- Synthesis and Analysis of Bio-based Polyurethanes with Different Polyester Polyols
*School of Energy, Materials and Chemical Engineering, Korea University of Technology and Education, Cheonan, Chungnam 31253, Korea
**Research Center of Eco-friendly & High Performance Chemical Materials, Cheonan, Chungnam 31253, Korea- 바이오 폴리에스터 폴리올에 의한 폴리우레탄의 합성 및 물성에 대한 연구
*한국기술교육대학교 에너지, 신소재, 화학공학부, **친환경고성능화학소재연구소
Four kinds of bio-based polyester polyols had been synthesized by esterification of two kinds of fatty acids (azelaic acid and adipic acid) and two kinds of diols (isosorbide and 1,3-propanediol (1,3-PD)). After the esterification reaction, the bio-based polyurethanes were synthesized with bio-based polyester polyols and isocyanates (MDI) with a mixing ratio of 1:1.5. The bio-based TPUs were characterized by FTIR, TGA, NMR and GPC. Viscoelastic properties had been tested with strain sweep test mode using rubber process analyzer (RPA), mechanical properties (tensile strength and hardness value) was characterized by UTM and shore A hardness tester. The bio-based polyester polyol which synthesized by adipic acid and 1,3-PD the resulting bio-based TPU showed the best mechanical and viscoelastic properties, when mixed with MDI. The two kinds of bio-based TPUs which contained isosorbide showed narrower molecular weight distribution than the bio-based TPUs containing 1,3-PD. This work provides a new way to develop and research the eco-friendly TPU materials.
2가 산인 azelaic acid, adipic acid와 2가 알코올인 isosorbide, 1,3-propanediol(1,3-PD)를 사용하여 4 가지 바이오 폴리에스터 폴리올을 합성하였다. 합성된 바이오 폴리에스터 폴리올을 4,4'-methylene bis(phenyl isocyanate)(MDI)과 1:1.5의 비율로 혼합하여 바이오 폴리우레탄을 합성하였다. 바이오 폴리우레탄의 구조를 분석하기 위해서 FTIR, TGA, NMR 및 GPC를 사용하였다. 고분자 가공분석기(RPA)의 변형 스윕(strain sweep) 기능을 사용하여 바이오 폴리우레탄의 점탄성을 조사하였다. UTM, Shore A 기기를 사용하여 바이오 폴리우레탄의 인장강도, 경도를 측정하였다. Adipic acid와 1,3-PD에 의한 바이오 폴리에스터 폴리올과 MDI에 의한 바이오 폴리우레탄이 가장 좋은 기계적 물성과 점탄성을 보여주었다. Isosorbide에 의한 2가지 바이오 폴리우레탄이 1,3-PD에 의한 바이오 폴리우레탄보다 더 작은 분자량 분포를 보였다. 본 연구는 미래의 친환경 폴리우레탄 재료의 개발 및 연구에 새로운 방향을 제공한다.
Bio-based polyester polyol had been synthesized by the esterification method by 2 kinds of fatty acids (azelaic acid and adipic acid) and 2 kinds of diols (isosorbide and 1,3-propandiol), bio-based polyurethanes were synthesized with bio-based polyester polyols and isocyanates (MDI), the bio-thermoplastic polyurethanes which contained isosorbide showed narrower molecular weight distribution.
Keywords: bio-polyurethane, isosorbide, synthesis, mechanical properties, viscoelastic properties
The traditional non-biodegradable polyurethanes (PUs), which are produced from fossil fuels, have considerably disturbed and damaged the ecosystem of nature.1 They are used in a broad range of applications, for example, as elastomers, sealants, fibers, foams, coatings, adhesives, and biomedical materials.2 The synthesis of PUs is carried out by a variety of methods, although the most widely used method starts from di- or poly-functional hydroxyl-compounds (polyol) with di- or poly-functional isocyanates, which are usually industrially produced from petroleum.3 The progressive dwindling of fossil resources, coupled with the drastic increase in oil prices in the long-term, have driven researches to develop alternatives based on renewable resources for the production of polymer materials.4 Recently, the utilization of renewable materials such as plant oils and natural fatty acids derivative (such as polyester polyols and polyether polyols) for replacing petroleum derived raw materials for the production of polymeric materials has attracted great attention, due to social, environmental and economic considerations.5 Regarding bio-based PUs, almost reports have been focused on preparing polymers using polyol derived from vegetable oil and bio-based polyester polyol in combination with petrochemical-based di-isocyanates.6 It has been demonstrated that these bio-based PUs have comparable properties in many aspects with PUs derived from petrochemical polyol.7 Over the past years, worldwide interest in developing methodologies has been growing for the synthesis of bio-based polyester polyol PUs.8
In this article, bio-based polyester polyols had been synthesized by esterification using azelaic acid, adipic acid as fatty acids, isosorbide and 1,3-propanediol (1,3-PD) used as polyols. After the esterification, the bio-based PUs were mixed with bio-based polyester polyols and isocyanate (MDI) to obtain thermoplastic polyurethane (TPU). The chemical structure of the bio-PU products were confirmed with FTIR, NMR spectra and GPC. The thermal decomposition behaviors of products were characterized by TGA. The mechanical properties (tensile strength, hardness value) and viscoelastic properties (storage modulus and loss modulus) also had been tested.
Materials and Reagents. The reagtents in this experiment were shown in Table 1. All the reagents were of industrial pure class, and the nitrogen gas which used as a protecting gas in polyester synthesis process was from Daesung Co.
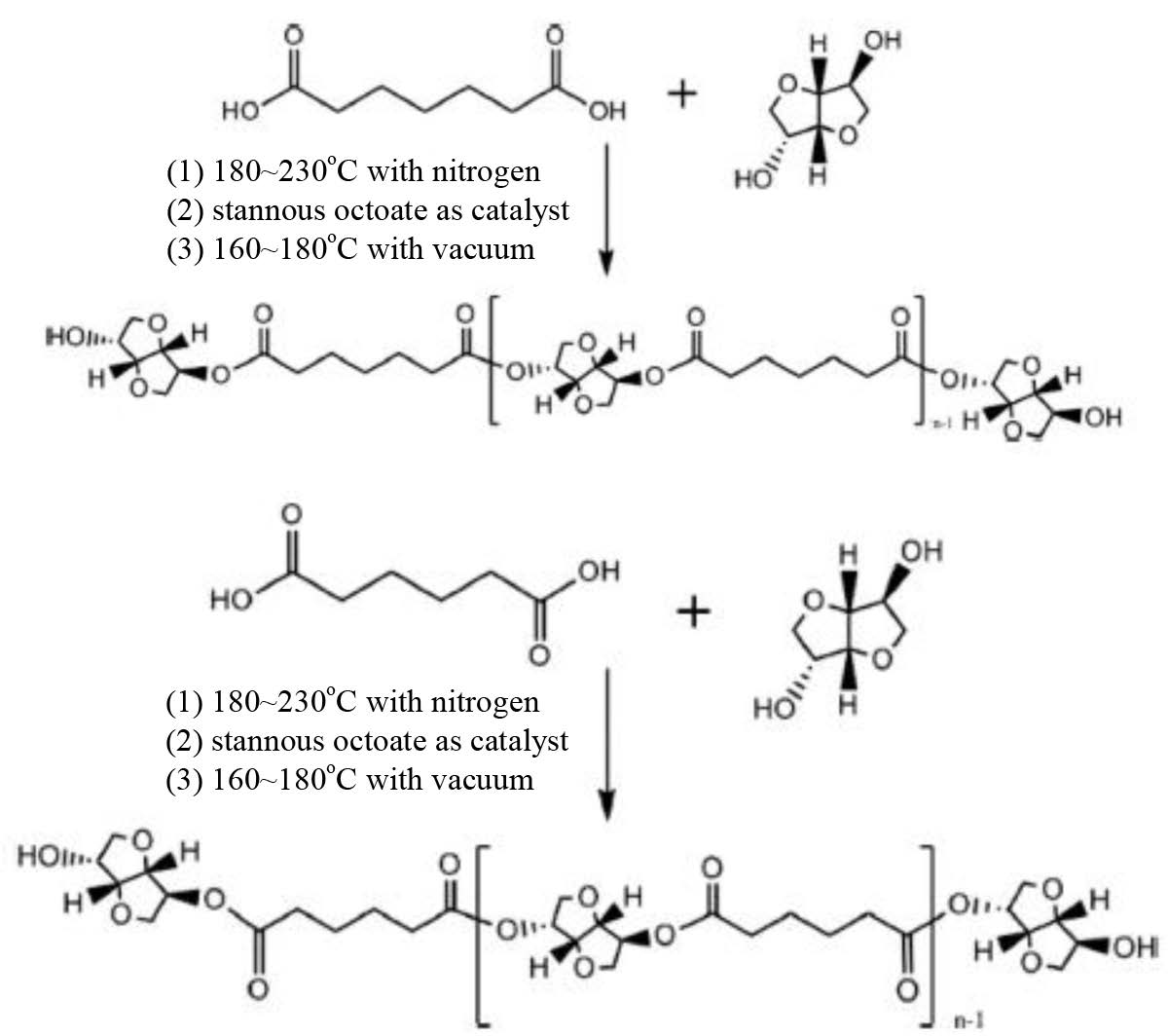
Scheme 1. Mechanisms for polymerization of PE 1.

Scheme 2. Mechanisms for polymerization of PE 2.
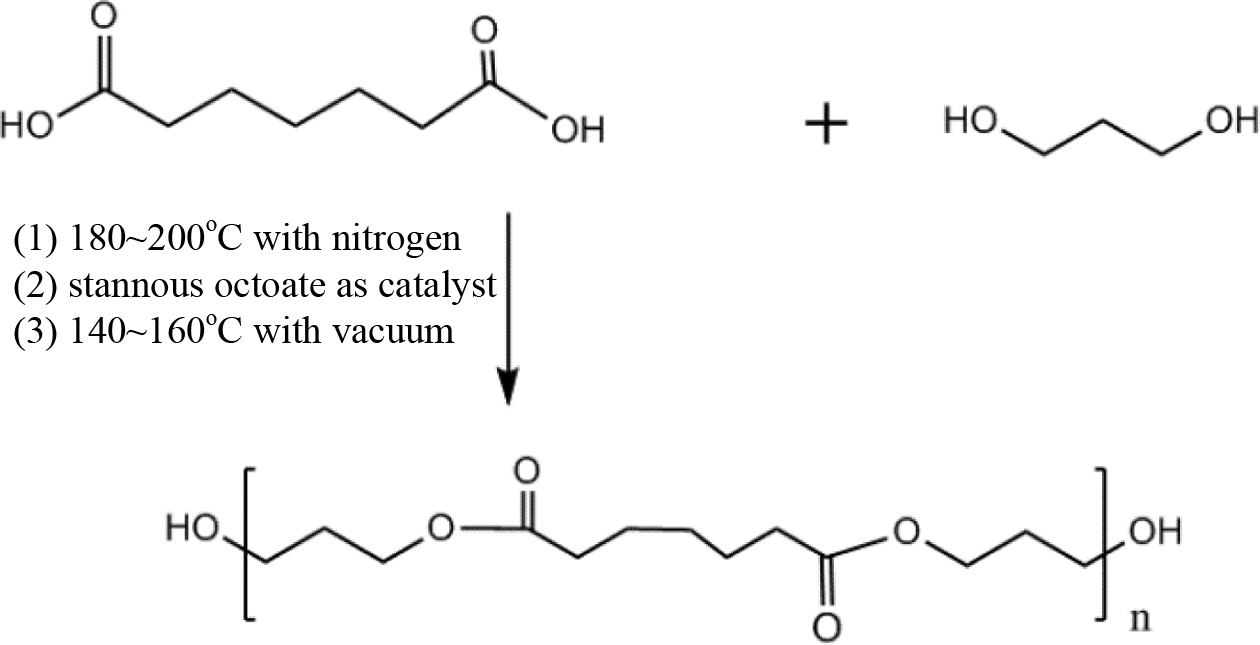
The Polymerization of Polyester Polyols. The mechanisms for polymerization of polyester polyols were shown in Schemes 1, 2, 3 and 4. And the formulation was shown in Table 2. A typical polymerization reaction was carried out as following: mixed the fatty acid and polyol into the reaction system which was shown with the amount in Table 2. The mixture was stirred by a magnetic stirrer at 400 rpm. Before reaction time of 4 h, the mixture was maintained under nitrogen, and then tin(II) 2-ethylhexanoate (stannous octoate) was added as catalyst.9 At the last 4 h, nitrogen atmosphere was removed and vacuum was induced (2 torr). The reaction mixture was cooled down below 60 oC, and the polyester polyol was obtained.
The Polymerization of Polyurethanes. The ratios of the components for PU polymerization were shown in Table 3. Polyester polyols, diisocyanates and chain extender was mixed with stirring at 200 rpm at room temperature, and the products had been synthesized after 5 min stirring.
Characterization. Thermal gravimetric analysis (TGA) of TPU samples were performed with TA Instruments TGA Q500 V20 from 30 to 800 ℃ at a heating rate of 10 ℃ min-1 under nitrogen atmosphere. Fourier transform infrared (FTIR) analysis of the filling materials was performed on a thin film of about 200-300 μm with Perkin Elmer Spectrum 100®. Samples were scanned in the wave range between 650 and 4000 cm-1 with a resolution of 4 cm-1. The nuclear magnetic resonance (NMR) results of samples were performed with Aglient NMR VNMRS 400 with DMSO solvent. Viscoelastic properties were determined with a rubber process analyzer (RPA-V1, U-Can Dynatex Inc., Taiwan). The strain sweep test from 0.01 degree to 20 degrees was operated at 60 oC and 1 Hz on RPA according to ASTM D 6204-97. The hardness was obtained by a shore durometer type A under ASTM D22-40. Tensile strength test was measured three times on a Tinius Olsen H5KT-0401 testing machine at a speed of 500 mm/min according to ASTM D412. The samples were fabricated into a dumb-bell shape with the dimensions of 25×6×1 mm using a heating press machine.
The results of TGA wereshown in Figure 1 and Figure 2. From the figures, TPU 4 showed the largest knee point before 400 ℃, suggesting that it showed the best thermal stability in the low temperature range, resulting from the shortest molecular chain and regular structure, which provided great mechanical properties. But above 400 ℃, because of the ring-like structure and branch chain of bio-polyol, the second decomposition temperature of TPU 2 and TPU 3 was higher than TPU 1 and TPU 4.
The results of FTIR were presented in Figure 3. From this figure, it could be seen the typical characteristic bands of PU were obaserved, in which 1735 cm-1 corresponds to C=O, 1540 cm-1 and 3200 cm-1 to N-H, But characteristic band of NCO at 2245 cm-1 was not obaserved, meaing all the –NCO groups were reacted.
Figure 4 showed the NMR spectra of the TPU samples. The difference in proton peaks were observed from the different polyester polyols. In the spectra of TPU 2 and TPU 3, the typical peaks of isosorbide structure could be found the chemical shift range from 2 to 5 with many peaks. In the cases of TPU 1 and TPU 4, due to the simple linear structure, the peaks of these two samples were simple.
The GPC results of TPU samples were presented in Table 4. TPU 3 showed the largest Mn, and TPU 2, TPU 3 showed the PDI about 1.45, which meant TPU 2 and TPU 3 were relative homogeneous due to the ring-like structure and more oxygen atoms in isosorbide, which could cause spatial steric hindrance and charge repulsion to make uniform molecular weight distribution of TPU samples.10,11
Figure 5 and Figure 6 showed the storage and loss modulus of TPU samples during the strain sweep. The internal interactions in the TPU samples were analyzed using the dynamic deformation. It could be found in Figure 5 and Figure 6, TPU 4 and TPU 1 always showed the higher storage and loss modulus during all strain sweep process, meaning that the internal interactions were high in these two polymers . In the cases of TPU 2 and TPU 3, the curves showed the smaller values, meaning that the internal interactions in these two polymers were smaller, showing the weaker mechanical properties and viscoelastic properties.12
Figure 7 showed the loss factor results of TPU samples in the strain sweep test. From the curves, TPU 3 showed the largest loss factor value, indicating the loose structure of TPU matrix and weak deformation resistance. But for TPU 4, the curve was located at the bottom, meaning the best deformation resistance and compact structure.13,14
Figure 8 and Figure 9 showed the elastic torque and viscous torque of TPU samples in the strain sweep test. At the low strain range, TPU 1 and TPU 4 showed the similar elastic and viscous torques, but at high strain range, with the increase of external force from the RPA, all the samples showed the larger elastic and viscous torques than before, which meant all the TPU samples in this research had great viscoelastic properties, especially for TPU 4, showed the largest torque values in this characterization, which means this TPU had the best viscoelastic properties.15,16
Table 5 showed the tensile strength results of all the samples. PE 4 provided the best tensile strength compared to the other samples. The difference of TPU 1 and TPU 4 was the polyester polyol, and PE 1 is synthesized using azelaic acid and 1,3-PD, and PE 4 using adipic acid and 1,3-PD. PE 1 shows the longer soft-segments chain and higher carbon number than PE 4. In TPU, the soft segment is made by polyol and isocyanate. The longer polyol molecule provided higher soft segment content, lower Tg, and less hardness value, and theoretical mechanical reinforcing. On the other hand, longer molecular chains might increase the probability of molecular entanglement, and caused the higher Tg, which could increase molecular crystallinity of TPU molecular, reduced the viscoelasticity of TPU, and this effect offsets the theoretical mechanical properties reinforcement. Thus, TPU 4 showed better mechanical properties and viscoelastic properties than TPU 1.
Figure 10 showed the hardness value results in this research. Also due to compact internal structure formation, TPU 1 and TPU 4 showed higher hardness value, but TPU 4 showed the smallest floating range, which meant the most stable structure.17

|
Figure 1 TGA results of TPU samples. |

|
Figure 2 Derivative of weight according to temperature changes of TPU samples. |

|
Figure 3 FTIR spectra of TPU samples. |

|
Figure 4 NMR spectra of TPU samples. |

|
Figure 5 Storage modulus of TPU samples. |

|
Figure 6 Loss modulus of TPU samples. |

|
Figure 7 Loss factor of TPU samples. |

|
Figure 8 Elastic torque of TPU samples. |

|
Figure 9 Viscous torque of TPU samples. |

|
Figure 10 Hardness of TPU samples. |
Bio-based polyester polyols had been synthesized by esterification using azelaic acid, adipic acid as fatty acidsand isosorbide and 1,3-PD used as polyols. Then the bio-based PUs were synthesized with bio-based polyester polyols and MDI. The chemical structures of bio-based PUs were confirmed with FTIR, NMR spectra and GPC. The thermal decomposition behaviors of products were characterized by TGA. The mechanical properties (tensile strength, hardness value) and viscoelastic properties (storage modulus and loss modulus) also had been tested. From the results of the characterizations, all the bio-based TPUs were successfully synthesized. TPU 4 showed the best viscoelastic properties in RPA characterizations, TPU 2 and TPU 3 showed the narrower molecular weight distribution in GPC results, resulting from the spatial steric hindrance and charge repulsion of isosorbide structure. Because of the compact structure of TPU matrix, TPU 4 showed the best results of tensile strength and hardness value.
- 1. K. M. Zia, M. Zuber, M. Barikani, A. Jabbar, and M. K. Khosa, Carbohyd. Polym., 80, 539 (2010).
-

- 2. A. Rahimi and P. Shokrolahi, J. Inorg. Mater., 3, 843 (2001).
-

- 3. P. L. Nayak, J. Macromol. Sci. C, 40, 1 (2000).
-

- 4. S. Miao, P. Wang, Z. Su, and S. Zhang, Acta Biomater., 10, 1692 (2014).
-

- 5. P. S. Nigam and A. Singh, Prog. Energ. Combust., 37, 52 (2011).
-

- 6. L. M. de Espinosa and M. A. Meier, Eur. Polym. J., 47, 837 (2011).
-

- 7. L. Hojabri, X. Kong, and S. S. Narine, J. Polym. Sci., Part A: Polym. Chem., 48, 3302 (2010).
-

- 8. N. Mahmood, Z. Yuan, J. Schmidt, and C. C. Xu, Renew. Sust. Energ. Rev., 60, 317 (2016).
-

- 9. A. Kowalski, A. Duda, and S. Penczek, Macromolecules, 33, 689 (2000).
-

- 10. D. K. Chattopadhyay and K. V. S. N. Raju, Prog. Polym. Sci., 32, 352 (2007).
-

- 11. F. Chambon, Z. S. Petrovic, W. J. MacKnight, and H. H. Winter, Macromolecules, 19, 2146 (1986).
-

- 12. S. Thakur and N. Karak, Prog. Org. Coat., 76, 157 (2013).
-

- 13. J. G. Kirkwood, Recl. Trav. Chim. Pays-Bas, 68, 649 (1949).
-

- 14. M. Barikani and M. Barmar, Iran. Polym. J., 5, 231 (1996).
-

- 15. K. H. Jin and U. R. Cho, Elastomers Compos., 49, 31 (2014).
-

- 16. K. H. Jin, M. S. Kim, and U. R. Cho, Elastomers Compos., 48, 190 (2013).
-

- 17. S. Y. Choi and U. R. Cho, Elastomers Compos., 40, 249 (2005).
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2019; 43(4): 595-601
Published online Jul 25, 2019
- 10.7317/pk.2019.43.4.595
- Received on Mar 15, 2019
- Revised on May 22, 2019
- Accepted on May 22, 2019
 Services
Services
Shared
 Correspondence to
Correspondence to
- Ryong Cho
-
*School of Energy, Materials and Chemical Engineering, Korea University of Technology and Education, Cheonan, Chungnam 31253, Korea
**Research Center of Eco-friendly & High Performance Chemical Materials, Cheonan, Chungnam 31253, Korea - E-mail: urcho@koreatech.ac.kr
- ORCID:
0000-0003-4866-8109














 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.