample was analyzed by the fixed angle specular reflection method. The wavenumber range was 400 cm-1 to 4000 cm-1.
Nuclear magnetic hydrogen spectrum analysis: The nuclear magnetic resonance (NMR) analysis reflects the nuclear magnetic resonance effect of H-1 in the molecule. This method can be used to determine the molecular structure. When a sample contains hydrogen, especially the isotope H-1, HNMR spectroscopy can be used to determine the structure of the molecule. The H-1 atom is also called a protium. A simple hydrogen spectrum is derived from a solution containing the sample. To avoid the interference of protons in the solvent, deuterated solvents are usually used. Deuterium chloroform was selected for this study. Fig. 1

|
Figure 1 ARGET-ATRP polymerization reaction. |
Effect of the Reaction Temperature on the Polymerization Kinetics. With a fixed monomer ratio MMA:BA:EA:2-EHA: KH-570 of 66.5:15:15:3:0.5, an initiator concentration of 6.67 × 10-3 mol·L-1 (0.67% of mass), a catalyst dosage of 0.03% (mass) and a reaction time of 4 h, the effects of different reaction temperatures on the polymerization were determined, as shown in
Figure 2. The conversion increased with increasing temperature. After 90 min of reaction, the conversion wad generally stable; a lower polymerization temperature correlated to a lower conversion within the same reaction time, and the conversion slowly increased when the polymerization temperature was 75 ℃; the conversion was approximately 95% after four hours of reaction at the four temperatures.
The characteristics of the controllable free radical polymerization were examined.18 In this study, the polymerization kinetics were studied at 4 stages with conversion rates of 10, 20, 30 and 45 min, and the results are shown in Figure 3; ln([M0]/[M]) has a linear relationship with time, indicating that the polymerization process conformed to first-order kinetics, and a lower polymerization temperature correlated to a better linear relationship. At polymerization temperatures of 75, 80, 85, and 90 ℃, the apparent growth rate constants Kapp were 0.01451, 0.02332, 0.03361, and 0.04535 min-1, respectively; additionally, the polymerization rate increased with increasing polymerization temperature. The apparent rate constant Kapp could be calculated by the kinetic formula ln([M]0/M)=Kapp·t, and this is plotted against T -1, as shown in Figure 4. We obtain the line y = -9.58x + 23.34, where the slope of the line is -Ea/R and the intercept is the frequency factor A. According to the Arrhenius formula Kapp=Aexp (-Ea/RT), the apparent activation energy Ea of the emulsion can be calculated and was determined to be 79.65 kJ·mol-1; this value is greater than those of common homopolymers,19 mainly because many monomers are present in the system and the activity of the monomers such as silicone is low.20-21
Effect of the Initiator on the Polymerization Kinetics. Chain initiation is the key reaction for controlling the polymerization rate and molecular weight; thus, the appropriate amount of initiator needs to be determined. In this study, EBIB was selected as the initiator, and the monomer ratio MMA:BA:EA:2-EHA: KH570=66.5:15:15: 3: 0.5 was fixed. With a catalyst dosage of 0.05% (mass), a reaction time of 4 h and a reaction temperature of 80 ℃, the conversion at different temperatures and the effect of initiator dosage with different monomer ratios on the polymerization were determined. As shown in Figure 5, before 90 min, a higher initiator concentration correlated to a greater monomer conversion; however, after 90 min, the conversion became stable, and the initiator had a minimal effect on the conversion within a certain range. Because the initiator is directly related to the molecular weight of the polymer, the effects of different concentrations of initiator on the molecular weight are examined. The test results are provided in
Table 1. With increasing amounts of initiator, the molecular weight gradually decreased, and the change in the polydispersity index (PDI) was small. In this system, the effect was improved when the ratio of initiator to monomer was 0.6:100.
The results from the study of the polymerization kinetics after 60 min are shown in Figure 6. When the initiator dosage was 100:0.6, 100:0.8, 100:1.0, and 100:1.4, the corresponding apparent growth rate constants Kapp were 0.02070, 0.02770, 0.02916, and 0.03244 min-1, respectively. With increasing initiator dosage, the apparent growth rate constant of the reaction increased; specifically, the reaction rate increased. ln([M]0/[M]) showed a linear relationship with time, which confirmed that polymerization was a first-order reaction; moreover, the linear relationship was better when the initiator concentration was low.
Effect of the Silicone Content on the Polymerization Kinetics. By selecting 0.0, 0.5, 1.0, 2.0, and 4.0% (total monomer mass ratio) KH-570 for polymerization, the effects of the different organosilicon contents on the polymerization conversion and the logarithm of the monomer concentration (ln([M0]/[M])) were examined. The effect of the KH-570 dose on the monomer conversion is shown in Figure 7. With increasing silicone content, the conversion rate gradually decreased, which was likely caused by the excessive cross-linking during the reaction process, resulting in the formation of a gel. After 60 min of polymerization, the conversion rate slowly increased. During the experiment, a greater amount of silicone correlated to more difficulty in controlling the reaction.
The influence of the amount of KH-570 on the logarithm of the monomer concentration (ln([M0]/[M])) is shown in Figure 8; with the addition of silicone, the linear relationship of ln([M0]/[M]) vs. t deteriorated. Thus, a greater silicone content correlated to more difficultly in controlling the reaction. ln([M0]/[M]) vs. t showed a linear relationship, indicating that the apparent rate constant was a first-order reaction with respect to the monomer concentration; specifically, the concentration of the increased free radicals remained basically unchanged during the polymerization. A larger slope from the first-order kinetic curve correlated to a faster reaction rate. The apparent polymerization rate constants corresponding to the KH-570 dosages of 0.0, 0.5, 1.0, 2.0, and 4.0% were 0.01306, 0.01098, 0.01019, 0.00913, and 0.00807 min-1, respectively. These results showed that the polymerization rate of the reaction was negatively correlated with the increase in the amount of silicone. The low polymerization rate occurred because when the organosilicon content was high, the cross-linking of the system was large, resulting in a partial coating of the monomers and an incomplete reaction.
The effect of different silicone contents on the molecular weight and PDI of the polymer is shown in
Table 2. With increasing silicone content, the molecular weight and PDI increased, which was consistent with the results reported in the literature; this result was mainly caused by the increase in the molecular weight due to the cross-linking of silicone; when the silicone content was 4%, tetrahydrofuran could not dissolve the polymer, and the polymer could not be detected by GPC.
Effect of the Amount of Bipyridine on the Polymerization Kinetics. The proportions of0.2, 0.4, 0.6, and 0.8% bipyridine (monomer mass ratio) were selected to study the effects of the different amounts of bipyridine on the conversion and the logarithm of the monomer concentration, as shown in Figure 9. The addition amounts of bipyridine of 0.6% and 0.8% were slightly greater than the addition amounts of 0.2% and 0.4%. By simultaneously comparing the addition of 0.6% and 0.8% bipyridine, the conversion rate was greater when slightly excess 2-pyridine-bipyridine was added; however, increasing the content of the 2-pyridine-bipyridine ligand had a minimal effect on the final conversion rate. 2-Pyrrolidine-bipyridine is the ligand in the reaction and forms a complex with copper bromide at a molar ratio of 2:1. The complex has a catalytic effect. The formation of more complexes correlate to an increase in the formation of the free radicals, which can speed up the polymerization.
The effect of the amount of pyridine on the polymerization rate is shown in Figure 10. The linear relationship between the content of bipyridine and the polymerization rate indicated that the polymerization rate was a first-order reaction with respect to the monomer concentration; specifically, the increased concentration of free radicals in the polymerization process remained basically unchanged. A greater slope from the first-order kinetic curve correlates to a faster reaction rate. The values were 0.00976, 0.01003, 0.01074, and 0.01102 min-1, respectively; these results indicated that the polymerization rate increased with the addition of bipyridine.
Characterization of the Polymers. As shown in Figure 11, vibrational absorption peaks were observed at 3400, 1735, 2875, and 2950 cm-1; these corresponded to the stretching vibrations of -OH, C=O, -CH2 and -CH3, respectively. The expansion vibration absorption peak of the S=O double bond at 1633 cm-1 indicated that the reactive emulsifier SR-10 participated in the polymerization reaction and was successfully grafted onto the polymer molecular chain. The characteristic peak of the stretching vibration of the Si-O bond in the silane coupling agent KH-570 was observed at approximately 1066 cm-1, and the characteristic absorption peak of Si-CH3 was observed at approximately 1260 cm-1. These results showed that KH-570 was introduced into the polymer molecular chain, and the polymerization of KH-570 with acrylate was confirmed.
The 1H NMR spectra in Figure 12 showed the characteristic peaks for the deuterated reagent CDCl3, the H on the double bond in its molecular structure, the methylene H connected to the ester group, and the methylene H on the main chain of the polymer molecule (the wide peak at d = 1.74 ppm). The characteristic peaks of the OmurCH2murCH2-methylene H at δ = 3.53 ppm and δ = 3.58 ppm were methylene and methyl. As shown in
Figure 13, a triple peak for the deuterated reagent CDCl3 was observed at d = 77 ppm; additionally, the saturated hydrocarbon carbon at d = 51.80 ppm and 13.74 pm due to -CH2-, the saturated hydrocarbon carbon at d = 44 and a single peak from the Si-C bond at d = 19.16 ppm indicated that silicone KH-570 was successfully grafted into the molecular chain of the acrylate emulsion.22-23

|
Figure 2 Changes in the conversion rate with time at different temperatures. |

|
Figure 3 ln [M]0/[M] diagram at different temperatures. |

|
Figure 4 Relationship between In Kapp and T -1. |

|
Figure 5 Effect of the initiator dosage on the conversio. |

|
Figure 6 ln([M]0/[M]) diagram under different initiation dosages. |

|
Figure 7 Variation diagram of the conversion with reaction time for the different organosilicon contents. |

|
Figure 8 Variation in the organosilicon content with reaction time. |

|
Figure 9 Variation in the conversion with respect to the reaction time at different bipyridine contents. |

|
Figure 10 Variation in the bipyridine content with respect to the reaction time. |

|
Figure 11 Infrared spectrum of the silicon-acrylic emulsion. |

|
Figure 12 1H NMR spectra of silicon-acrylic emulsions. |

|
Figure 13 13C NMR spectra of the silicon-acrylic emulsions. |
Using MMA, BA, EA and 2-EHA as raw materials, a series of silicone-modified acrylate emulsions were successfully prepared by changing the experimental conditions, such as initiation dose and reaction temperature. The ARGET-ATRP method could be used for polymerization under complex conditions, and this method provides a wide and controllable molecular weight distribution.
The following conclusions were drawn: with increasing initiator dosage, the final conversion rate of the emulsion increased, the initial reaction rate increased, the reaction rate increased linearly with time and initiator dosage. When the reaction time was 4 h, the conversion rate of the emulsion was 95.52%. As the reaction time continued to increase, the emulsion conversion rate was basically unchanged, but the molecular dispersion coefficient increased. The conversion rate increased with increasing reaction temperature, and the reaction temperature had a linear relationship with the conversion rate of the emulsion. An apparent activation energy Ea of 79.65 kJ·mol-1 was obtained. The conversion rate and reaction rate increased when the amount of silicone was 0.5% and 1%, respectively. The gel content, molecular weight and PDI of the polymer increased with increasing silicone content. When the silicone content was 4%, the polymer could not be dissolved and was not detected by GPC. As the amount of bipyridine increased, the conversion rate slightly changed, and the reaction rate decreased.
- 1. Dhar, A.; Koiry, B. P.; Haloi, D. J. Synthesis of Poly(methyl methacrylate) via ARGET ATRP and Study of the Effect of Solvents and Temperatures on Its Polymerization Kinetics. Int. J. Chem. Kinet. 2018, 50, 10,757-763.
-

- 2. Matyjaszewski, K.; Kajiwara, A. EPR Study of Atom Transfer Radical Polymerization (ATRP) of Styrene. Macromolecules 1998, 31, 548-550.
-

- 3. Jakubowski, W.; Min, K.; Matyjaszewski, K. Activators Regenerated by Electron Transfer for Atom Transfer Radical Polymerization of Styrene. Macromolecules 2006, 39, 39-45.
-

- 4. Cunningham, M. F. Controlled/living Radical Polymerization in Aqueous Dispersed Systems. Prog. Polym. Sci. 2008, 33, 365-398.
-

- 5. Schwartz, P. O.; Moingeon, F.; Roeser, J.; Couzigné, E.; Voirin, E.; Masson, P.; Méry, S. Preparation of Multi-allylic Dendronized Polymers via Atom-transfer Radical Polymerization. Eur. Polym. J. 2019, 118, 358-364.
-

- 6. Bai, L. J.; Zhang, L. F.; Cheng, Z. P.; Zhu, X. L. Activators Generated by Electron Transfer for Atom Transfer Radical Polymerization: Recent Advances in Catalyst and Polymer Chemistry. Polym. Chem. 2012, 3, 2685-2697.
-

- 7. Lin, W. J.; Nie, S. Y.; Zhong, Q.; Yang, Y. Q.; Cai, C. Z.; Wang, J. F.; Zhang, L. J. Amphiphilic Miktoarm Star Copolymer (PCL)3- (PDEAEMA-b-PPEGMA)3 as pH-sensitive Micelles in the Delivery of Anticancer Drug. J. Mater. Chem. B. 2014, 2, 4008-4020.
-

- 8. Jian, L.; Chen, H. J.; Mu, G. M.; Sun, J. P.; Sun, Y. L.; Wang, C. Y.; Ren, Q.; Ji, J. L. Synthesis of Amphiphilic Block Copolymers via ARGET ATRP Using An Inexpensive Ligand of PMDETA. React. Funct. Polym. 2013, 73, 1517-1522.
-

- 9. Krol, P.; Chmielarz, P. Synthesis of PMMA-b-PU-b-PMMA Tri-block Copolymers Through ARGET ATRP in the Presence of Air. Express. Polym. Lett. 2013, 7, 249-260.
-

- 10. Shu, J. B.; Cheng, C. J.; Zheng, Y.; Shen, L.; Qiao, Y. L.; Fu, C. H. One Pot” Synthesis of Fluorinated Block Copolymers Using a Surface-active ATRP Initiator Under Emulsion Polymerization Conditions. Polym. Bull. 2011, 67, 1185-1200.
-

- 11. Hao, L. F.; An, Q. F.; Xu, W.; Huang, L. X. Synthesis Film Morphology and Hydrophobicity of Novel Fluorinated Acrylate Emulsion and Solution on Silicon Wafer. Colloid Surface A. 2012, 396, 83-89.
-

- 12. Taran, E.; Donose, B.; Higashitani, K.; Asandei, A. D.; Hurduc, N. ATRP Grafting of Styrene From Benzyl Chloride Functionalized Polysiloxanes: An AFM and TGA study of the Cu(0)/bpy Catalyst. Eur. Polym. J. 2006, 42, 119-125.
-

- 13. Yang, X. B.; Lei, T.; Lu, W.; Huang, X.; Jiang, L. Characterization and Study of the Vulcanization Characteristics of Ultra-cold-resistant Reactive Chlorinated Acrylic Rubber Prepared by Emulsion Polymerization. Polym Int. 2022, 72, 189-194.
-

- 14. Cintora, A.; Kfer, F.; Yuan, C.; Ober, C. K. Effect of Monomer Hydrophilicity on ARGET-ATRP Kinetics in Aqueous Mini-emulsion Polymerization. J. Polym. Sci. 2021, 60, 666-673.
-

- 15. Shao, M. L., Yue, X. G.; Yue, T. Q.; He, J. Regulating Gelation Time and Kinetics Analysis Based on the Arget Atrp Mechanism. J. Polym. Sci. 2020, 58, 519-527.
-

- 16. Regmi, KNU.; Mehrvar, M.; Dhib, R.; Dhib, R. Experimental Design and Statistical Analysis of AGET ATRP of MMA in Emulsion Polymer Reactor. Macromol. React. Eng. 2019, 13, 4.
-

- 17. Awad, M.; Duever, T. A.; Dhib, R. Experimental Investigation of Methyl Methacrylate in Stirred Batch Emulsion Reactor: AGET ATRP Approach. Materials 2020, 13, 5793.
-

- 18. Nitschke, A.; Riemann, L.; Kollenbach, L.; Braun,V.; Buback, M.; Vana, P. Investigation into the Kinetics of n-Pentyl Methacrylate Radical Polymerization. Macromol. Chem. Phys. 2019, 221, 1.
-

- 19. Fazli, Y.; Kulani, E.; Khezri, K.; Alijani, H. PMMA-grafted Silica Aerogel Nanoparticles via in situ SR&NI ATRP: Grafting Through Approach. Micropor Mesopor Mat. 2015, 214, 70-79.
-

- 20. Dhar, A.; Koiry, B.; Haloi, D. Synthesis of Poly(methyl methacrylate) via ARGET ATRP and Study of the Effect of Solvents and Temperatures on Its Polymerization Kinetics. Int. J. Chem. Kinet. 2018, 50, 757-763.
-

- 21. Shao, M.; Yue, X.; Yue, T.; He, J. Regulating Gelation Time and Kinetics Analysis Based on the ARGET ATRP Mechanism. J. Polym. Sci. 2020, 58, 519-527.
-

- 22. Yang, J.; Zhou, S.; Bo, Y.; Wu, L. The Preparation and Surface Properties of Silicone-grafted Acrylic Copolymer Coatings. High Perform. Polym. 2005, 17, 85-102.
-

- 23. Ma, C.; Li, Y.; Zhang, J.; Ning, F.; Qiu, Z. Preparation and Characterization of Polyacrylate Composite and Its Application in Superhydrophobic Coating Based on Silicone-modified ZnO. J. Coat. Technol. Res. 2021, 18, 415-433.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2024; 48(5): 467-474
Published online Sep 25, 2024
- 10.7317/pk.2024.48.5.467
- Received on Jan 30, 2024
- Revised on May 13, 2024
- Accepted on May 17, 2024
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Conflict of Interest
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Xingbing Yang
-
*College of Chemical and Chemical Engineering, Sichuan Institute of Arts and Science, Dazhou, 635000, China.
**Special Polymer Materials for Automobile Key Laboratory of Sichuan Province, Sichuan Institute of Arts and Science, Dazhou, 635000, China.
***Key Laboratory of Low-cost Rural Environmental Treatment Technology, Sichuan Institute of Arts and Science, Dazhou, 635000, China. - E-mail: yxb19830528@163.com
- ORCID:
0000-0002-0254-6184










 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.