- Influence of Water Absorption Behavior on Mechanical and Tribological Performance of Polymer Materials
Shengpeng Zhan*, **, Ruize Li*, **, Tian Yang*, **, Dan Jia*, **, Jiesong Tu*, **, lixin Ma*, **, Bingxue Cheng***, and Haitao Duan*, **,†

*State Key Laboratory of Special Surface Protection Materials and Application Technology, Wuhan Research Institute of Materials Protection, Wuhan 430030, Hubei, China
**Hubei Longzhong Laboratory, Xiangyang 441000, Hubei, China
***State Key Laboratory of Tribology, Tsinghua University, Beijing 100084, China- 물 흡수에 따른 다양한 고분자 재료의 기계적/트라이볼로지적 물성 연구
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
The physical, chemical and mechanical properties of polymer materials used in water environment for a long time will change due to their water absorption characteristics, which will affect their tribological properties. This work studied the changes of physicochemical, mechanical, and tribological properties for five kinds of polymer materials (polyethylene, polyformaldehyde,polyamide, polytetrafluoroethylene, and poly-ether-etherketone) before and after water absorption. The mechanism of water absorption for polymer materials was revealed by molecular dynamics simulation. The results show that the higher the water absorption of polymer, the larger the swelling volume, the more the surface hardness and compressive strength decrease, and the toughness and friction coefficient of the material increase obviously. The water absorption status of polymer materials is closely related to the polarity of molecular chain. Water molecules are easy to form hydrogen bonds with polymer molecular chains of high polarity, which is the main factor of water absorption.
This work studied the changes of physicochemical, mechanical and tribological properties for five kinds of polymer materials [polyethylene (UHMWPE), polyformaldehyde (POM), polyamide (PA6), polytetrafluoroethylene (PTFE), and poly-ether-etherketone (PEEK)] before and after water absorption. The mechanism of water absorption for polymer materials was revealed by molecular dynamics simulation.

Keywords: polymer, water absorption, tribological performance, molecular dynamics.
This work was supported by the National Natural Science Foundation of China (Grant No. 52175183 and 51805377), Project of enterprise technology innovation in Wuhan (2020010602012060).
The authors declare that there is no conflict of interest.
Polymer materials usually have the advantages of corrosion resistance, self-lubrication, high insulation, vibration resistance and embedded sand. It is widely used that replaced metal materials as friction components in water wading engineering equipment.1-5 Such as bush of water lubricated bearing, sliding rail, gears and sealing parts, etc. Polymer materials for long-term service in aqueous environments will undergo varying degrees of reversible (changes of physical properties) or irreversible (changes of chemical properties) degradation, which will affect the mechanical and tribological properties of polymer materials.6-8 Different polymer materials have different water-absorbing characteristics, so the working conditions of selecting them as components of friction pair are also different. In regard to the friction pair parts, such as water lubricated bearing, sliding rail, etc., it should be avoided that the materials swelling after water absorption will cause the "stuck" phenomenon of the friction pair matching deformation.9 However, such as seals, fasteners, etc., it is more reliable that swelling effects make the sealing or fastening for the parts.10 Therefore, the research on the water absorption behavior of polymer friction pair materials has great value of engineering application.
At present, the research on water absorption of polymer materials mainly focuses on the diffusion mechanism. Broudin et al.11 characterized the diffusion of water in polyamide 6.6 (PA66) under a wide range of temperature (from 25 to 80 ℃) and various humidities using dynamic vapor sorption machine. It is found that the classic Arrhenius behavior can be used to describe the diffusion coefficient of water in PA66 when the amorphous phase is in a glassy state. When the amorphous phase is in a rubbery state, the water diffusion coefficient on temperature and water activity can be described using the free volume theory. Courvoisier et al.12investigated water absorption of PEEK and PEI, and found that both polymer materials showed typical Henry and Fick diffusion behavior. Simultaneously, the relationship between semi-empirical structure and water transport properties was provided for polymer materials with group of high polar (hydroxyls and sulfones), medium polar (carbonyls of imides, amides and ketones) and low polar (carbonyls of esters). Pogany, Roy and Gilormini et al.13-16 found that the diffusion of water in polymers does not fully obey the Fick’s law, and conducted a systematic study of the non-Fick diffusion behavior of water in polymer materials. The author's research team Chen et al.17 studied the influence of hydrostatic pressure on water absorption of polyoxymethylene by water absorption test and molecular dynamics. The results show that the water absorption of polyoxymethylene decreases with the increase of hydrostatic pressure in the range of 0-3.0 MPa, it subsequently increases with the increase of hydrostatic pressure in the range of 3.0-5.0 MPa. Water diffusion coefficient in polyoxymethylene gives trend of firstly decreasing and then increasing with the increase of hydrostatic pressure. The simulation results are in good agreement with the experimental results. However, these studies on the diffusion mechanism of water in polymer materials are still controversial, it is unclear that the leading relationship between water absorption of polymer and its molecular free volume and molecular polar groups.
In addition, the influence of water absorption for polymer on its physical and chemical properties, mechanical properties and tribological properties has also been reported by domestic and foreign scholars. Bouvet et al.18 observed that plasticization of polymer materials because of water absorption, resulting in the decrease of stiffness and glass transition temperature (Tg) for polymer materials. Das et al.19 found that the mechanical properties of linear low-density polyethylene for grafted with maleic anhydride (LLDPE-g-MA) were significantly reduced after water absorption. Alamri et al.20 found that the flexural strength and modulus of epoxy-based nanocomposites decreased with increasing water absorption, but the fracture toughness and impact strength of the material increased after water absorption. Dhakal et al.21 found that the tensile and flexural properties of fiber-reinforced unsaturated polyester composites decreased with the increasing moisture absorption. At the same time, high temperature significantly enhanced the degradation of the composites and the water absorption showed non-Fick, while the water absorption at room temperature conformed to Fick law. Yamamoto et al.22 found that the attraction of carbonyl groups in the molecular structure of poly-ether-ether-ketone (PEEK) materials to water molecule is the main reason for the decrease of hardness caused by surface plasticization deformation during water lubrication, and the reduction of hardness will aggravate surface wear of PEEK materials. Zhao et al.23 found that water absorption increased the elongation and significantly reduced the flexural strength of PTFE/PA6 bends. Wang et al.24 found that polyamide (PA) materials have high water absorption due to the easy formation of hydrogen bonds between amide groups and water, resulting in lower mechanical properties and abrasion resistance. Cao et al.25 found that the invading water molecules led to a sharp decrease in the hardness of PPESK surface, resulting in very high wear rate of PPESK in water. The wear processes of CF/PPESK in water were no longer dominated by high water absorption but by the load-carrying effect of CFs. Kawaljit et al.26 reviewed the effect of environmental humidity/water absorption on the tribo-mechanical performance of polyamides, epoxy resins, PMMA and their composites, and found that the mechanical and tribological properties of polymers and polymer composites varied with environmental humidity, the pure polymer substrates exhibit a somewhat complex mixing behavior in humid environments. It can be seen that water absorption behavior of polymer materials is a relatively complex process and has a great influence on the physicochemical, mechanical and tribological properties of polymer, and there are many related research reports. However, the polymer materials used in wading engineering are usually immersed in water for a long time. Currently, there are few studies on the physicochemical, mechanical and tribological properties of polymer materials after long-term immersion in water. Simultaneously, the research that interaction between behavior of water absorption and the physicochemical, mechanical and tribological properties of polymer materials in water environment is rarely reported.
In this work, the long-term immersion test of water absorption will be carried out for five kinds of typical engineered polymer materials, such as ultra-high molecular weight polyethylene (UHMWPE), polyformaldehyde (POM), polyamide (PA6), polytetrafluoroethylene (PTFE), and poly-ether-ether-ketone (PEEK). The crystallinity, molecular structure, mechanical properties and tribological properties of the polymer materials before and after water absorption were analyzed and discussed. At the same time, molecular simulation was used to study the change mechanism of water molecules in molecular chain of polymer at microscale. This work is not only helpful to enrich and perfect the theory of polymer tribology, but also it has important scientific significance and engineering value for the application of polymer materials in wading tribology.
Details of the Experiment. Experimental Materials: UHMWPE and PTFE are non-polar, POM is weakly polar, and PA6 and PEEK are polar among the five kinds of typical friction pair materials of polymer for marine engineering. The material performance parameters are as shown in Table 1.
The chemical structure formula is shown in Figure 1.
Experimental Method-Test Method of Water Absorption: Test of water absorption for polymer materials is conducted according to standard of Plastics-Determination of water absorption (ISO 62: 2008). The sample size is Φ40 mm×13 mm, and three samples of each polymer material are prepared for water absorption test. The specimens shall be carefully dried prior to testing in an oven at 50±2 ℃ for at least 24 hours and allow them to cool to room temperature in the desiccator before weighing them to the nearest 0.1 mg. Repeat this process until the mass of the specimens is constant to within ±0.1 mg. Then place the test specimens in a container filled with distilled water maintained at 23±2 ℃ and a relative humidity of (50±5)%. After immersion for every period of time (24 h, 48 h, 96 h…), take the test specimens from the water and remove all surface water with filter paper. Reweigh the test specimens to the nearest 0.1 mg. The formula for calculating the mass fraction of water absorption is as follows:

Cabsorpis the mass fraction of water absorption for sample, miand m0are the first mass and the i-th mass of the sample respectively.
Physicochemical Characterization of Material Water Absorption: Heat flow curve of materials for before and after immersion were tested using Differential Scanning Calorimeter (DSC, NETZSH DSC-204 HP, Germany) according to the ASTM F2625-10 standard. Test parameters: the weight of test sample is about 5.0-6.5 mg, the temperature is 60-200 ℃, the heating rate is 10 ℃/min, and the nitrogen atmosphere environment. The crystallinity of the polymer material before and after water absorption can be obtained by calculating the area of the heat flow curve. Calculated as follows:

c is the percentage content of crystallized area in the formula. ΔHmwas the melting enthalpy of test materials. ΔHm0
the melting enthalpy of polymer material with 100% crystallinity, and the UHMWPE, POM, PA6, PTFE and PEEK were 293, 185, 190, 82, and 130 J/g, respectively. In addition, The Fourier Transform Infrared Spectrometer (FTIR, NICOLET iS10, ThermoFisher, Waltham, MA, USA) was used to characterize and analyze the chemical structure of the polymer materials before and after water absorption, and obtain the chemical structure change of the materials.
Mechanical Properties Testing of Polymer Materials: In order to evaluate the changes of mechanical properties for polymer materials before and after water absorption, five kinds of polymer materials including UHMWPE, POM, PA6, PTFE and PEEK were carried out by Universal Testing Machine. The test was performed according to standard of Plastics-Determination of compressive properties (ISO 604: 2002). The sample size was (10±0.2) mm×(10±0.2) mm×(4±0.2) mm and the loading rate was 1.0 mm/min. The surface hardness of polymer materials will be measure by Shore D hardness tester. The 5 points were measured every 6 mm along the radial direction of the sample and their average value was calculated to obtain the material hardness.
Tribological Test of Samples: Tribological tests of UHMWPE, POM, PA6, PTFE and PEEK materials (before and after water absorption) were carried out on the reciprocating friction testing machine of MFT 5000 by Retc Company. The friction pair is the form of ball-on-disk and dry friction environment. The ball samples are the material of GCr15 and the disk samples are polymer materials. The test environment temperature is 25±2 ℃ and the relative humidity is (50±5)%. The test parameters are shown in Table 2.
The morphology of worn traces was measured by Micromeasure 2 confocal scanning optical microscope (STIL, Sciences et Techniques Industrielles de la Lumière, Aix en Provence, France). The scanning area is 12.0 mm×4.0 mm. The scanning steps in X direction and Y direction are 20 μm and 10 μm, respectively. Moreover, the microstructure of the worn area was observed by scanning electron microscope (SEM, JSM-6510LV, JEOL, Japan) and the elemental of the surface was analyzed by energy-dispersive spectroscopy (EDS).
Detail of Molecular Dynamics. In order to reveal the water absorption mechanism of five kinds of polymer materials, molecular dynamics method27,28 is used to study the change mechanism of water molecules inside the micro-structure of polymer materials. The simulation is based on the software of Materials Studio. The model building of molecular dynamics is mainly included the following six steps: Firstly, the models of molecular chain were constructed based on the chemical structure of polymer materials. The degree of polymerization for UHMWPE, POM and PTFE molecular chains is 100, respectively. The degree of polymerization of PA6 and PEEK was set at 50 because there are more atoms of monomer. Secondly, the energy of the chain was minimized by using the method of maximum velocity descent to obtain the conformation of the chain with low-energy stability. Thirdly, the amorphous cells were composed of three polymer chains and different concentrations of water molecules (number of H2O: 0, 5, 10, 20, 30, 60, 90), as shown in Figure 2. The initial density of cell was determined according to the density of polymer material, and the mass fraction of water molecules in the polymer material is shown in Table 3. Fourthly, the global minimum energy of amorphous cell (the most stable structure) was got by using the method of annealing kinetics (the range of annealing temperature is 300-500 K). Fifthly, a molecular dynamic simulation of 500 ps was performed by using the NPT ensemble (P=0.1 MPa, T=298 K) and control method of Nosé-Hoover thermal bath,29,30 which resulted in the actual density for the water absorption model of polymer material. Lastly, molecular dynamics simulations were performed on the optimized cell structure under the NVT ensemble, and the simulation parameters are shown in Table 4.

|
Figure 1 Chemical structure formula of polymer. |

|
Figure 2 Molecular dynamics model of water absorption of polymer materials: (a) UHMWPE; (b) POM; (c) PA6; (d) PTFE; (e) PEEK. |
|
Table 2 Sample Information and Parameters of Ball-disc Reciprocating Friction Testing |

Analysis of Water Absorption Characteristics of Polymer Materials. Figure 3(a) shows the variation of mass fraction of water absorption for five polymer materials, UHMWPE, POM, PA6, PTFE and PEEK. It can be seen that the water absorption mass fraction increases gradually with the increase of immersion time and finally tends to saturation state. The material of PA6 had the highest water absorption mass fraction, which was still not saturated after immersing for 13872 h (578 days), followed by POM, PEEK, respectively. The materials of UHMWPE and PTFE had the lowest mass fraction of water absorption. Figure 3(b) displays the variation of rate of water absorption for polymer materials. The rate of water absorption for POM, PA6, and PEEK started to be larger, and the rate of water absorption became smaller and smaller and finally stabilized with the increase of time. However, the rate of water absorption fluctuates in the early stage of immersion, and then the rate of water absorption basically does not change.
According to the curve of mass fraction of water absorption, the relationship between mass fraction of water absorption and immersing time is a power function. The corresponding relationship between water absorption mass fraction of POM, PA6, PEEK and time is shown below:
The relationship of water absorption mass fraction for POM:
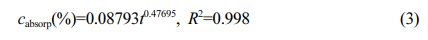
The relationship of water absorption mass fraction for PA6:
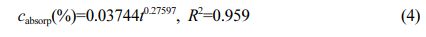
The relationship of water absorption mass fraction for PEEK:
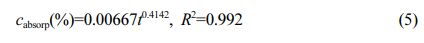
Where cabsorp is water absorption mass fraction -%; t is water absorption time (t ≥ 24 h)-h; R2 is the degree of fit.
Figure 4 shows the average water absorption mass fraction and volume change of the five polymer materials after immersing for 13872 h. It can be seen that the volume change of the polymer materials is basically in a positive proportion to the average water absorption mass fraction. The water absorption mass fraction and volume change of PA6 is the largest, while that of PTFE is the smallest. The shows that the swelling effect of polymer is closely related to its water absorption, and the change of volume and size has a great influence on the fitting accuracy of the friction pair, thus affecting its tribological properties.
Analysis of Physicochemical Properties of Polymer Materials After Water Absorption. Figure 5 shows the DSC heat flux curves and the crystallinity changes of five polymers materials. It can be seen that PTFE and PEEK were more temperature-resistant among the five typical polymer materials, followed by PA6, POM and UHMWPE. According to melting enthalpy and the calculation of crystallinity (formula 2), the crystallization of UHMWPE and POM materials decreased after water absorption, and the crystallization of PA6, PTFE and PEEK increased after water absorption. The molecular chain of UHMWPE and POM with high crystallinity will be destroyed by the effect of water molecular hydroxyl, and the crystallinity will be slightly decreased. For PA6, PTFE and PEEK materials with low crystallinity, most of the water molecules have amorphous regions. Under the force of water molecules, the disordered molecular chains may be arranged in order to a certain extent, resulting in a decrease in crystallinity. The main reason is that water molecules are inside the polymer, which affects the arrangement of polymer molecular chains. But in general, the water has little effect on the crystallinity of polymer materials.
Figure 6 shows the infrared spectra of the five polymer materials. It can be seen that there are no obvious new peak appears in the five polymer materials after water absorption. Near-infrared spectra showed that this increase in intensity of the O-H band is due to the increase of monomeric water molecules hydrogen bonded to polymer materials,31 the peak intensity is change due to the action of the O-H bond of water molecules in the polymer material.
Analysis of Microscopic Water Absorption of Polymer Materials. Calculation of Hydrogen Bond : Water is a polar molecule, and the hydrogen atoms (H) on water molecules are easy to form hydrogen bond interactions with more electronegative atoms (such as O, N, F atoms, etc.). Water absorption characteristics of polymer materials have a great relationship with hydrogen bond interactions. The number of hydrogen bonds of polymer molecular cell calculated with the perl script of Materials Studio software. Figure 7 shows the change curve of the number of hydrogen bonds with the concentration of Pwater molecules in polymer. It can be seen that the number of hydrogen bonds inevitably increase with the number of water molecules. The main reason is that not only hydrogen bonds can be formed between water molecules, but also hydrogen bonds can be formed between atoms (such as O, N, F) on the molecular chain of polymer materials and water molecules. In addition, the largest number of hydrogen bonds formed between water molecules and the molecular chains of PA6 under the same concentration of water molecules, followed by POM. The simulation results are consistent with the test of water absorption. It shows that water molecules are more likely to form hydrogen bonds with the molecular chains of PA6 (nitrogen atom on amide group, -NHCO-) and POM (nitrogen atom on ether, C-O-C) materials.32 Figure 8 shows the internal distribution state of water molecules in five polymer materials, The ball-and-stick model shows water molecules, the linear structure model shows the molecular chains of the polymer material, and the dashed lines represent hydrogen bonds. It can be observed that hydrogen bonds are formed between water molecules inside UHMWPE and PTFE, and forming water cluster structure. Although hydrogen bonds are easy to form between the water molecules and fluorine atoms, it is difficult for water molecules to disperse uniformly within the PTFE chain because of the helical conformation (a protective layer was formed by fluorine atoms on the outside of the carbon chain) of PTFE molecular chain.33 The water molecules are uniform distributed inside the PA6, POM, and PEEK materials due to the hydrogen bonding between water molecules and their molecular chains of polymer. It is main reason for the higher water absorption of PA6, POM, and PEEK polymer materials.
Calculation of Diffusion Coefficient: The movement state of water molecules in the polymer material can be expressed by the diffusion coefficient.34 The formula is as follows:
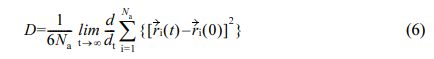
D is the diffusion coefficient, Narepresents the number of water molecules, ri(0), ri(t) are the positions of atom i at the initial and t time, respectively.
Figure 9 presents the diffusion coefficients of different concentrations of water molecules in five polymer materials. It can be seen that the diffusion coefficient almost increases with the increase of water concentration, which because that more water molecules are easy to form water clusters at a higher water concentration. There are fewer hydrogen bonds formed between water molecules and molecular chains of polymer, and the aggregated molecules of water cluster are more likely to move, which increases the diffusion coefficient. In addition, Due to the effect of hydrogen bonds between water molecules and chains of polymer, the water molecules are uniformly distributed inside the material, making it difficult for the water molecules to move. Therefore, the diffusion coefficient of water molecules in POM, PA6, and PEEK is relatively small. In contrast, the water molecules form water clusters in the UHMWPE and PTFE materials, which are easy to diffuse and move, so their diffusion coefficients are high.
Calculation of Fractional Free Volume: The polymer system after water absorption is mainly composed of the occupied space volume (the volume occupied by water molecules and polymer molecular chains) and the unoccupied free space volume. Fractional free volume (FFV)35 is the ratio of the free space volume of the polymer system after water absorption to the total volume of the molecular cell, which can be used to evaluate the movable space of water molecules in the polymer material. The formula is as follows:

Vfree is free volume, Vocc is the occupied volume. As can be seen from Figure 10, the variation trend of fractional free volume of the five polymer materials is consistent with their diffusion coefficients. The larger the fractional free volume, the larger the diffusion coefficient. The POM has the smallest fractional free volume, which indicates that water molecules are not easy to diffuse and stay in the POM material, followed by PA6, PEEK, UHMWPE and PTFE. Moreover, the fractional free volume of water absorbing polymer water initially decreased with the increase of water concentration and then increased, and finally remained basically stable. The main reason is that the free volume inside the polymer material decreases when the concentration of water molecules is small. However, with the further increase of the water concentration in the later stage, the volume of the polymer material expands so that the fractional free volume increases.
Calculation of Cohesive Energy Density: Cohesive Energy Density (CED) is a physical quantity to evaluate the intermolecular force, which mainly characterizes the interaction between molecular groups. In general, the greater the polarity of the groups contained in a molecule, the greater the interchain force and the corresponding cohesive energy density. Figure 11 shows the CED of the five polymer materials, and it shows that CED of five polymer materials increased with the increase of water molecule concentration. Meanwhile, the water absorption of polymer materials is proportional to its change of CED. It can be seen that the water absorption of polymer materials is closely related to the polarity of its structure of molecular chain. Figure 12 shows the change of CED for different numbers of water molecules, the verifies the phenomenon that the CED of the five polymer materials increases with the increase of the concentration of water molecules. The CED of non-polar UHMWPE and PTFE molecular chains is significantly lower than that of polar POM, PA6, and PEEK molecular chains.
Through the molecular dynamics simulation of the microscopic water absorption process of polymer materials, it can be seen that the water absorption of polymer materials is closely related to the interaction between the molecular chain group and the hydrogen bond formed by water molecules. The hydrogen bond force makes it difficult for water molecules to diffuse in the polymer materials, thus affecting the water absorption performance of the materials. At the same time, water molecules will increase the spacing of polymer molecular chain arrangement, thus affecting the mechanical properties of materials.
Analysis of Mechanical Properties of Polymer Materials After Water Absorption. Figure 13 shows the hardness contrast of five polymer materials before and after water absorption. As can be seen from the figure, the hardness of PA6 decreased the most after water absorption after water absorption, followed by POM and PEEK. the change of UHMWPE and PTFE hardness is the least. Thus, the higher the water absorption, the lower the surface hardness due to the water absorption and plasticization of the polymer material, which will affect the mechanical and tribological properties of the polymer material.
Figure 14 shows the curves of compressive stress-strain and compressive elastic modulus changes of five typical polymer materials before and after water absorption. It can be seen from Figure 14(a-e) that UHMWPE and PTFE have little difference in elastic stage and yield stage before and after water absorption due to their low water absorption. However, in the strain hardening stage (when the strain reaches about 35%), the stress of UHMWPE and PTFE materials without water absorption decreased sharply after reaching the maximum stress until destruction, while the samples after water absorption are still in the strain hardening stage. In addition, the maximum compressive stress points of POM, PA6, and PEEK moved backward obviously, which indicates that water absorption of the material causes swelling of the polymer materials and their toughness increases significantly. It can also be seen from Figure 14(f) that the compressive elastic modulus of PA6 material decreased the most after water absorption, reaching 75.36%. followed by PEEK, POM, and UHMWPE, which decrease by 35.97, 28.62, and 22.10% respectively. The elastic modulus of PTFE compression has little change. It can be seen that water absorption has an obvious effect on the mechanical properties of polymer materials.
Analysis of Tribological Properties of Polymer Materials After Water Absorption. The influence of water absorption on the mechanical properties of the material is significant, and the tribological properties of the material are also changed. Figure 15 shows the average friction coefficient curves of the five polymer materials after 200s sliding under 20 N and 50 N loading, respectively. It can be seen that the load is inversely proportional to the friction coefficient, which conforms to the basic laws of tribology. In addition, water absorption has a most significant effect on the friction coefficient of PA6 among the five kinds of materials, while the other four kinds of materials have a lower effect due to the low water absorption. There are two main reasons for the increase of friction coefficient of PA6 material after water absorption. On the one hand, the surface hardness and elastic modulus of PA6 material decrease due to the effect of water absorption and plasticizing, which makes the material deformation more seriously and the friction coefficient of furrow component increase under the same load. On the other hand, the steel ball needs to overcome large interaction force between molecular chains and hydrogen bond interaction force during sliding friction process, which makes the component of the adhesive friction coefficient larger under the same load.
Figure 16 shows the morphology of worn traces of polymer materials before and after water absorption under different test loading conditions. The abrasion depth of UHMWPE and POM materials are very shallow, the abrasion depth of PA6 material increased obviously after absorbing water. In order to quantitatively compare the wear resistance of the polymer material before and after water absorption, SPIP software was used to analyze and calculate the surface wear volume. The wear volume changes of the five kinds of polymer materials before and after water absorption under normal loading conditions of 20 N and 50 N are shown in Figure 17. Both POM and UHMWPE have the smallest wear volume under normal loads of 20 N and 50 N, which indicates that they have better wear resistance. The wear volume of PA 6 decreases by 38.5% and 28.8% under 20 N and 50 N normal loads, respectively. The main reason is that the interaction force between molecular chains of PA6 material increases after water absorption, and the interaction force between molecular chains increases, and the surface molecular chains are easily pulled out during the friction process. Because of the small water absorption of PTFE, the wear volume of the material has little change before and after water absorption. PEEK have weaker interaction between molecular chains compared with PA6 material, so the influence of water absorption on its wear resistance is not as great as that of PA6 material. The wear resistance of PEEK also decreased. The wear volume decreases by 5.3% and 7.6% under 20 N and 50 N normal loads, respectively.
Figure 18 shows the microstructure morphology of the wear surface of the five kinds of polymer materials before and after water absorption under 50 N normal loads. It can be seen that the wear surface of UHMWPE without water immersion is obviously rough and furrow abrasive wear occurs, while the wear surface of UHMWPE after water absorption is relatively smooth. The wear surface of POM material has little change before and after water absorption, and there are microcracks in the wear region. The wear surface of PA6 without water immersion is obviously different from that of immersed PA6 material. The wear surface of PA6 material without immersion has less adhesive wear region, while the wear surface of immersed PA6 material has larger adhesive wear area and it has tearing phenomenon. The surface of PTFE material immersed in water is smoother than that of the material not immersed in water. Adhesive wear was observed on the wear surface of both unsoaked and soaked for PEEK materials, but the adhesive wear degree of PEEK after water absorption was lighter than that of unabsorbent PEEK materials.

|
Figure 3 (a) Water absorption mass fraction; (b) water absorption rate of the five materials. |

|
Figure 4 (a) Average water absorption mass fraction; (b) volume change of five polymer materials after water. |

|
Figure 5 DSC heat flux curves and the crystallinity changes of five polymers materials before and after water absorption. |

|
Figure 6 IR spectra of polymer materials before and after water absorption |

|
Figure 7 Number of hydrogen bonds varies with the concentration of water molecule molecules. |

|
Figure 8 Distribution of water molecules in the polymer material. |

|
Figure 9 Diffusion coefficients of different concentrations of water molecules in polymer. |

|
Figure 10 Fractional free volume of different concentrations water molecular in polymer. |

|
Figure 11 Cohesive energy density of polymer materials at different water molecular concentrations. |

|
Figure 12 CED changes of different amount of water molecules. |

|
Figure 13 Hardness comparison of polymer material before and after water absorption. |

|
Figure 14 Compressive stress-strain curve of polymer material before and after water absorption |

|
Figure 15 Average friction coefficient of polymer material before and after water absorption for different normal force: (a) 20 N; (b) 50 N |

|
Figure 16 Morphology of worn traces of polymer materials before and after water absorption under different test loading conditions. |

|
Figure 17 Wear volume of polymer material before and after water absorption for different normal force: (a) 20 N; (b) 50 N. |

|
Figure 18 Microscopic surface of wear region of polymer material before and after water absorption. |
In this work, the water absorption characteristics of five typical polymer materials (UHMWPE, POM, PA6, PTFE, PEEK) were studied by water absorption test. The changes of physicochemical, mechanical and tribological properties of polymer materials before and after water absorption were systematically investigated. Combined with molecular dynamics simulation method, the mechanism of water absorption of polymer materials, the relationship between water absorption and molecular structure of materials are revealed from microscope to macroscope scale. The main conclusions of this work are as follows:
(1) Among the five kinds of polymer materials, the water absorption rate from the largest to the smallest is PA6, POM, PEEK, UHMWPE, PTFE. The water absorption mass fraction of PA6, POM, and PEEK materials was a power function of immersing time. With the increase of immersion time, the water absorption of the material gradually tends to saturation state. The swelling volume of the five kinds of polymer materials is proportional to their water absorption.
(2) The water absorption of polymer materials is closely related to the structure of molecular chains (including polar groups of molecular chains, diffusion coefficient, cohesive energy density, etc.). Because the molecular chains of POM, PA6, and PEEK are weakly polar or polar groups, water molecules can easily form hydrogen bonds with their molecular chains. The effect of hydrogen bond force makes water molecules evenly distributed in the interior of the material, and it is not easy to diffuse in the interior of PA6, POM, and PEEK materials, resulting in high water absorption. However, it is difficult for water molecules to form hydrogen bonds with non-polar UHMWPE and PTFE material molecular chains. Water molecules are distributed in clusters inside the materials due to the effect of hydrogen bond force between water molecules.
(3) The higher the water absorption mass fraction of the five polymer materials, the more the surface hardness decreases, the more serious the plasticization degree, the compressive strength is greatly reduced, and the toughness of the materials is significantly improved. The longer the water absorption time of five kinds of polymer materials, the more their mechanical properties will decline, and the decline of mechanical properties is the main factor affecting the increase of their friction coefficient and wear amount.
- 1. Olabisi, O.; Kolapo, A. Handbook of Thermoplastics; CRC press: Boca Raton, 2016.
- 2. Yan, Z. M.; Zhou, X. C.; Yuan, C. Q.; Zhang, Q., Ma, J. J.; Chen, J. X. Development and Study of Ship Stern Bearing Materials of American Naval Vessels. Ship Eng. 2015, 37, 1-5.
- 3. Abdulaziz, K.; Li, C. Recent Advances in High Performance Polymers—tribological Aspects. Lubricants 2019, 7, 2.
- 4. Wang, C. P.; Wang, S. H.; Wang, H. Z.; Ruan, G. L.; Xiao, Y. X.; Jiang, Z. G.; Li, X. P. Research Status and Prospects of Tribological Behaviors of Key Friction Pairs of Materials in Marine Equipment. Mater. Sci.-Medžiagotyra 2021, 27, 148-154.
-

- 5. Wu, K. P.; Zhou, G. W.; Mi; X. W.; Zhong, P.; Liao, D. X. Tribological and Vibration Properties of Three Different Polymer Materials for Water-lubricated Bearings. Materials 2020, 13, 3154.
-

- 6. Wang, C. H. Plastic Tribology—Theory and Practice of Friction, Wear and Lubrication; China Machine Press: Beijing, 1994.
- 7. Gonçalves, E. S.; Poulsen, L.; Ogilby, P. R. Mechanism of the Temperature-dependent Degradation of Polyamide 66 Films Exposed to Water. Polym. Degrad. Stab. 1985, 92, 1977-1985.
-

- 8. Song, Y.; Wei, M. J.; Xu, F.; Wang, Y. Transport Mechanism of Water Molecules Passing Through Polyamide/COF Mixed Matrix Membranes. Phys. Chem. Chem. Phys. 2019, 21, 26591-26597.
-

- 9. Jiang, S. Y.; Zhang, S. W. Progress in the Key Technologies of High Speed Motorized Spindle Supported by Water Lubricated Bearings. Mach. Des. Manuf. Eng. 2016, 45, 11-17.
- 10. Zhang, J. H.; Zhang, J. Q.; Cui, T., Wang, D. L.; Liu, J. C. Preparation and Properties of EPDM/WSPU Water-swellable Rubber. Polyurethane Industry 2022, 37, 12-15.
- 11. Broudin, M.; Gac, P. Y. L.; Saux, V. L.; Champy, C.; Robert, G.; Charrier, P.; Marco, Y. Water Diffusivity in PA66: Experimental Characterization and Modeling Based on Free Volume Theory. Eur. Polym. J. 2015, 67, 326-334.
-

- 12. Courvoisier, E.; Bicaba, Y.; Colin, X. Water absorption in PEEK and PEI Matrices. Contribution to the Understanding of Water-polar Group Interactions. AIP Conf. Proc. 2016, 1736, 020036.
-

- 13. Pogany, G. A. Anomalous Diffusion of Water in Glassy Polymers. Polymer 1976, 17, 690-694.
-

- 14. Roy, S.; Xu, W. X.; Park, S. J. Liechti K M. Anomalous Moisture Diffusion in Viscoelastic Polymers: Modeling and Testing. J. Appl. Mech. 2000, 67, 391-396.
-

- 15. Carter, H. G.; Kibler, K. G. Langmuir-type Model for Anomalous Moisture Diffusion in Composite Resins. J. Compos. Mater. 1978, 12, 118-131.
-

- 16. Pierre, G.; Jacques, V. On the Role of Hydrogen Bonding on Water Absorption in Polymers. Polymer 2018, 142, 164-169.
- 17. Chen, S.; Xu, H. P.; Duan, H. T.; Hua, M.; Lei, W.; hang, H. F.; Li, J. Influence of Hydrostatic Pressure on Water Absorption of Polyoxymethylene: Experiment and Molecular Dynamics simulation. Polym. Adv. Technol. 2017, 28, 59-65.
-

- 18. Bouvet, G.; Cohendoz, S.; Feaugas, X.; Touzain, S.; Mallarino, S. Microstructural Reorganization in Model Epoxy Network During Cyclic Hygrothermal Ageing. Polymer 2017, 122, 1-11.
-

- 19. Das, V.; Kumar, V.; Singh, A. Compatibilization Efficacy of LLDPE-g-MA on Mechanical, Thermal, Morphological and Water Absorption Properties of Nylon6/LLDPE Blends. Polym. Plast. Technol. Eng. 2012, 51, 446-454.
-

- 20. Alamri, H.; Low, I. Effect of Water Absorption on the Mechanical Properties of Nano-filler Reinforced Epoxy Nanocomposites. Mater. Des. 2012, 42, 214-222.
-

- 21. Dhakal, H.; Zhang, Z.; Richardson, M. Effect of Water Absorption on the Mechanical Properties of Hemp Fibre Reinforced Unsaturated Polyester Composites. Compos. Sci. Technol. 2007, 67, 1674-1683.
-

- 22. Yamamoto, Y.; Takashima, T. Friction and Wear of Water Lubricated PEEK and PPS Sliding Contacts. Wear 2002, 253, 820-826.
-

- 23. Zhao, R.; Luo, W.; Xiao, H.; Zhong, G. Water-absorptivity and Mechanical Behaviors of PTFE/PA6 and PTFE/PA66 Blends. Trans. Nonferrous Met. Soc. China 2006, 16, 498-503.
-

- 24. Wang, J. X.; Gu, M. Y. Wear Properties and Mechanisms of Nylon and Carbon-fiber-reinforced Nylon in Dry and Wet Conditions. J. Appl. Polym. Sci. 2004, 93, 789-795.
-

- 25. Cao, F. X.; Wang, J. Z.; Yan, F. Y. Probing the Tribological Behaviors in Water of Novel Poly(phthalazione ether sulfone ketone) and Its Composites Based on the State of Aggregation and the Microstructure. Polym. Adv. Technol. 2017, 28, 1071-1077.
-

- 26. Kawaljit, S. R.; Ashwin, P. The Effect of Environmental Humidity/water Absorption on Tribo-mechanical Performance of Polymers and Polymer Composites-a Review. Ind. Lub. Tribol. 2021, 73, 1146-1158.
-

- 27. Allen, M. P.; Tildesley, D. J. Computer Simulation of Liquids.; Clarendon Press: Oxford, 1987.
- 28. Frenkel, D.; Smit, B. Understanding Molecular Simulation: from Algorithms to Applications, 2nd ed.; Academic Press: San Diego, 1996.
- 29. Nosé, S. A. Unified Formulation of the Constant Temperature Molecular Dynamics Methods. J. Chem. Phys. 1984, 81, 511-519.
-

- 30. Hoover, W. G. Canonical Dynamics: Equilibrium Phase-space Distributions. Phys. Rev. A, 1985, 31, 1695-1697.
-

- 31. Tadanaga, K.; Ellis, B.; Seddon, A. B. Near- and Mid-infrared Spectroscopy of Sol-gel Derived or Mosil Films for Photonics from Tetramethoxysilane and Trimethoxysilylpropylmethacrylate. J. Sol-Gel Sci. Technol. 2000, 19, 687-690.
-

- 32. Goudeau, S.; Charlot, M.; Vergelati, C.; Muller-Plathe, F. Atomistic Simulation of the Water Influence on the Local Structure of Polyamide 6,6. Macromolecules, 2004, 37, 8072-8081.
-

- 33. Xu, J. L.; Gowen, A. A. FTIR Spectroscopy For Molecular Level Description of Water Vapor Sorption In Two Hydrophobic Polymers, 2019 10th Workshop on Hyperspectral Imaging and Signal Processing: Evolution in Remote Sensing (WHISPERS), Amsterdam, Netherlands, 2019, pp. 1-5.
-

- 34. Jost, S.; Biswas, P.; Schüring, A.; Karger, J.; Bopp, P.; Haberlandt, R.; Fritzsche, S. Structure and Self-diffusion of Water Molecules in Chabazite: A Molecular Dynamics Study. J. Phys. Chem. C 2007, 111, 14707-14712.
-

- 35. Frost, H.; Düren, T.; Snurr, R. Q. Effects of Surface Area, Free Volume, and Heat of Adsorption on Hydrogen Uptake in Metal-organic Frameworks. J. Phys. Chem. B 2006, 110, 9565-9570.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2023; 47(3): 264-277
Published online May 25, 2023
- 10.7317/pk.2023.47.3.264
- Received on Nov 5, 2022
- Revised on Feb 14, 2023
- Accepted on Mar 14, 2023
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Conflict of Interest
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Haitao Duan
-
*State Key Laboratory of Special Surface Protection Materials and Application Technology, Wuhan Research Institute of Materials Protection, Wuhan 430030, Hubei, China
**Hubei Longzhong Laboratory, Xiangyang 441000, Hubei, China - E-mail: duanhaitao2007@163.com
- ORCID:
0000-0002-6892-4897











 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.