- Polymerization of Vinyl Acetate Initiated by Hydrosilane in the Presence of Platinum Complex
Ming Yuan, Wenxian Zhu, Jiaxing Lv, Panpan Zhang*, and Huadong Tang†

Institute of Industrial Catalysis, College of Chemical Engineering, Zhejiang University of Technology, Hangzhou, Zhejiang 310014, China
*State Key Laboratory of Modern Optical Instrumentation, Zhejiang University, Hangzhou, Zhejiang 310027, China- 백금계 촉매와 Hydrosilane 개시제를 이용한 아세트산 비닐 합성
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
In this work, a series of hydrosilanes including phenylsilane, diphenylsilane, triethylsilane (TES), and tris(trimethylsilyl)silane combined with catalytical amount of platinum complex such as dichloro(1,5-cyclooctadiene)platinum (COD) and dichloro(dicyclopentadienyl)platinum have been used to initiate the polymerization of vinyl acetate (VAc) at 70-95 ºC. An ultrahigh molecular weight PVAc with number-average molecular weight (Mn) up to 1.17×106 Da and polydispersity index (PDI) of 2.59 has been synthesized using TES as an initiator in the presence of 9.7 ppm of COD. A kinetic study indicates that the polymerization at VAc/TES/COD molar ratio = 3.0×105 : 60 : 1 smoothly reached 81.0% conversion in 28.0 h at 85 ºC, producing PVAc with Mn increasing linearly from 1.41×105 to 6.01×105 Da with the increase of monomer conversion. A mechanistic investigation revealed that COD was firstly reduced by reductive TES, producing Pt nanoparticles and corresponding silane radicals to initiate the polymerization of VAc
An ultrahigh molecular weight poly(vinyl acetate) (PVAc) with number-average molecular weight (Mn) of 1.17x106 Da was synthesized by the polymerization of vinyl acetate (VAc) using triethylsilane (TES) as an initiator in the presence of catalytical amount (9.7 ppm) of dichloro(1,5-cyclooctadiene)platinum (COD). The polymerization of VAc at VAc/TES/COD molar ratio = 3.0x105:60:1 smoothly reached 81.0% conversion in 28.0 h at 85 oC, producing PVAc with Mn increasing linearly with the increase of monomer conversion.
Keywords: vinyl acetate, polymerization, hydrosilane, kinetics, mechanism
The authors thank the support from the National Natural Science Foundation of China (No. 21174133) and Zhejiang Natural Science Foundation for Distinguished Young Scholars (No. LR12B04002).
Vinyl acetate (VAc) is an important industrial vinyl monomer since poly(vinyl acetate) (PVAc) and its hydrolysis product, poly(vinyl alcohol) (PVA) have numerous applications in coating, fiber, textile, adhesive, and pharmaceutical industries.1-5 The molecular weight of PVAc has significant effects on its physical properties. For example, the glass transition temperature of PVAc raises from 17 to 29 oC when the molecular weight of PVAc increases from 1.50×104 to 1.12×106 Da.6 Properties such as tensile strength, viscosity,1 and adhesion of PVAc increase with the increase of molecular weight up to a certain point,7 after which these properties tends to gradually level off and become roughly constant at very high range of molecular weight. Therefore, it is still of great importance to develop a facile synthetic approach to PVAc with high and ultrahigh molecular weights.
Suspension polymerization, solution polymerization, and emulsion polymerization have been applied to prepare high molecular weight PVAc. Lyoo and coworkers reported that the suspension polymerization of VAc using 2,2'-azobis-(2,4-dimethylvaleronitrile) as an initiator and PVA as a suspending agent produced PVAc with a high number-average degree of polymerization Pn (Pn = 13500).8 However, completely removing the suspension agent from PVAc products was quite complex and costly. Lyoo et al. also synthesized PVAc with Pn ranging from 0.70×104 to 1.30×104 by solution polymerization,9 but it was difficult to obtain high molecular weight PVAc with monomer conversion over 80% by solution polymerization because the high solution viscosity and the branch formation resulted from chain transfer reactions were unfavorable to the formation of linear high molecular weight PVAc.9,10 Fukae and Kamachi et al. found that photo-emulsion polymerization of VAc could generate PVAc with high Pn of 7900-15600.11 Unfortunately, this polymerization had to be conducted at a low temperature condition (5 oC) and under the radiation of a high pressure mercury lamp. Imann and coworkers reported that Fe(III) 2-ethylhexanoate or Co(II) acetylacetonate combined with pentamethyldisiloxane could initiate the free radical polymerization of VAc. Regrettably, the polymerizations of VAc only achieved low monomer conversions of ~5% by Fe(III) 2-ethylhexanoate/pentamethyldisiloxane and ~20% by Co(II) acetylacetonate/pentamethyldisiloxane. In addition, the molecular weights of obtained PVAc were low (6450 Da ~ 77.4 kDa).12 Recently, synthetic approaches such as atom transfer radical polymerization (ATRP),13-17 nitroxide-mediated polymerization (NMP),18,19 reversible addition-fragmentation chain transfer (RAFT) polymerization,20-22 RAFT/MADIX polymerization,23,24 and degenerative transfer (DT) polymerization25-29 have been applied to the “living”/controlled polymerization of VAc. Nevertheless, the molecular weights of prepared PVAc by these methods were relatively low (≤ 1.60×105 Da), it still remains challenging to develop a feasible and facile polymerization strategy for the synthesis of high and ultrahigh molecular weight PVAc (Mn > 1.00×106 Da).
Platinum complex is an efficient catalyst for many organic reactions.30,31 Especially, platinum complex has been extensively used as an efficient catalyst for hydrosilylation reaction, which enables the addition of Si-H group to an unsaturated bond (e.g., vinyl monomer) for the formation of a large variety of organosilicon compounds.32-35 Generally, in a normal hydro-silylation reaction the molar ratio of vinyl monomer to hydrosilane is close to 1:1. In this work, we found that phenylsilane (PSH), diphenylsilane (DPS), triethylsilane (TES), and tris(trimethylsilyl)silane (TTSS) combined with catalytical amount of platinum complex such as dichloro(1,5-cycloocta-diene)platinum (COD) and dichloro(dicyclopentadienyl) platinum (CP) could initiate remarkable polymerization of vinyl acetate at mild conditions if VAc was much excess than hydrosilane. An ultrahigh molecular weight PVAc with number-average molecular weight (Mn) up to 1.17×106 Da and polydispersity index (PDI) of 2.59 has been prepared. Gel permeation chromatography (GPC), proton nuclear magnetic resonance (1H NMR), transmission electron microscope (TEM) and electron spin resonance (ESR) have been used to investigate the mechanism of initiation of the polymerization of VAc.
Materials. Vinyl acetate (VAc, 99%), phenylsilane (PSH, 98%), dichloro(1,5-cyclooctadiene)platinum (COD, 97%), dichloro(dicyclopentadienyl)platinum (CP, 97%), tris(trimethylsilyl)silane (TTSS, 96%), triethylsilane (TES, 98%), diphenylsilane (DPS, 99%), and aluminum oxide (200-300 mesh) were purchased from Shanghai Aladdin biochemical technology Co., LTD (China). N-tert-butyl-alpha-phenylnitrone (PBN, > 98%) was purchased from TCI (Tokyo, Japan). Methanol, chloroform-d, tetrahydrofuran (THF), n-hexane and other solvents were of analytical grades and also supplied byAladdin. The inhibitors in VAc were removed by a column filled with basic aluminum oxide and the purified monomer was preserved at -20 oC in a refrigerator.
Analysis and Measurements. A gel permeation chromatography (GPC) using THF as an eluent at a flow rate of 1.0 mL/min at 35 °C was employed to determine the number-average molecular weight (Mn), weight-average molecular weight (Mw) and polydispersity index (PDI) of synthesized PVAc. The GPC measurement was performed on a Malvern Viscotek 270max system (Malvern Panalytical, Malvern, England) equipped with Viscotek T6000M GPC column (8.0×300 mm, molecular weight range: 1.0×103-2.0×107 Da), Viscotek VE1122 solvent transfer unit, Viscotek 270 laser light scattering-differential viscometer detector and Viscotek VE 3580 refractive index detector. The GPC had been validated using a polystyrene standard (PDI = 1.02, Malvern Panalytical, Malvern, England).
Proton nuclear magnetic resonance (1H NMR) spectra of PVAc were recorded on a Bruker Avance III 500 MHz spectrometer (Bruker, Fällanden, Switzerland) using chloroform-d (CDCl3) as a solvent and tetramethylsilane (TMS, δ = 0 ppm) as an internal standard. The monomer conversion was determined by gravimetric method.
The morphology of in-situ formed platinum nanoparticle (Pt NP) during the process of polymerization of VAc was observed by Tecnai G2 F30 transmission electron microscope (TEM, acceleration voltage 300 kV, FEI, Hillsboro, OR, USA). The TEM is equipped with an energy dispersive X-ray spectrometer (EDX) accessory and has a point resolution of 0.20 nm, a line resolution of 0.10 nm, and a high-resolution STEM resolution of 0.17 nm.
The radical intermediate in the polymerization was detected by a Bruker A300 electron spin resonance (ESR) spectrometer (Bruker, Karlsruhe, Germany). The instrument was worked in X-band with a microwave power of 20.39 mW and frequency at 9.8374 GHz. PBN was used as a free radical trapping agent and added into the reaction solution during the polymerization of VAc (PBN final concentration: 0.075 mol/L). Finally, 40 μL of the solution was sealed into a 1.3 mm capillary tube for ESR detection.
Bulk Polymerization of VAc Initiated by Hydrosilanes in the Presence of Pt Complex. A general procedure for the bulk polymerization of VAc using hydrosilanes as initiators in the presence of Pt complex is as follow. An Ace pressure tube (#15 Ace-Thred) was charged with a stirring bar and Pt complex (COD or CP). VAc was then added into the reaction tube using a syringe equipped with a long needle, followed by the addition of hydrosilane (e.g., TES) using microsyringes. The colorless reaction solution quickly changed to a yellowish color. Then the reaction tube was put into an oil bath set at a desired temperature. At different time intervals, a small amount of reaction solution was withdrawn from the reaction tube via a syringe equipped with a long needle and the obtained samples were stored in a fridge for determination of monomer conversion and GPC analysis. The reaction solution was finally precipitated with n-hexane to obtain PVAc and the PVAc was dried at 50 oC under vacuum to give a yield of 67.1%.
Synthesis of Low Molecular Weight PVAc for End Group Analysis.The synthetic procedure for low molecular weight PVAc is essentially the same as the above-mentioned bulk polymerization of VAc except that the polymerization was carried out at 85 ºC with a molar ratio of [VAc] : [TES] : [COD] = 4.5×104 : 2.4×103 : 1. The polymerization was stopped at a reaction time of 4 h and then the reaction solution was diluted in THF and precipitated in n-hexane to remove unreacted monomer and initiator. The obtained PVAc precipitates were dried under vacuum at 100 oC for 20 h and then prepared for 1H NMR analysis of end groups.
In-situ Formation of Pt Nanoparticles for TEM Obser-vation. The collection and TEM observation of in-situ formed Pt nanoparticles in the polymerization of VAc is as follow. The polymerization procedure is essentially the same as the above-mentioned synthesis of low molecular weight PVAc except that the polymerization was stopped at the reaction time of 30 min. Then the reaction tube was cooled down to room temperature and a small drop of the sample solution was dripped on TEM copper grid and dried for observation.
Bulk Polymerization of VAc Initiated by Hydrosilanes in the Presence of Pt Complex. A series of hydrosilanes including TTSS, PSH, DPS, and TES were found to be able to initiate the bulk polymerization of VAc in the presence of catalytical amount of Pt complex such as CP and COD. The results are listed in Table 1. As the bond dissociation energy of the Si-H bond in TTSS is significantly lower than that of TES and many other hydrosilanes,36 TTSS initiated relative fast polymerization of VAc (51.4% conversion in 9 h at 80 oC) at VAc/TTSS/CP molar ratio = 4.5×104 : 60 : 1.0, producing PVAc with Mn = 3.79×104 Da and a broad molecular weight distribution (PDI = 4.01, Table 1, entry 1). Lowering the feeding ratio of TTSS and CP decreased the polymerization rate (42.0 h, 67.2% conversion) even though the obtained PVAc had higher molecular weight and narrower molecular weight distribution (Table 1, entry 2). The polymerization of VAc initiated by PSH at 70 ºC was definitely slower than that at 80 oC (Table 1, entry 3 & 4), generating PVAc with lower molecular weight and higher polydispersity index. In comparison with TTSS, DPS initiated slower polymerization of VAc at same reaction conditions (VAc/Initiator/CP molar ratio = 4.5×104 : 60 : 1.0, 80 ºC) due to the higher Si-H bond dissociation energy and electron withdrawing effect of phenyl group, but the produced PVAc had a significantly larger molecular weight (Mn = 1.13×105 Da, Table 1, entry 6) and lower polydispersity index (PDI = 2.44, Table 1, entry 6). Decreasing the CP feeding ratio had little effects on the polymerization rate of VAc when TES was used as an initiator (Table 1, entry 7 & 8). Table 1 (entry 9) shows that PSH initiated slower polymerization of VAc in the presence of COD than the polymerization initiated by TTSS in the presence of CP (Table 1, entry 1) at VAc/Initiator/Pt complex molar ratio = 4.5×104 : 60.0 : 1.0. The PSH initiated polymerization was significantly enhanced at lower VAc/PSH/COD ratio (4.5×104 : 6.0 : 0.2, Table 1, entry 10) at 85 ºC. Similar results were found when DPS was used as an initiator in the presence of COD (Table 1, entry 11 & 12). TES combined with COD was investigated and the polymerization initiated at VAc/TES/COD = 2.25×105 : 60.0 : 1.0 achieved 90% conversion in 72 h at 85 ºC, generating PVAc with a very high number-average molecular weight up to 5.64×105 Da and a PDI of 2.66 (Table 1, entry 14). Therefore, the VAc/TES/COD system was further optimized for the synthesis of ultrahigh molecular weight PVAc.
Synthesis of Ultrahigh Molecular Weight PVAc. The effects of TES concentration (Table 2, entry 3-5), COD concentration (Table 2, entry 6-8) and temperature (Table 2, entry 9-11) on the polymerization rate of VAc and the PVAc molecular weight had been studied. Table 2 (entry 1) shows that almost no VAc was polymerized in the presence of COD without TES. Although the VAc could be initiated by hydrosilane alone in the absence of COD,12,37,38 the polymerization only reached low monomer conversion and produced PVAc with low molecular weights (Table 2, entry 2). In the presence of COD, the polymerization rate was found to increase with the increase of TES concentration but the molecular weight decrease with the increase of TES concentration (Table 2, entry 3-5). Both the polymerization rate and the PVAc molecular weight decreased with the increase of COD concentration (Table 2, entry 6-8). The reaction temperature had notable effects on the molecular weight of PVAc (Table 2, entry 9 & 10). To obtain ultrahigh molecular weight PVAc, a two-step temperature-programmed polymerization of VAc at VAc/TES/COD molar ratio = 2.25×105 : 50.0 : 0.5 was conducted at a starting temperature of 85 ºC for 12 h, followed by 95 ºC for another 60 h (Table 2, entry 11). The resulting PVAc had an ultrahigh number-average molecular weight of 1.17×106 Da, weight-average molecular weight of 3.03×106 Da, and a polydispersity index of 2.59. In this case, the COD concentration was calculated to be only 9.7 ppm according to the VAc/TES/COD molar ratio, which is low enough to eliminate the necessity of post-purification and catalyst recovery from the PVAc products. These results indicate that the polymerization system is potentially promising for wide practical applications.
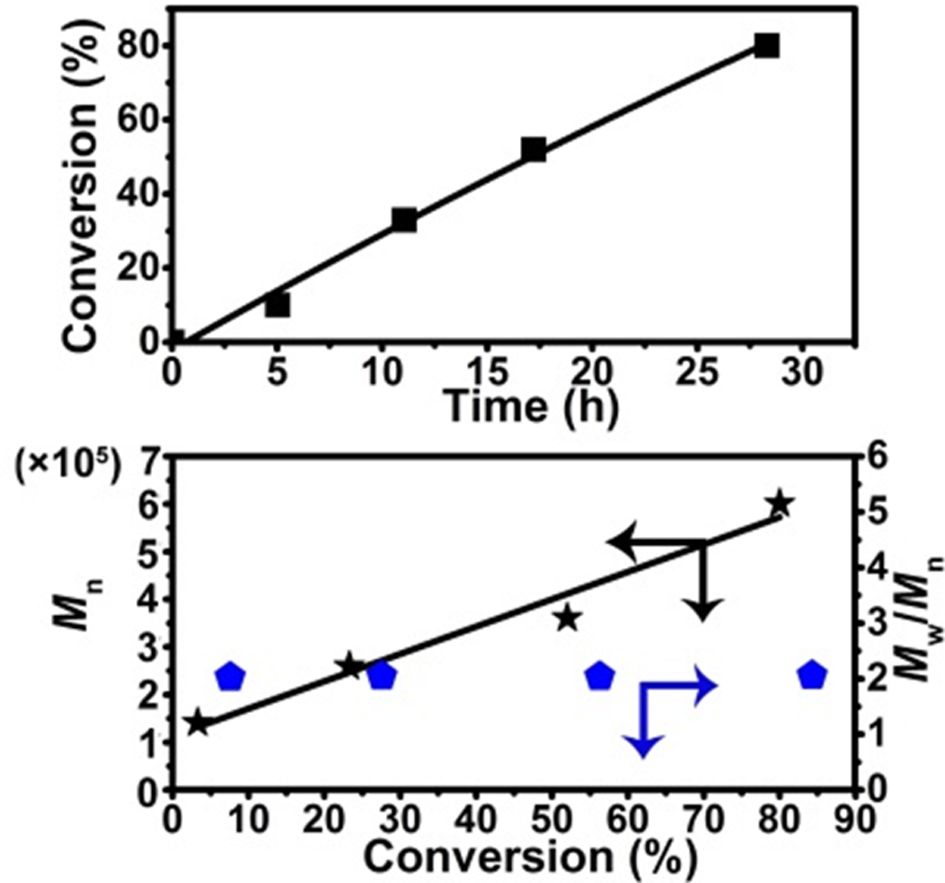
Kinetic Study of the Polymerization of VAc Initiated by TES in the Presence of COD.Tough ultrahigh molecular weight PVAc with a Mn up to 1.17×106 Da had been synthesized by using TES as an initiator in the presence of COD at VAc/TES/COD molar ratio = 2.25×105 : 50.0 : 0.5, the polymerization was too slow (72 h, 92.6% conversion), time-consuming and inefficientfor kinetic investigations. Therefore, the kinetic data was collected in the polymerization of VAc at VAc/TES/COD molar ratio = 3.0×105 : 60 : 1 and the results are shown in Figure 1. As can be seen from Figure 1(a), the polymerization of VAc smoothly reached 81% conversion in 28 h at 85 ºC. No induction time and auto-acceleration behavior were observed. The number-average molecular weight of obtained PVAc increased linearly from 1.41×105 to 6.01×105 Da with the increase of monomer conversion and the corresponding polydispersity index PDI was around 2.0 (Figure 1(b)). However, the molecular weightvs. conversion plot didn’t pass the original point of the axis, suggesting that though the polymerization of VAc using TES as an initiator in the presence of COD partially fulfilled the experimental criteria for a living/controlled polymerization,39 there were still irreversible chain transfer and chain termination reactions occurred in the polymerization. Figure 1(c) presents the GPC elution curve of produced PVAc (Mn = 6.01×105 Da, PDI = 2.03). Obviously, the GPC curve of PVAc was monomodal but not symmetric, indicating the formation of dead polymer chains during the polymerization process due to the irreversible chain transfer and chain termination reactions.
End Group Analysis of Low Molecular Weight PVAc. In order to improve the integration accuracy of 1H NMR spectrum in end group analysis, a low molecular weight PVAc was synthesized using TES as an initiator in the presence of COD (as described in section 2.4), and its number-average molecular weight Mn was measured to be 12750 Da by GPC. Figure 2 shows the 1H NMR spectrum of the prepared low molecular weight PVAc. There are clearly three strong peaks a, b, and c, respectively corresponding to the protons of methine, methylene and methyl groups from the repeating units of PVAc.40 The coupled small peaks d and e at d = 0.9 ppm and 0.5 ppm, respectively are unambiguously assigned to the protons of methyl, methylene groups from TES due to the electron-donating ability of silicon atoms. According to the integration ratio of peak a to peak d or e, the number-average molecular weight Mn of PVAc was calculated to be 12140 Da, which is very close to the molecular weight (Mn) measured by GPC, implying that the macromolecular chain of PVAc was essentially one end-capped with TES.
Mechanistic Study of the Polymerization Initiated by TES in the Presence of COD. Electron spin resonance (ESR) was used to identify the active species during the polymerization of VAc initiated by TES in the presence of COD. PBN was used as a trap reagent to capture the active species produced in the polymerization. The results are shown in Figure 3. Figure 3(a) and 3(b) display the ESR spectra respectively acquired at the reaction time of 30 min and 60 min during the polymerization of VAc. According to the literature,12 the triple doublet signal was ascribed to typical carbon-centered radicals trapped by PBN, indicating that the polymerization of VAc was basically via a radical mechanism and the active intermediates during the polymerization were PVAc chain carbon propagating radicals. The signal intensity was significantly reduced at the reaction time of 60 min, suggesting that there were irreversible chain transfer and chain termination side reactions during the polymerization.
According to the above ESR spectra and 1H NMR results, a presumable mechanism for the initiation of the polymerization of VAc by TES in the presence of COD is proposed in Figure 4. Since hydrosilane is a strong reductive agent, fast redox reaction between COD and TES occurred at the beginning of the reaction, producing a lot of Pt nanoparticles (Pt NPs) as observed by TEM. Figure 4(a) and 4(b) show the low-resolution and high-resolution TEM images of the in-situ formed Pt nanoparticles. The average diameter of Pt nanoparticles was determined to be 2.40 ± 0.26 nm with interplanar spacing of 0.226 nm, which is corresponding to Pt(111) crystal plane.41 In addition, there was an amorphous polymer-like substance existing on the surface of Pt nanoparticles or around the Pt nanoparticles, implying that the polymerization may occur around the surface or at the vicinity of the Pt nanoparticles. Therefore, a schematic illustration of the polymerization steps is presumed as follow (Figure 4(c)). Hydrosilane such as TES could adsorb on the surface of Pt colloid/nanoparticle to form an intermediate according to the literature,42 which would be activated at relatively high temperatures in polymerization to release silyl radicals around the Pt colloids/nanoparticles, followed by immediate addition to the double bond of VAc and startup of the polymerization at the vicinity of Pt nanoparticles. The Pt nanoparticles are assumed to provide certain protections to the chain propagating radicals due to their bulky size, and in a sense the chain radical termination and transfer side reactions are somewhat suppressed. So the polymerization in this work may not follow the same reaction mechanism as in conventional free radical polymerization (FRP), but to some extent, behave like a kind of “living”/controlled radical polymerization (e.g., NMP), which facilitates the synthesis of ultrahigh molecular weight PVAc due to the suppression of radical termination and transfer reactions. However, there are still noticeable irreversible chain transfer and chain termination side reactions in the polymerization, as evidenced by the relatively broad molecular weight distribution of PVAc (Mw/Mn) listing in Table 2.

|
Figure 1 (a) Conversion-time (■) plot; (b) Mn-conversion (★) and PDI-conversion (■) plots of the polymerization of VAc initiated by TES in the presence of COD; (c) the GPC elution curve of produced PVAc. Reaction condition: [VAc]/[TES]/[COD] = 3.0×105 : 60 : 1, T = 85 ºC |

|
Figure 2 1 H NMR spectrum of PVAc synthesized using TES as an initiator in the presence of COD. |

|
Figure 3 ESR spectra acquired at reaction time of (a) 30 min; (b) 60 min during the polymerization of VAc using TES as an initiator in the presence of COD. Conditions: [VAc] : [TES] : [COD] = 4.5×104 : 60 : 1, T = 80 ºC. |

|
Figure 4 (a) and (b) TEM images of in-situ formed Pt nanoparticles at the early stage of the polymerization; (c) the proposed mechanism for the polymerization of VAc initiated by TES in the presence of COD. |
|
Table 1 Bulk Polymerizations of VAc Initiated by Hydrosilanes in the Presence of Pt Complexa |

a Init. = initiator. [VAc] = 10.8 mol/L. |
|
Table 2 Bulk Polymerizations of VAc Using TES as an Initiator in the Presence of CODa |

a [VAc] = 10.8 mol/L. b The polymerization was conducted at 85 ºC for 12 h, followed by 95 ºC for another 60 h. |
The polymerization of VAc initiated by hydrosilanes in the presence of platinum complex has been investigated in this work. An ultrahigh molecular weight PVAc with a number-average molecular weight up to 1.17×106 Da and PDI of 2.59 has been synthesized using TES as an initiator in the presence of catalytical amount (9.7 ppm) of COD. The polymerization of VAc at VAc/TES/COD molar ratio = 3.0×105 : 60 : 1 smoothly reached 81.0% conversion in 28.0 h at 85 ºC. The Mn of produced PVAc increased linearly with the increase of monomer conversion and the corresponding polydispersity index (PDI) was around 2.0, suggesting that though the polymerization of VAc using TES as an initiator in the presence of COD partially fulfill the experimental criteria for living/controlled polymerization, irreversible chain transfer and chain termination reactions occurred in the polymerization. A mechanistic investigation revealed that the polymerization of VAc was practically via a radical mechanism. Platinum complexes such as COD was firstly reduced by reductive TES, producing Pt nanoparticles and corresponding silane radicals to initiate the polymerization of VAc.
- 1. Mark, H. F. Encyclopedia of Polymer Science and Technology; Wiley-Interscience: Hoboken, New Jersey, USA, 2004.
- 2. Cowan, D. A.; Blankenhorn, P. R.; Murphey, W. K. Effects of Relative Humidity and Shelf-Life on Selected Properties of Polyvinyl Acetate Adhesive Films. Wood Fiber 1978, 10, 138-146.
- 3. Salvini, A.; Saija, L. M.; Lugli, M.; Cipriani, G.; Giannelli, C. Synthesis of Modified Poly(vinyl acetate) Adhesives. J. Adhes. Sci. Technol. 2010, 24, 1629-1651.
-

- 4. Amann, M.; Minge, O. Biodegradability of Poly(vinyl acetate) and Related Polymers. Adv. Polym. Sci. 2012, 245, 137-172.
-

- 5. DeMerlis, C. C.; Schoneker, D. R. Review of the Oral Toxicity of Polyvinyl Alcohol (PVA). Food Chem. Toxicol. 2003, 41, 319-326.
-

- 6. Wiley, R. H.; Brauer, G. M. Refractometric Determination of Second Order Transition Temperatures in Polymers. VI. Effect of Molecular Weight on the Transition Temperature of Polyvinyl Acetate. J. Polym. Sci. 1953, 11, 221-224.
-

- 7. Lasoski, S. W.; Kraus, G. Polymer to Metal Adhesion. The System Polyvinyl Acetates Steel. J. Polym. Sci. 1955, 18, 359-376.
-

- 8. Lyoo, W. S.; Lee, S. G.; Kim, J. P.; Han, S. S.; Lee, C. J. Low Temperature Suspension Polymerization of Vinyl Acetate Using 2,2'-azobis(2,4-dimethylvaleronitrile) for the Preparation of High Molecular Weight Poly(vinyl alcohol) with High Yield. Colloid Polym. Sci. 1998, 276, 951-959.
-

- 9. Lyoo, W. S.; Han, S. S.; Choi, J. H.; Ghim, H. D.; Yoo, S. W.; Lee, J.; Hong, S. I.; Ha, W. S. Preparation of High Molecular Weight Poly(vinyl alcohol) with High Yield Using Low-Temperature Solution Polymerization of Vinyl acetate. J. Appl. Polym. Sci. 2001, 80, 1003-1012.
-

- 10. Lyoo, W. S.; Lee, H. W. Synthesis of High-Molecular-Weight Poly(vinyl alcohol) with High Yield by Novel One-Batch Suspension Polymerization of Vinyl Acetate and Saponification. Colloid Polym. Sci. 2002, 280, 835-840.
-

- 11. Yamamoto, T.; Seki, S.; Fukae, R.; Sangen, O.; Kamachi, M. High Molecular Weight Poly(vinyl alcohol) through Photo-Emulsion Polymerization of Vinyl Acetate. Polym. J. 1990, 22, 567-571.
-

- 12. Imann, R.; Bandermann, F.; Korth, H. G. Free-Radical Polymerization of Acrylates and Vinyl Acetates Initiated by Transition Metal/Hydrosilane Two-Component Initiation Systems. Macromol. Chem. Phys. 1996, 197, 921-935.
-

- 13. Matyjaszewski, K.; Xia, J. H. Atom Transfer Radical Polymerization. Chem. Rev. 2001, 101, 2921-2990.
-

- 14. Kamigaito, M.; Ando, T.; Sawamoto, M. Metal-Catalyzed Living Radical Polymerization. Chem. Rev. 2001, 101, 3689-3745.
-

- 15. Mazzotti, G.; Benelli, T.; Lanzi, M.; Mazzocchetti, L.; Giorgini, L. Straightforward Synthesis of Well-Defined Poly(vinyl acetate) and Its Block Copolymers by Atom Transfer Radical Polymerization. Eur. Polym. J. 2016, 77, 75-87.
-

- 16. Islam, M. T.; Haldorai, Y.; Nguyen, V. H.; Islam, M. N.; Ra, C. S.; Shim, J.-J. Controlled Radical Polymerization of Vinyl Acetate in Supercritical CO2 Catalyzed by CuBr/Terpyridine. Korean J. Chem. Eng. 2014, 31, 1088-1094.
-

- 17. Champouret, Y.; MacLeod, K. C.; Smith, K. M.; Patrick, B. O.; Poli, R. Controlled Radical Polymerization of Vinyl Acetate with Cyclopentadienyl Chromium β-Diketiminate Complexes: ATRP vs OMRP. Organometallics 2010, 29, 3125-3132.
-

- 18. Yoshida, E. Nitroxide-Mediated Photo-Living Radical Polymerization of Vinyl Acetate. Colloid Polym. Sci. 2010, 288, 73-78.
-

- 19. Banerjee, S.; Domenichelli, I.; Ameduri, B. Nitroxide-Mediated Alternating Copolymerization of Vinyl Acetate with tert-Butyl-2-trifluoromethacrylate Using a SG1-Based Alkoxyamine. ACS Macro Lett. 2016, 5, 1232-1236.
-

- 20. Destarac, M.; Charmot, D.; Franck, X.; Zard, S. Z. Dithiocarbamates as Universal Reversible Addition-Fragmentation Chain Transfer Agents. Macromol. Rapid Commun. 2000, 21, 1035-1039.
-

- 21. Stenzel, M. H.; Davis, T. P.; Barner-Kowollik, C. Poly(vinyl alcohol) Star Polymers Prepared via MADIX/RAFT Polymerization. Chem. Commun. 2004, 10, 1546-1547.
-

- 22. Simms, R. W.; Davis, T. P.; Cunningham, M. F. Xanthate-Mediated Living Radical Polymerization of Vinyl Acetate in Miniemulsion. Macromol. Rapid Commun. 2005, 26, 592-596.
-

- 23. Levere, M. E.; Chambon, P.; Rannard, S. P.; McDonald, T. O. MADIX Polymerization of Vinyl Acetate Using Ethyl Acetate as a Green Solvent; Near-Complete Monomer Conversion with Molecular Weight Control. J. Polym. Sci., Part A: Polym. Chem. 2017, 55, 2427-2431.
-

- 24. Segura, T.; Peralta, R. D.; Menes-Arzate, M.; León, F.; Mendoza, R. RAFT/MADIX Miniemulsion Polymerization of Vinyl Acetate: Influence of Oil Soluble Initiators, Temperature, and Type of Chain Transfer Agent in Nanodroplets. Colloid Polym. Sci. 2018, 296, 271-283.
-

- 25. Iovu, M. C.; Matyjaszewski, K. Controlled/Living Radical Polymerization of Vinyl Acetate by Degenerative Transfer with Alkyl Iodides. Macromolecules 2003, 36, 9346-9354.
-

- 26. Borkar, S.; Sen, A. Controlled Copolymerization of Vinyl Acetate with 1-Alkenes and Their Fluoro Derivatives by Degenerative Transfer. J. Polym. Sci., Part A: Polym. Chem. 2005, 43, 3728-3736.
-

- 27. Peng, C. H.; Scricco, J.; Li, S.; Fryd, M. Organo-Cobalt Mediated Living Radical Polymerization of Vinyl Acetate. Macromolecules 2008, 41, 2368-2373.
-

- 28. Xu, W.; Zhang, W.; Li, W.; Yan, J.; Shen, G.; Li, J. Synthesis of Poly(vinyl acetate) by Degenerative Transfer Polymerization in the Presence of Iodine. J. Appl. Polym. Sci. 2012, 126, 104-109.
-

- 29. Peng, C.-H.; Li, S.; Wayland, B. B. Aspects of Living Radical Polymerization Mediated by Cobalt Porphyrin Complexes. J. Chin. Chem. Soc. 2009, 56, 219-233.
-

- 30. Harrod, J. F.; Chalk, A. J. Homogeneous Catalysis I. Double Bond Migration in n-Olefins Catalyzed by Group VIII Metal Complexes. J. Am. Chem. Soc. 1964, 86, 1776-1779.
-

- 31. Chalk, A. J.; Harrod, J. F. Homogeneous Catalysis. II. The Mechanism of the Hydrosilation of Olefins Catalyzed by Group VIII Metal Complexes. J. Am. Chem. Soc. 1965, 87, 16-21.
-

- 32. Buch, F.; Brettar, J.; Harder, S. Hydrosilylation of Alkenes with Early Main-Group Metal Catalysts. Angew. Chem. 2006, 118, 2807-2811.
-

- 33. Kim, D. W.; Joung, S.; Kim, J. G.; Chang, S. Metal-Free Hydrosilylation Polymerization by Borane Catalyst. Angew. Chem. Int. Ed. 2015, 54, 14805-14809.
-

- 34. Nakajima, Y.; Shimada, S. Hydrosilylation Reaction of Olefins: Recent Advances and Perspectives. RSC Adv. 2015, 5, 20603-20616.
-

- 35. Hofmann, R. J.; Vlatković, M.; Wiesbrock, F. Fifty Years of Hydrosilylation in Polymer Science: A Review of Current Trends of Low-Cost Transition-Metal and Metal-Free Catalysts, Non-Thermally Triggered Hydrosilylation Reactions, and Industrial Applications. Polymers 2017, 9, 534.
-

- 36. Chatgilialoglu, C.; Griller, D.; Lesage, M. Tris(trimethylsilyl)silane. A New Reducing Agent. J. Org. Chem. 1988, 53, 3641-3642.
-

- 37. Laleveé, J.; Allonas, X.; Fouassier, J. P. Tris(trimethylsilyl)silane (TTMSS)-Derived Radical Reactivity Toward Alkenes: A Combined Quantum Mechanical and Laser Flash Photolysis Study. J. Org. Chem. 2007, 72, 6434-6439.
-

- 38. Laleveé, J.; El-Roz, M.; Morlet-Savary, F.; Graff, B.; Allonas, X.; Fouassier, J. P. New Highly Efficient Radical Photoinitiators Based on Si-Si Bond Cleavage. Macromolecules 2007, 40, 8527-8530.
-

- 39. Quirk, R. P.; Lee, B. Experimental Criteria for Living Polymerizations. Polym. Int. 1992, 27, 359-367.
-

- 40. Debuigne, A.; Caille, J.-R.; Jéróme, R. Synthesis of End-Functional Poly(vinyl acetate) by Cobalt-Mediated Radical Polymerization. Macromolecules 2005, 38, 5452-5458.
-

- 41. Chen, J.; Herricks, T.; Geissler, M.; Xia, Y. Single-Crystal Nanowires of Platinum can be Synthesized by Controlling the Reaction Rate of a Polyol Process. J. Am. Chem. Soc. 2004, 126, 10854-10855.
-

- 42. Lewis, L. N. On the Mechanism of Metal Colloid Catalyzed Hydrosilylation: Proposed Explanations for Electronic Effects and Oxygen Cocatalysis. J. Am. Chem. Soc. 1990, 112, 5998-6004.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2021; 45(3): 428-436
Published online May 31, 2021
- 10.7317/pk.2021.45.3.428
- Received on Dec 24, 2020
- Revised on Jan 31, 2021
- Accepted on Feb 22, 2021
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Huadong Tang
-
Institute of Industrial Catalysis, College of Chemical Engineering, Zhejiang University of Technology, Hangzhou, Zhejiang 310014, China
- E-mail: thd@zjut.edu.cn
- ORCID:
0000-0002-9936-2444








 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.