- [Review]
- Development of Hemoglobin-based Oxygen Delivery System Using PEGylation
Hyemin Cho*, Kidong Kim*, Jaeeun Oh*, Young-Joon Park**, and Sejin Son*, ***,†

*Department of Biological Sciences and Bioengineering, Inha University, Incheon22212, Korea
**College of Pharmacy, Ajou University, 206, World Cup-ro, Yeongtong-gu, Suwon-si, Gyeonggi-do, Korea
***Department of Biological Sciences, Inha University, Incheon 22212, Korea- 페길화를 이용한 헤모글로빈 기반 산소 전달 시스템 개발
*인하대학교 바이오시스템융합학과, **아주대학교 약학대학, ***인하대학교 생명과학과
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
Hemoglobin-based oxygen carriers can be used not only as blood substitutes but also as oxygen therapeutics for the treatment of hypoxemia, altitude sickness, carbon monoxide poisoning, chronic lung disease, and cancer. As COVID-19, population decline, and aging populations have raised concerns about the unreliability of the blood supply, hemoglobin-based oxygen carriers have become increasingly important in the development of artificial blood to replace donated blood. The strategy of conjugating polyethylene glycol (PEG) to hemoglobin has been used to mitigate the inherent problems of cell-free and substrate-free hemoglobin, such as toxicity, vasoconstriction, short half-life, and impairment of the immune system. In this review, we introduced the strategy of PEGylation as one of the methods for developing hemoglobin-based oxygen carriers.
헤모글로빈 기반 산소운반체는 혈액 대체제로 사용될 수 있을 뿐 아니라 저산소혈증, 고산병 및 일산화 탄소 중독, 만성 폐 질환 및 암 치료를 위한 산소 치료제로써 사용될 수 있다. COVID-19, 인구 감소, 고령화 등의 문제로 혈액 수급의 불안정성에 대한 문제가 대두되면서 헌혈 혈액을 대체할 인공 혈액을 개발하기 위해 헤모글로빈 기반 산소 운반체의 중요성이 더욱 강조되고 있다. 독성 유발, 혈관 수축, 짧은 반감기, 면역 체계 손상 등 무세포 및 기질이 없는 헤모글로빈의 고유한 문제를 완화하기 위하여 poly(ethylene glycol)(PEG)을 헤모글로빈에 접합하는 PEGylation 전략이 연구되어 왔다. 본 리뷰에서는 헤모글로빈 기반 산소운반체 개발의 방법 중 하나인 PEG를 이용한 PEGylation 전략과 이 전략을 이용한 헤모글로빈 기반 산소운반체에 대해 소개하였다.
Hemoglobin-based oxygen carriers serve not only as blood substitutes but also as therapies for hypoxemia, altitude sickness, carbon monoxide poisoning, chronic lung disease, and cancer. Conjugating polyethylene glycol (PEG) to hemoglobin addresses issues of toxicity, vasoconstriction, short half-life, and immune system effects associated with cell-free and substrate-free hemoglobin. This review explores PEGylation as a key method for developing hemoglobin-based oxygen carriers and discusses their applications in medical treatment.
Keywords: hemoglobin, polyethylene glycol, PEGylation, hemoglobin-based oxygen carrier, blood substitute, oxygen therapeutics.
This work was supported in part by an INHA University Research Grant.
The authors declare no conflicts of interest.
In recent years, the importance of hemoglobin-based oxygen carriers has been increasingly emphasized not only as a potential blood substitute but also as an oxygen therapeutic for conditions such as hypoxemia,1 sickle cell disease,2 carbon monoxide poisoning,3 chronic lung diseases, and cancer. As blood supplies become unreliable owing to issues such as COVID-19, population decline, and an aging population, there is an urgent need to develop artificial blood substitutes to complement traditional blood donations.4,5 Hemoglobin-based oxygen carriers, which utilize cell-free or stroma-free hemoglobin, offer a promising solution to these challenges by providing stable and reliable sources of oxygen. They can be utilized in various applications, from emergency blood transfusions to continuous oxygen therapy, in critical care settings.
Hemoglobin, an oxygen-transporting protein found in red blood cells, has the intrinsic ability to bind and release oxygen efficiently.6 However, when hemoglobin is used outside the protective environment of red blood cells, it can cause a range of adverse effects. Free hemoglobin rapidly disassociates into dimers and monomers, leading to renal toxicity and oxidative damage. Additionally, it can scavenge nitric oxide (NO), a crucial vasodilator, resulting in vasoconstriction and increased blood pressure.7 To mitigate these problems, chemical modifications of hemoglobin have been extensively investigated, including PEGylation, which involves binding polyethylene glycol (PEG) to proteins,8,9 conjugation, which involves attaching polymers to specific amino acid functional groups on the surface of proteins,10cross-linking, which involves cross-linking between proteins to create complexes,11,12polymerization, which involves attaching proteins to polymer chains to polymerize protein,13-15 and encapsulation which involves loading proteins into capsules.16,17 Among these modifications PEGylation, the process of attaching PEG chains to hemoglobin molecules, has shown considerable potential.18 PEGylation enhances the biocompatibility, stability, and circulation time of therapeutic proteins by shielding them from the immune system and reducing their renal clearance in the blood circulation.19 When applied to hemoglobin, PEGylation could potentially serve as a more effective and safer oxygen carrier.
This review aims to provide a comprehensive overview of the development of PEG-based oxygen carriers in basic, pre-, and clinical research. We delve into the physicochemical properties of hemoglobin, including its conformational structure, oxygen binding capacity, the limitations of cell-free hemoglobin as an oxygen carrier under in vivo conditions, and the potential benefits of PEGylation. We also introduce research on hemoglobin-based oxygen carriers that utilize PEGylation and discuss PEGylated hemoglobin oxygen carriers that have entered clinical trials.
Hemoglobin. The primary function of red blood cells (RBCs) is to transport oxygen from the lungs to body tissues and carbon dioxide from the tissues to the lungs. RBCs use the gas-binding ability of hemoglobin to function as oxygen carriers.20 The average amount of hemoglobin in adult human RBCs, known as the mean corpuscular hemoglobin, ranges from 27 to 31 picograms per cell, which is approximately 250 million Hb molecules per cell. Hemoglobin is a tetrameric protein consisting of two α polypeptide chains and two β polypeptide chains with each chain bound to an iron-containing heme group capable of binding one oxygen molecule (O2)5 (Figure 1a).
In RBCs, hemoglobin is concentrated to approximately 2 mM, which aids in stabilizing its tetrameric structure. This tetramer is a weak complex of two α-β dimers, with each α-subunit strongly bound to a β-subunit. RBCs also produce 2,3-bisphosphoglycerate (2,3-BPG), a metabolite that cross-links the two β-chains of hemoglobin, enhancing its stability and modulating RBCs function.21,22
RBCs contain various antioxidant enzymes, including catalase and superoxide dismutase, which degrade the superoxide (O2•–) radicals and hydrogen peroxide (H2O2) generated by the spontaneous oxidation of ferrous heme iron (Fe2+) in hemoglobin. In addition, RBCs reductase systems convert ferric iron (Fe3+) back to its ferrous state, which is the only form that can reversibly bind to oxygen. Despite these protective measures, approximately 1–3% of the hemoglobin in RBCs is oxidized at any given moment.21,22
Oxygen Binding and Carrying Capacity of Hemoglobin. The oxygen O2-binding kinetics of Hb are positively cooperative, meaning that small changes in oxygen partial pressure as blood travels from the lungs to tissues can lead to significant variations in the amount of oxygen bound (in the lungs) or released (in tissues) by Hb. This is demonstrated by the characteristic sigmoidal shape of the oxygen dissociation curve (Figure 1b).23,24
Each subunit has an iron-containing heme group to which one oxygen molecule can bind.25 Iron in hemoglobin normally exists in the reduced ferrous (Fe2+) state. When iron is oxidized to the ferric state (Fe3+), it dissociates to form oxygen (Figure 1d).26 In natural RBCs, the oxygen transport mechanism of Hb is tightly linked to the redox cycles, ensuring that Fe2+-containing Hb remains in its oxygen-binding state. This unique state change in hemoglobin allows it to bind more oxygen in the lungs (higher oxygen affinity) and release more oxygen in tissue capillaries (lower oxygen affinity). The reversible regulation of the oxygen-binding affinity of hemoglobin is facilitated by an allosteric effector molecule, 2,3-BPG, which forms inside RBCs as an intermediate in the process. Therefore, when designing an oxygen carrier as a substitute for human blood, it is essential to maintain the oxygen-carrying thermodynamic and kinetic characteristics of the hemoglobin molecule inside the cell, maintain a redox metabolic environment, and minimize irreversible methemoglobin formation.5

|
Figure 1 The structure of hemoglobin and the limitations of cell-free hemoglobin: (a) structure of hemoglobin and oxygen binding and dissociation; (b) oxygen dissociation curve of hemoglobin; (c) limitations of cell-free hemoglobin; (d) iron ion oxidation of heme. Created with BioRender.com |
Loss of Oxygen-binding Capacity. Cell-free or stroma-free hemoglobin lacks the protective and regulatory effects of enzymes within RBCs, such as 2,3-BPG, which modulates oxygen affinity. 2,3-BPG is a highly negatively charged small molecule that is present in high concentrations (4.5-5.0 mM) in RBCs. It is synthesized by the enzyme diphosphoglycerate mutase, which acts as a key regulator of oxygen release from hemoglobin by transferring the phosphate group from C1 to C2 on 1,3-bisphosphoglycerate, a metabolic intermediate product of the process.27 In RBCs, the ability of hemoglobin to bind and release oxygen is finely tuned to efficiently deliver oxygen to tissues. In RBCs, 2,3-BPG functions by binding to and stabilizing deoxyhemoglobin, shifting the equilibrium toward deoxyhemoglobin over oxyhemoglobin. This interaction shifts the oxygen dissociation curve to the right, reducing the oxygen affinity of hemoglobin, which enhances oxygen release into the tissue. Consequently, low levels of 2,3-BPG shift the oxygen dissociation curve to the left, reducing oxygen delivery to the tissue.28 However, without these regulatory mechanisms, the ability of cell-free Hb to oxygenate tissues is unregulated and is significantly less efficient.5
In addition, without the enzymatic environment of RBCs, cell-free hemoglobin is highly susceptible to the rapid and irreversible oxidation of its iron content from the ferrous (Fe2+) state to the ferric (Fe3+) state, resulting in the formation of methemoglobin (MetHb). MetHb is unable to bind oxygen, which significantly reduces its oxygen-carrying capacity. This irreversible oxidation of the ferrous (Fe2+) state to the ferric (Fe3+) state impairs the functional performance of hemoglobin, making it difficult to use cell-free hemoglobin as an effective oxygen carrier without RBCs protective environment.4
Nitric Oxide (NO) Scavenges. Hemoglobin infusions have been reported to increase arterial blood pressure in many animal and human studies, primarily as a result of vasoconstriction, which is the shrinking of blood vessels due to the constriction of the blood vessel walls.7
Cell-free or stroma-free hemoglobin functions as a powerful scavenger of NO, a critical molecule produced by vascular endothelial cells that plays a vital role in regulating vasodilation. In a normal physiological environment, NO is responsible for maintaining the vascular tone by promoting the relaxation of blood vessels, thereby facilitating proper blood flow and reducing blood pressure. However, when hemoglobin is present outside the RBCs, it exhibits a high affinity for NO, leading to its rapid and efficient scavenging both within and outside the blood vessels. Heme in hemoglobin has a high affinity for NO; however, within RBCs, the scavenging of NO by hemoglobin is 1000 times slower than that of cell-free hemoglobin.29,30 First, RBCs acts as a physical barrier due to the cell membrane, delaying the penetration of NO and slowing diffusion rates.31,32 Second, NO diffusion to RBCs is restricted by an unstirred layer around the RBCs, where NO is rapidly removed. During blood flow, RBCs are pushed towards the center of vessels, creating a cell-free zone that separates them from the endothelial cells where NO is produced, thereby regulating NO scavenging by hemoglobin inside the RBCs.33,34 Cell-free or substrate-free hemoglobin, which lacks the protective barrier of red blood cells, has direct access to endothelial cells in the blood vessels, resulting in rapid NO scavenging.
This scavenging activity disrupts the delicate balance of NO levels, thereby impeding its vasodilatory function. A reduction in available NO leads to vasoconstriction, a condition in which blood vessels constrict and reduce their diameter, which, in turn, increases vascular resistance and elevates blood pressure. This phenomenon is believed to be a primary contributor to the vasoconstriction and hypertensive side effects observed with the use of cell-free hemoglobin.
The excessive removal of NO by cell-free hemoglobin not only compromises its role in vascular homeostasis, but also poses significant challenges in the therapeutic application of hemoglobin-based oxygen carriers.22
Toxicity and Short Circulation Time. Cell-free hemoglobin lacks a protective shield against RBCs and the biological environment necessary for stability. In the absence of RBCs, cell-free or stroma-free hemoglobin tetramers rapidly break down into dimers and monomers owing to dynamic equilibrium (Figure 1c). Subsequently, small hemoglobin dimers (32 kDa) move across tissue barriers such as the vascular endothelium, renal glomeruli, and ependymal epithelium, separating the ventricular cerebrospinal fluid (CSF) space from the brain parenchyma. This process leads to toxic accumulation of hemoglobin and its breakdown products near crucial parenchymal tissues.35
Cell-free hemoglobin is primarily removed from the bloodstream via renal clearance. Therefore, the kidneys bear the brunt of exposure during intravascular hemolysis. Following filtration, progressive acidification of urine accelerates hemoglobin oxidation, destabilizes globin structures, and releases heme.36 This sequence of events explains why most observations regarding oxidation-driven hemoglobin toxicity are associated with hemoglobinuria-induced kidney injury.37
As a result, cell-free hemoglobin degrades rapidly in the blood from tetramers to dimers and monomers, has a short circulating half-life, and can penetrate tissue barriers, leading to toxicity.
Immune System Impairment. MetHb is formed when iron in hemoglobin is oxidized from its ferrous state (Fe2+) to its ferric state (Fe3+). This oxidation significantly impacts the structural integrity of the hemoglobin molecule, leading to destabilization of the heme-globin association. Consequently, heme groups, which are normally tightly bound to the hemoglobin molecule, can dissociate and exist freely within the bloodstream.35,38
Heme has been identified as a catalyst for immune dysregulation induced by hemolysis and a disruptor of essential cellular structures, including the proteasome and cytoskeleton. Studies have shown that heme binding to toll-like receptor 4 (TLR4) triggers inflammatory NF-kB signaling in murine models of sickle cell anemia (SCA).39 This activation exposes P-selectin, a critical mediator of cell-adhesion interactions in SCA, providing a plausible framework for the initiation and progression of vasoocclusive crises (VOC) and acute chest syndrome. Conversely, the adverse effects of hemolysis in immune cells exacerbate hyposplenism, a condition in which splenic function is compromised, leading to increased vulnerability to infections in patients with SCA and other forms of hemolytic anemia.
The immunosuppressive effects of heme are mediated by heme oxygenase 1 gene (Hmox1) and the activation of the transcription factor nuclear factor erythroid 2-related factor 2 (NRF2), which suppresses inflammatory cytokine responses in macrophages.40 Moreover, heme-NRF2 signaling in myeloid progenitors disrupts normal myeloid differentiation pathways, resulting in reduced dendritic cell numbers and impaired antigen presentation, thus hindering CD4 T-cell activation in murine SCA models.41 Heme-induced alterations in actin-cytoskeletal dynamics further impair phagocytosis and leukocyte migration, increasing the susceptibility to gram-negative sepsis in mice.
Consequently, in endothelial cells and leukocytes, Hb oxidation, generation of free heme, and heme binding by TLR4 activate adhesion pathways. Exposure of the immune system to Hb and heme leads to phagocytic dysfunction, inflammation suppression, and impaired antigen presentation.35
PEG. PEG is an inert, long-chain, amphiphilic molecule produced by linking repeating units of ethylene oxide.43 PEGs are highly water-soluble, flexible, and noncharged polymers suitable as biomaterials.19 PEGs have a wide variety of highly active and multifunctional terminal groups at their ends, resulting in many different structures, including linear, branched, and multiarmed structures. PEG is thermally stable over a wide pH range.
PEG molecules can vary in size depending on the number of repeated units (Table 1). PEG is excreted from the body intact, with smaller PEGs (less than 30 kDa) eliminated via the kidneys and larger PEGs (> 20 kDa) through fecal excretion.
The size and number of PEG molecules attached to these compounds can be tailored for specific purposes. Single linear or branched methoxy PEG (mPEG) molecules ranging from 12 to 60 kDa are attached to peptides, nucleotides, and small recombinant proteins to increase the hydrodynamic diameter and reduce renal absorption. Conversely, multiple mPEG 5000 molecules can be attached to the surface of foreign enzymes to enhance their stability within the body and block the binding of anti-enzyme antibodies. Hundreds or thousands of mPEG 2000 lipid molecules integrated into liposomes and nanoparticles reduce absorption by Kupffer cells in the liver.
PEG is commonly described as a small linear molecule similar in size to small proteins; however, the actual sizes vary significantly, ranging from approximately 12.5 nm for PEG 2000 to approximately 253 nm for linear PEG 40000 molecules.44
In protein therapeutics, PEGylation shields antigenic epitopes through steric hindrance by conjugating PEG to the therapeutic proteins. This shielding mechanism reduces the immunogenicity of the protein, preventing its recognition by the immune system and subsequent degradation by proteolytic enzymes. PEGylation can also inhibit mis-oxidation, avoiding clearance by the mononuclear phagocytic system (MPS).19
Over the past few decades, PEGs have been considered as non-immunogenic molecules with very low toxicity that can be safely used as drugs and food additives.45 However, recent studies have indicated instances of immune responses against PEG, indicating its potential immunogenicity.46 Wan, Xue, et al. reported that higher molecular weight generally, increase immune responses, and a correlation has been found between higher molecular weights and increased immunogenicity.47 This highlights the importance of carefully selecting the molecular weight in PEGylation strategies because a higher molecular weight can enhance both the immunogenicity and circulating half-life of conjugated therapeutic molecules.48
PEG has significant therapeutic limitations owing to its nonbiodegradability. Currently, approved PEGylated protein therapeutics employ PEGs with molecular masses of £ 40 kDa, close to the glomerular filtration threshold of approximately 50 kDa.49 While higher molecular masses typically result in longer circulation times, concerns regarding the build-up of nonbiodegradable PEG restrict the optimization of polymer molecular mass and subsequent pharmacokinetics.50 Consequently, chemical approaches, such as incorporating biodegradable units into the main chain, have been developed to impart biodegradability to PEG.19,51
PEGylation. PEGylation is the method of attaching one or more PEG chains to a protein, peptide, or non-peptide molecule. Proteins and peptides hold great promise as therapeutic agents and carriers. However, they have the disadvantages of being easily degraded by proteolytic enzymes, rapidly cleared by the kidneys, short shelf life, low solubility, and a propensity to generate neutralizing antibodies.52 PEGylation allows PEG to chemically attach to proteins through multiple reactions because it has a variety of functional groups. When polymeric PEG is conjugated to a protein, the steric hindrance caused by PEG protects the protein from adsorption by enzymes and plasma proteins, improves its in vivo stability, and extends its circulation time.53 The size of the PEG-protein conjugate increases, preventing it from being cleared by the kidneys.18 Studies on PEG solutions have revealed that each ethylene glycol subunit is tightly associated with 2-3 water molecules, which increases the water solubility of the protein. The binding of water molecules makes the PEGylated protein conjugate appear 5 to 10 times larger than the corresponding water-soluble protein of similar molecular weight, as confirmed by SDS-PAGE or size exclusion chromatography.54 PEGylated
protein conjugates are also more stable against a wide range of pH and temperature changes than non-PEGylated proteins. Consequently, PEGylation confers several properties on proteins, peptide therapeutics, and carriers, including improved in vivo stability, reduced side effects, and increased shelf life.55
Early PEGylation researchers considered amino groups as suitable conjugation sites because they are the most representative groups of proteins and can be modified through a variety of chemical strategies. Chemical strategies include acylation, which involves the loss of charge on the amino group of a protein after conjugation with PEG-carboxylate or PEG-carbonate, and alkylation, which involves the formation of a secondary amine after conjugation with alkylated PEG, thus maintaining the positive charge of the amino group. Various chemical strategies, such as the thiolation of amino groups, can be used to modify the amino groups of proteins for PEGylation.56
PEGylation using the thiol group of cysteine, which is not involved in the formation of disulfide bonds, is one of the most specific PEGylation methods, because cysteine is rarely present in proteins or peptides. PEGylation is performed using PEGs with functional groups that react with thiol groups, such as PEG-maleimide, PEG-pyridildisulfide, and PEG-vinyl sulfone. Because of its hydrophobic nature, cysteine is often zburied inside protein structures, making it only partially accessible to reagents. If cysteine is inaccessible, heterobifunctional low molecular weight PEGs with a thiol reactive group at one end and an azide group at the other end with low steric hindrance can be used to increase reactivity.56
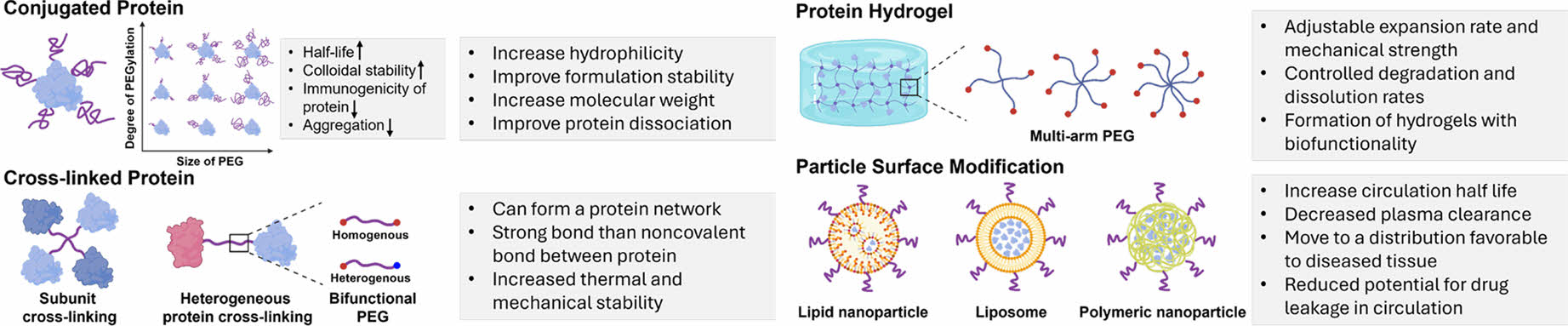
Protein PEGylation Strategies. There are multiple strategies for PEGylation of proteins, each tailored to achieve specific outcomes in terms of stability, solubility, and bioavailability (Figure 2).
PEG conjugation is the process of attaching one or more PEG chains to a protein. Conjugation with PEG enhances the hydrophilicity of molecules, which improves their dispersion, prevents aggregation, and increases the stability of the formulations. It also increases molecular weight, which decreases clearance by the kidneys and extends the circulation half-life. Additionally, conjugation reduces the immunogenicity and antigenicity, helping molecules evade the reticuloendothelial system.18
Proteins are linked to each other using PEG as a cross-linking agent. Cross-linking increases the hydration capacity of molecules because of their hydrophilic properties, expanding the fluid radius of the conjugate 5 to 10 times. It also reduces the frequency of administration by increasing the dosage capacity. Furthermore, crosslinking can create a protein network with stronger bonds than noncovalent interactions, providing enhanced thermal and mechanical stability.57,58
It is possible to create a protein hydrogel by employing multi-arm PEG as a crosslinker between proteins. This method involves attaching PEG molecules with multiple arms to protein chains, thereby facilitating the formation of a three-dimensional network. The properties of protein hydrogels can be fine-tuned by adjusting the molecular weight and specific gravity of PEG, which affect the expansion ratio and mechanical strength.59,60 These hydrogels offer improved structural stability, influence drug release profiles, and enhance the stability of sustained drug release systems.61 The characteristics of these particles can be enhanced by conjugating PEG to the surfaces of lipid nanoparticles, liposomes, and polymeric nanoparticles, the characteristics of these particles can be enhanced. Surface modification techniques can extend the circulatory half-life of molecules and reduce their plasma clearance. They help direct molecules to tissues where they are required. For example, in liposomes, surface modifications create a hydrated outer shell that integrates into the lipid bilayer, protecting against destruction by proteins and reducing uptake by the reticuloendothelial system, while minimizing the likelihood of medication leakage during circulation.62

|
Figure 2 Potential protein PEGylation strategies for developing hemoglobin-based oxygen carriers. Created with BioRender.com |
|
Table 1 Characteristics of PEGs by Size |

a The ethylene oxide subunit length is considered to be 0.278 nm in water.60 |
Various forms of Hemoglobin-based Oxygen Carriers Using PEG. Many researchers have actively investigated various strategies for the PEGylation of proteins. Conjugation typically involves attaching PEG molecules to specific amino acid residues on the protein surface, enhancing its pharmacokinetic profile and reducing its immunogenicity. Crosslinking PEG to proteins results in the formation of PEG-protein complexes or conjugates that can alter protein interactions and creating versatile biomaterials. Hydrogel formation using PEGylation allows proteins to be encapsulated within a three-dimensional network, facilitating controlled release and applications in tissue engineering. Modifying the surface of particles, such as liposomes, with PEG improves biocompatibility, reduces aggregation, and enhances targeting precision in drug delivery systems.18
Hemoglobin-based oxygen carriers are systems that utilize natural hemoglobin as an oxygen carrier through chemical methods such as conjugation, cross-linking, and encapsulation of hemoglobin with polymers.63 Typically, the hemoglobin used to develop hemoglobin-based oxygen carriers is human or bovine hemoglobin.64,65 The use of cell-free hemoglobin has the advantage that it is not interfered by cell membranes, allowing for more efficient contact with oxygen; however, it can cause a number of side effects and has a short circulating half-life in the body.66,67 The production of hemoglobin-based oxygen carriers using the PEGylation strategy is expected to overcome the disadvantages of cell-free hemoglobin.
Cooper et al. recently engineered a hemoglobin molecule with a single reactive cysteine residue on the surface of the α-subunit to create a single PEGylation site (bCys93Ala/aAla19Cys).9 When the maleimide-PEG adducts were compared to mono-sulfone-PEG (reacting at aAla19Cys), the mono-sulfone-PEG adducts were found to be significantly more stable when incubated at 37°C for 7 days in the presence of 1 mM reduced glutathione. The authors believed that although maleimide-PEGylation appeared to be sufficiently stable for use in acute oxygen therapy, monosulfone-PEG may be more suitable for longer vascularization.4
Advances in PEGylation-based research have led to the development of formulations that have progressed to clinical trials. These formulations aim to optimize the stability, circulation time, and therapeutic efficacy of hemoglobin-based oxygen carriers, thereby enhancing their potential applications in clinical settings, such as transfusion medicine and critical care.
Clinical Trials of Hemoglobin-based Oxygen Carriers Using PEGylation. Hemospan: When Maleimide was PEGylated by modifying the amine group of human hemoglobin with a thiol group, the Hemospan exhibited increased molecular size and improved oxygen affinity.8 Owing to the high level of water hydration by PEG, Hemospan has a larger molecular radius than proteins of similar molecular weight, rendering it unable to cross the intercellular junctions. Therefore, Hemospan appears to have fewer toxicity issues due to tissue penetration and fewer side effects, such as vasoconstriction and hypertension, owing to its low nitric oxide scavenging effect. However, it failed to meet the clinical feasibility criteria for phase 3 clinical trials and was terminated in 2015.4 Hemospan was well tolerated among volunteers and patients in phase 1 and phase 2 clinical trials, with no serious adverse events associated with the product and no signs of hypertension or gastrointestinal side effects. However, two phase 3 clinical trials (one in Europe and one in the U.S.) comparing Hemospan to Voluven, a plasma volume substitute used to restore blood volume, did not produce the expected results when comparing its effects on volume expansion and hypotension during surgery.68
Sanguinate: Sanguinate, a pegylated carboxyhemoglobin, is a hemoglobin-based oxygen carrier that is PEGylated using bovine hemoglobin and can bind not only oxygen, but also carbon monoxide.69 Owing to its structural characteristics and altered hemoglobin-oxygen binding affinity, it can bypass obstructions in the microcirculation and effectively deliver oxygen to ischemic tissue. Sanguinate can also endogenously deliver carbon monoxide, which reduces inflammation and oxidative stress, mitigates ischemia-reperfusion injury and promotes vasodilation.70 Although several phase II trials have been completed, only the results of phase I trials of Sanguinate have been published. These trials suggested a possible risk of myocardial injury; however, there is little evidence that this is due to Sanguinate.4
Hemoglobin Vesicle: A Hemoglobin Vesicle (HbV) is a cellular-type HBOC consisting of PEG and phospholipid vesicles encapsulating approximately 35–40 g/dL human hemoglobin, resulting in a HbV suspension with a hemoglobin concentration of 10 g/dL. Each HbV particle, approximately 250 nm in diameter, contains approximately 30000 Hb molecules.71
Animal studies have validated the safety and efficacy of HbV as a red blood cell substitute. When HbV suspended in an albumin solution replaced 90% of the circulating blood in rats,72 stable hemodynamics were observed, and effective resuscitation was achieved in rats, rabbits, and dogs. Additionally, HbV has a circulatory half-life of 32 h after administration. Preclinical studies, including single- and repeated-dose toxicity, safety pharmacology, and immunogenicity tests in rodents and dogs, have confirmed the safety of HbV as a transfusion alternative. HbV manufacturing began under Good Manufacturing Practices (GMP) and is currently in phase 1 clinical trials.73
Hemoglobin-based oxygen carriers can be useful not only as blood substitutes but also in a variety of applications, including the treatment of hypoxemia, hyperoxia, carbon monoxide poisoning, chronic lung disease, and cancer. Hemoglobin is considered an ideal candidate for these applications because of its unique oxygen binding property and oxygen carrying capacity. However, cell-free hemoglobin has limitations such as instability, rapid clearance, toxicity and the potential for vasoconstriction.
One promising strategy for addressing these issues is PEGylation, which involves the attachment of PEG chains to Hb. PEGylation can improve the stability, biocompatibility, and circulating half-life of hemoglobin while retaining its essential oxygen-carrying function, which can mitigate the side effects associated with cell-free hemoglobin. Numerous studies have demonstrated the potential of PEGylated hemoglobin-based oxygen carriers, such as Hemospan and Sanguinate, to improve oxygen delivery to tissues and reduce side effects.
However, the clinical application of PEGylated hemoglobin-based oxygen carriers faces several challenges. For example, the discontinuation of Hemospan's phase III clinical trial highlights the need for continued research and optimization to improve PEGylation technology and evaluate its safety and efficacy.
Future research should focus on advancing PEGylation technologies to further improve the stability and functionality of hemoglobin-based oxygen carriers and validate their safety and efficacy through comprehensive preclinical and clinical trials. By addressing these challenges and optimizing the design of PEGylated hemoglobin-based oxygen carriers, we will be able to develop viable hemoglobin-based oxygen carriers that meet the clinical needs of patients, reduce reliance on donated blood, and improve outcomes in emergency and chronic care settings.
- 1. Cao, M.; Zhao, Y.; He, H.; Yue, R.; Pan, L.; Hu, H.; Ren, Y.; Qin, Q.; Yi, X.; Yin, T. New Applications of HBOC-201: a 25-year Review of the Literature. Front Med 2021, 8, 794561.
-

- 2. Alayash, A. I. Hemoglobin-based Blood Substitutes and the Treatment of Sickle Cell Disease: More Harm Than Help? Biomolecules 2017, 7, 2.
-

- 3. Chung, S.; Nguyen, T.; Wollocko, H.; Daneshvar, M. 559: Effectiveness of OxyVita Novel Hemoglobin-Based Oxygen Carrier (HBOC) in Treatment of CO Poisoning. Crit. Care Med 2021, 49, 272.
-

- 4. Chen, L.; Yang, Z.; Liu, H. Hemoglobin-based Oxygen Carriers: Where are We Now in 2023? Medicina 2023, 59, 396.
-

- 5. Gupta, A. S. Hemoglobin-based Oxygen Carriers: Current State-of-the-art and Novel Molecules. Shock 2019, 52, 70-83.
-

- 6. Yu, S. Hemoglobin: Physiology and Hemoglobinopathy. In Blood Substitutes and Oxygen Biotherapeutics;Henry Liu, Alan D. Kaye, Jonathan S. Jahr;Springer: Pennsylvania, 2022; pp 45-51.
-

- 7. Gulati, A.; Barve, A.; Sen, A. P. Pharmacology of Hemoglobin Therapeutics. J. Clin. Lab. Med. 1999, 133, 112-119.
-

- 8. Vandegriff, K. D.; Winslow, R. M. Hemospan: Design Principles for a New Class of Oxygen Therapeutic. Artif. Organs. 2009, 33, 133-138.
-

- 9. Cooper, C. E.; Bird, M.; Sheng, X.; Choi, J.-W.; Silkstone, G. G.; Simons, M.; Syrett, N.; Piano, R.; Ronda, L.; Bettati, S. Stability of Maleimide-PEG and Mono-sulfone-PEG Conjugation to a Novel Engineered Cysteine in the Human Hemoglobin Alpha Subunit. Front. Chem. 2021, 9, 707797.
-

- 10. Priavalle, C.; De Angelo, J. Hemoximer: History, Pharmacology, Pre-Clinical Studies, Clinical Trials, and Lessons Learned. In Blood Substitutes and Oxygen Biotherapeutics; Henry Liu, Alan D. Kaye, Jonathan S. Jahr;Springer: Pennsylvania, 2022; pp 375-382.
-

- 11. Estep, T. N. HemAssist: development, clinical trials, lessons learned. In Blood Substitutes and Oxygen Biotherapeutics;Henry Liu, Alan D. Kaye, Jonathan S. Jahr;Springer: Pennsylvania, 2022; pp 287-292.
-

- 12. Harris, D. R.; Palmer, A. F. Modern Cross-linking Strategies for Synthesizing Acellular Hemoglobin-based Oxygen Carriers. Biotechnol. Prog. 2008, 24, 1215-1225.
-

- 13. Prempeh, A. B.; Cheng, D. C. O-Raffinose Cross-Linked Human Hemoglobin (Hemolink): History, Clinical Trials and Lessons Learned. In Blood Substitutes and Oxygen Biotherapeutics; Henry Liu, Alan D. Kaye, Jonathan S. Jahr;Springer: Pennsylvania, 2022; pp 305-312.
-

- 14. Moore, E. E.; Moore, F. A.; Fabian, T. C.; Bernard, A. C.; Fulda, G. J.; Hoyt, D. B.; Duane, T. M.; Weireter Jr, L. J.; Gomez, G. A.; Cipolle, M. D. Human Polymerized Hemoglobin for the Treatment of Hemorrhagic Shock When Blood is Unavailable: the USA Multicenter Trial. J. Am. Coll. Surg. 2009, 208, 1-13.
-

- 15. Jahr, J. S.; Moallempour, M.; Lim, J. C. HBOC-201, Hemoglobin Glutamer-250 (bovine), Hemopure® (Biopure Corporation). Expert Opin. Biol. Ther. 2008, 8, 1425-1433.
-

- 16. Rudolph, A. S. Encapsulated Hemoglobin: Current Issues and Future Goals. Artif. Cells. Nanomed Biotechnol. 1994, 22, 347-360.
-

- 17. Kaneda, S.; Ishizuka, T.; Goto, H.; Kimura, T.; Inaba, K.; Kasukawa, H. Liposome-encapsulated Hemoglobin, TRM-645: Current Status of the Development and Important Issues for Clinical Application. Artif. Organs. 2009, 33, 146-152.
-

- 18. Harris, J. M.; Chess, R. B. Effect of Pegylation on Pharmaceuticals. Nat. Rev. Drug. Discov. 2003, 2, 214-221.
-

- 19. Ekladious, I.; Colson, Y. L.; Grinstaff, M. W. Polymer–drug Conjugate Therapeutics: Advances, Insights and Prospects. Nat. Rev. Drug. Discov. 2019, 18, 273-294.
-

- 20. Cherian, V. T. Physiological Functions of Blood. In Blood Substitutes and Oxygen Biotherapeutics; Henry Liu, Alan D. Kaye, Jonathan S. Jahr;Springer: Pennsylvania, 2022; pp 33-43.
-

- 21. Bunn, H. F.; Forget, B. G. Hemoglobin-molecular, Genetic, and Clinical Aspects; 1986.
- 22. Alayash, A. I. Hemoglobin-based Blood Substitutes: Oxygen Carriers, Pressor Agents, or Oxidants? Nat. Biotechnol. 1999, 17, 545-549.
-

- 23. Klotz, I. M. Hemoglobin–oxygen Equilibria: Retrospective and Phenomenological Perspective. Biophys Chem. 2002, 100, 123-129.
-

- 24. Goutelle, S.; Maurin, M.; Rougier, F.; Barbaut, X.; Bourguignon, L.; Ducher, M.; Maire, P. The Hill Equation: a Review of Its Capabilities in Pharmacological Modelling. Fundam Clin. Pharmacol. 2008, 22, 633-648.
-

- 25. Henry Liu, Alan D. Kaye, Jonathan S. Jahr; In Blood Substitutes and Oxygen Biotherapeutics; Springer: Pennsylvania, 2022.
-

- 26. Umbreit, J. Methemoglobin—it's Not Just Blue: a Concise Review. Am. J. Hematol. 2007, 82, 134-144.
-

- 27. Tellone, E.; Barreca, D.; Russo, A.; Galtieri, A.; Ficarra, S. New Role for An Old Molecule: The 2, 3-diphosphoglycerate Case. Biochim. Biophys Acta. Gen. Subj. 2019, 1863, 1602-1607.
-

- 28. Campbell-Lee, S. A.; Ness, P. M. Packed Red Blood Cells and Related Products. Blood Banking and Transfusion Medicine: Basic Principles and Practice: Second Edition; Christopher D. Hillyer, Leslie E. Silberstein, Paul M. Ness, Kenneth C. Anderson, John D. Roback; Elsevier: Philadelphia, 2006; pp 250-258.
-

- 29. Vaughn, M. W.; Kuo, L.; Liao, J. C. Effective Diffusion Distance of Nitric Oxide in the Microcirculation. Am. J. Physiol. Heart Circ. Physiol. 1998, 274, H1705-H1714.
-

- 30. Liu, C.; Liu, X.; Janes, J.; Stapley, R.; Patel, R. P.; Gladwin, M. T.; Kim-Shapiro, D. B. Mechanism of Faster NO Scavenging by Older Stored Red Blood Cells. Redox. Biol. 2014, 2, 211-219.
-

- 31. Vaughn, M. W.; Huang, K.-T.; Kuo, L.; Liao, J. C. Erythrocytes Possess An Intrinsic Barrier to Nitric Oxide Consumption. J. Biol. Chem. 2000, 275, 2342-2348.
-

- 32. Vaughn, M. W.; Huang, K.-T.; Kuo, L.; Liao, J. C. Erythrocyte Consumption of Nitric Oxide: Competition Experiment and Model Analysis. Nitric. Oxide. 2001, 5, 18-31.
-

- 33. Butler, A. R.; Megson, I. L.; Wright, P. G. Diffusion of Nitric Oxide and Scavenging by Blood in the Vasculature Biochim Biophys Acta. Gen. Sub. 1998, 1425, 168-176.
-

- 34. Liao, J. C.; W. Hein, T.; Vaughn, M. W.; Huang, K.-T.; Kuo, L. Intravascular Flow Decreases Erythrocyte Consumption of Nitric Oxide. Proc. Natl. Acad. Sci. 1999, 96, 8757-8761.
-

- 35. Vallelian, F.; Buehler, P. W.; Schaer, D. J. Hemolysis, Free Hemoglobin Toxicity, and Scavenger Protein Therapeutics. Blood. Adv. 2022, 140, 1837-1844.
-

- 36. Deuel, J. W.; Schaer, C. A.; Boretti, F. S.; Opitz, L.; García-Rubio, I.; Baek, J.; Spahn, D. R.; Buehler, P. W.; Schaer, D. J. Hemoglobinuria-Related Acute Kidney Injury is Driven by Intrarenal Oxidative Reactions Triggering a Heme Toxicity Response. Cell. Death. Dis. 2016, 7, e2064-e2064.
-

- 37. Baek, J. H.; Yalamanoglu, A.; Gao, Y.; Guenster, R.; Spahn, D. R.; Schaer, D. J.; Buehler, P. W. Iron Accelerates Hemoglobin Oxidation Increasing Mortality in Vascular Diseased Guinea Pigs Following Transfusion of Stored Blood. JCI. Insight. 2017, 2.
-

- 38. Bunn, H. F.; Jandl, J. Exchange of Heme Among Hemoglobins and Between Hemoglobin and Albumin. J. Biol. Chem. 1968, 243, 465-475.
-

- 39. Belcher, J. D.; Chen, C.; Nguyen, J.; Milbauer, L.; Abdulla, F.; Alayash, A. I.; Smith, A.; Nath, K. A.; Hebbel, R. P.; Vercellotti, G. M. Heme Triggers TLR4 Signaling Leading to Endothelial Cell Activation and Vaso-occlusion in Murine Sickle Cell Disease. Blood. Adv. 2014, 123, 377-390.
-

- 40. Pfefferlé, M.; Ingoglia, G.; Schaer, C. A.; Yalamanoglu, A.; Buzzi, R.; Dubach, I. L.; Tan, G.; López-Cano, E. Y.; Schulthess, N.; Hansen, K. Hemolysis Transforms Liver Macrophages Into Antiinflammatory Erythrophagocytes. J. Clin. Invest. 2020, 130, 5576-5590.
-

- 41. Martins, R.; Maier, J.; Gorki, A.-D.; Huber, K. V.; Sharif, O.; Starkl, P.; Saluzzo, S.; Quattrone, F.; Gawish, R.; Lakovits, K. Heme Drives Hemolysis-induced Susceptibility to Infection via Disruption of Phagocyte Functions. Nat. Immunol. 2016, 17, 1361-1372.
-

- 42. Oesterhelt, F.; Rief, M.; Gaub, H. Single Molecule Force Spectroscopy by AFM Indicates Helical Structure of Poly(ethylene-glycol) in Water. New. J. Phys. 1999, 1, 6.
-

- 43. Ibrahim, M.; Ramadan, E.; Elsadek, N. E.; Emam, S. E.; Shimizu, T.; Ando, H.; Ishima, Y.; Elgarhy, O. H.; Sarhan, H. A.; Hussein, A. K. Polyethylene Glycol (PEG): The Nature, Immunogenicity, and Role in the Hypersensitivity of PEGylated Products. J. Control. Release. 2022, 351, 215-230.
-

- 44. Chen, B.-M.; Cheng, T.-L.; Roffler, S. R. Polyethylene Glycol Immunogenicity: Theoretical, Clinical, and Practical Aspects of Anti-polyethylene Glycol Antibodies. ACS Nano. 2021, 15, 14022-14048.
-

- 45. P Garay, R.; P Labaune, J. Immunogenicity of Polyethylene Glycol (PEG). Open. Conf. Proc. J. 2011; Vol. 2.
-

- 46. d'Avanzo, N.; Celia, C.; Barone, A.; Carafa, M.; Di Marzio, L.; Santos, H. A.; Fresta, M. Immunogenicity of Polyethylene Glycol-based Nanomedicines: Mechanisms, Clinical Implications and Systematic Approach. Adv. Ther. 2020, 3, 1900170.
-

- 47. Wan, X.; Zhang, J.; Yu, W.; Shen, L.; Ji, S.; Hu, T. Effect of Protein Immunogenicity and PEG Size and Branching on the Anti-PEG Immune Response to PEGylated Proteins. Process Biochem. 2017, 52, 183-191.
-

- 48. Zhai, Y.; Zhao, Y.; Lei, J.; Su, Z.; Ma, G. Enhanced Circulation Half-life of Site-specific PEGylated rhG-CSF: Optimization of PEG Molecular Weight. J. Biotech. 2009, 142, 259-266.
-

- 49. Turecek, P. L.; Bossard, M. J.; Schoetens, F.; Ivens, I. A. PEGylation of Biopharmaceuticals: a Review of Chemistry and Nonclinical Safety Information of Approved Drugs. J. Pharm. Sci.2016, 105, 460-475.
-

- 50. Zhou, Y.; Kopeček, J. Biological Rationale for the Design of Polymeric Anti-cancer Nanomedicines. J. Drug. Target. 2013, 21, 1-26.
-

- 51. Lundberg, P.; Lee, B. F.; van den Berg, S. A.; Pressly, E. D.; Lee, A.; Hawker, C. J.; Lynd, N. A. Poly[(ethylene oxide)-co-(methylene ethylene oxide)]: A Hydrolytically Degradable Poly(ethylene oxide) Platform. ACS Macro. Lett. 2012, 1, 1240-1243.
-

- 52. Roberts, M.; Bentley, M.; Harris, J. Chemistry for Peptide and Protein PEGylation. Adv. Drug. Deliv Rev. 2002, 54, 459-476.
-

- 53. Milla, P.; Dosio, F.; Cattel, L. PEGylation of Proteins and Liposomes: a Powerful and Flexible Strategy to Improve the Drug Delivery. Curr. Drug. Metab. 2012, 13, 105-119.
-

- 54. Kozlowski, A.; Harris, J. M. Improvements in Protein PEGylation: Pegylated Interferons for Treatment of Hepatitis C. J. Control. Release. 2001, 72, 217-224.
-

- 55. Kozlowski, A.; Charles, S. A.; Harris, J. M. Development of Pegylated Interferons for the Treatment of Chronic Hepatitis C. Bio. Drugs. 2001, 15, 419-429.
-

- 56. Veronese, F. M.; Pasut, G. PEGylation, Successful Approach to Drug Delivery. Drug. Discov. Today. 2005, 10, 1451-1458.
-

- 57. Gupta, V.; Bhavanasi, S.; Quadir, M.; Singh, K.; Ghosh, G.; Vasamreddy, K.; Ghosh, A.; Siahaan, T. J.; Banerjee, S.; Banerjee, S. K. Protein PEGylation for Cancer Therapy: Bench to Bedside. J. Cell. Commun. Signal. 2019, 13, 319-330.
-

- 58. Knoff, D. S.; Kim, S.; Fajardo Cortes, K. A.; Rivera, J.; Cathey, M. V.; Altamirano, D.; Camp, C.; Kim, M. Non-Covalently Associated Streptavidin Multi-Arm Nanohubs Exhibit Mechanical and Thermal Stability in Cross-Linked Protein-Network Materials. Biomacromolecules 2022, 23, 4130-4140.
-

- 59. Kono, H. Characterization and Properties of Carboxymethyl Cellulose Hydrogels Crosslinked by Polyethylene Glycol. Carbohydr. Polym. 2014, 106, 84-93.
-

- 60. Kamaraj, M.; Sreevani, G.; Prabusankar, G.; Rath, S. N. Mechanically Tunable Photo-cross-linkable Bioinks for Osteogenic Differentiation of MSCs in 3D Bioprinted Constructs. Mater. Sci. Eng. C 2021, 131, 112478.
-

- 61. Li, X.; Ding, J.; Zhang, Z.; Yang, M.; Yu, J.; Wang, J.; Chang, F.; Chen, X. Kartogenin-incorporated Thermogel Supports Stem Cells for Significant Cartilage Regeneration. ACS Appl. Mater. Interfaces 2016, 8, 5148-5159.
-

- 62. Harris, J. M.; Martin, N. E.; Modi, M. Pegylation: a Novel Process for Modifying Pharmacokinetics. Clin. Pharmacokinet. 2001, 40, 539-551.
-

- 63. Chang, T. M. S. Blood Substitutes Based on Nanobiotechnology. Trends. Biotechnol. 2006, 24, 372-377.
-

- 64. Stowell, C. P.; Levin, J.; Spiess, B. D.; Winslow, R. M. Progress in the Development of RBC Substitutes. Transfusion 2001, 41, 287-299.
-

- 65. Winslow, R. M. Cell-free Oxygen Carriers: Scientific Foundations, Clinical Development, and New Directions. Biochim Biophys Acta Proteins Proteom 2008, 1784, 1382-1386.
-

- 66. Alayash, A. I. Setbacks in Blood Substitutes Research and Development: a Biochemical Perspective. Clin. Lab. Med. 2010, 30, 381-389.
-

- 67. Buehler, P. W.; D’Agnillo, F.; Schaer, D. J. Hemoglobin-based Oxygen Carriers: from Mechanisms of Toxicity and Clearance to Rational Drug Design. Trends. Mol. Med. 2010, 16, 447-457.
-

- 68. Smani, Y. Hemospan: a Hemoglobin-based Oxygen Carrier for Potential Use as a Blood Substitute and for the Potential Treatment of Critical Limb Ischemia. Curr Opin Investig Drugs 2008, 9, 1009-1019.
- 69. Plastini, T.; Locantore-Ford, P.; Bergmann, H. Sanguinate: a Novel Blood Substitute Product. Blood. 2017, 130, 1120.
-

- 70. Romito, B. T.; Romito, J. W.; Abuchowski, A. Sanguinate: History and Clinical Evaluation of a Multimodal HBOCs. In Blood Substitutes and Oxygen Biotherapeutics; Henry Liu, Alan D. Kaye, Jonathan S. Jahr;Springer: Pennsylvania, 2022; pp 335-343.
-

- 71. Sakai, H.; Sou, K.; Tsuchida, E. Hemoglobin-vesicles as An Artificial Oxygen Carrier. Methods Enzymol 2009, 465, 363-384.
-

- 72. Sakai, H.; Takeoka, S.; Park, S. I.; Kose, T.; Nishide, H.; Izumi, Y.; Yoshizu, A.; Kobayashi, K.; Tsuchida, E. Surface Modification of Hemoglobin Vesicles with Poly(ethylene glycol) and Effects on Aggregation, Viscosity, and Blood Flow During 90 Exchange Transfusion in Anesthetized Rats. Bioconjug Chem 1997, 8, 23-30.
-

- 73. Matsuhira, T.; Sakai, H. Artificial Oxygen Carriers, from Nanometer-to Micrometer-sized Particles, Made of Hemoglobin Composites Substituting for Red Blood Cells. Particuology 2022, 64, 43-55.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2024; 48(5): 449-459
Published online Sep 25, 2024
- 10.7317/pk.2024.48.5.449
- Received on Jul 3, 2024
- Revised on Aug 27, 2024
- Accepted on Aug 27, 2024
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Conflict of Interest
Introduction
Hemoglobin as an Oxygen Carrier
Limitation of Hemoglobin as Oxygen Carrier
PEGylation: Conjugation Strategy Using PEG
Hemoglobin Based Oxygen Carrier
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- and Sejin Son
-
*Department of Biological Sciences and Bioengineering, Inha University, Incheon22212, Korea
***Department of Biological Sciences, Inha University, Incheon 22212, Korea - E-mail: ssejin@inha.ac.kr
- ORCID:
0000-0003-1523-2990








 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.