- Carbon Nanotubes Modified by Ionic Liquid and Its Effect on Properties of Silicon Rubber Composites
College of Civil Engineering, Qingdao University of Technology, Qingdao, 266033, P.R. China
*College of Electromechanical Engineering, Qingdao University of Science and Technology,Qingdao 266061, P.R. China- 이온성 액체로 처리된 탄소 나노튜브가 실리콘 고무 복합체의 물성에 미치는 영향
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
In this paper, multi-walled carbon nanotubes (MWCNTs) were modified using ionic liquids (ILs), and silicone rubber/carbon nanotube composites were prepared. The surface of carbon nanotubes is coated with a layer of ionic liquid by non-covalent bond modification, and the van der Waals force between the carbon nanotubes is reduced to achieve the purpose of improving their dispersibility. The electrical conductivity of silicone rubber/carbon nanotube composites prepared by modified carbon nanotubes is improved. The dispersion of modified carbon nanotubes in silicone rubber matrix and the interface interaction between carbon nanotubes and silicone rubber are analyzed by rubber process analyzer and scanning electron microscopy (SEM) tests. The effects of modified carbon nanotubes on electrical conductivity, mechanical properties and electromagnetic shielding properties of silicone rubber composites were studied. The results show that the dispersion of carbon nanotubes in silicone rubber matrix is improved effectively through the modification of ionic liquid, the conductive property of the composite is improved, the electromagnetic wave reflection of the composite is increased, and the electromagnetic shielding efficiency of the composite is improved, and the shielding efficiency is up to 26 dB.
In this paper, multi-walled carbon nanotubes (MWCNTs) were modified using ionic liquids (ILs), and silicone rubber/carbon nanotube composites were prepared. The surface of carbon nanotubes is coated with a layer of ionic liquid by non-covalent bond modification, and the van der Waals force between the carbon nanotubes is reduced to achieve the purpose of improving their dispersibility. The electrical conductivity of silicone rubber/carbon nanotube composites prepared by modified carbon nanotubes is improved. The dispersion of modified carbon nanotubes in silicone rubber matrix and the interface interaction between carbon nanotubes and silicone rubber are analyzed by RPA and SEM tests. The effects of modified carbon nanotubes on electrical conductivity, mechanical properties and electromagnetic shielding properties of silicone rubber composites were studied. The results show that the dispersion of carbon nanotubes in silicone rubber matrix is improved effectively through the modification of ionic liquid, the conductive property of the composite is improved, the electromagnetic wave reflection of the composite is increased, and the electromagnetic shielding efficiency of the composite is improved, and the shielding efficiency is up to 26 dB.
Keywords: ionic liquid, carbon nanotubes, silicone rubber, electromagnetic shielding.
This study was supported by the Key Research and Development Plan of Shandong Province (Major Scientific and Technological Innovation Project, 2020CXGC010312) and Ocean Science and Technology of Collaborative Innovation Center Project (22-05-CXZX-04-04-28).
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Guangyi Lin reports financial support was provided by Key Research and Development Plan of Shandong Province and Collaborative Innovation Center for Marine Science and Technology Project.
Carbon nanotubes (CNTs) have been widely used as conductive fillers in conducting composites since their appearance due to their unique structure and excellent electrical properties. Multi-walled carbon nanotubes (MWCNT) are one-dimensional tubular materials composed of single or multi-layer graphite sheets. CNTs have a large specific surface area, thermal conductivity, and electrical conductivity, and the conductive composite prepared by using CNTs as conductive filler has an extremely low percolation threshold.1,2
According to the conductive pathway theory, the conductivity of composite materials is that when the conductive filler is added to a certain amount in the matrix, the conductive network is formed by connecting and contacting each other in the matrix.3
Conductive path theory regarding the conductivity of the composite material is good or bad about how many of the conductive paths, and as the number of conductive filler composites conductivity increased, but the conductive filler has a specific point in the process of increase, more than conductivity change happens after this point, the point is called the percolation threshold of the conductive fillers.4
Due to the length-diameter ratio of CNTs and the interaction of van der Waals forces, CNTs are very easy to aggregate and tangle, which also leads to certain limitations in the application of CNTs. In order to reduce the aggregation of CNTs, increase their compatibility with the composite matrix, and make them evenly dispersed in the composite matrix, it is necessary to modify the surface of CNTs.5,6
The surface modification methods of nano-fillers mainly include covalent bond, and non-covalent bond modification. Covalent bond modification is to modify the surface of the filler by exploiting the chemical reaction between the reactive functional groups on the filler surface and specific small molecules or polymers. Jiang et al.
used a KH550 silane coupling agent to modify CNTs and explored the influence of the amount of KH550 on the conductivity of CNTs/silicone rubber composites. They found that when a small amount of KH550 was added, CNTs were well dispersed in the silicone rubber matrix and were easy to form a conductive network, thus improving the conductivity of the composites. When the number of KH550 components continues to increase, the conductivity of the composite is decreased. This is because CNTs are covered by excessive silane coupling agent and rubber molecular chain, which hinders the direct contact between CNTs and silicone rubber matrix and makes it difficult to form a conductive network. Non-covalent bonding modification is realized by hydrogen bonding, π-π interaction, and other physical methods. Compared with covalent bond modification, non-covalent bond modification method is relatively simple and retains the intrinsic properties of nanofillers to a large extent. Jiang et al.
compared the influence of covalent bond modification and non-covalent bond modification of CNTs on the electrical conductivity of silicone rubber composites, and found that both modification methods can improve the dispersion of CNTs in the matrix of composites. When the CNTs content was 0.9%, the conductivity of the composites filled with non-covalently modified CNTs was four orders of magnitude higher than that of the composites filled with covalently modified CNTs, because the rubber molecular chains in the covalently modified composites were strongly adsorbed on the surface of CNTs, which hindered the direct contact of CNTs.7-10
The commonly used nanofiller modifier is generally not conductive, and its adsorption to the filler surface through chemical reaction or physical action will hinder the direct contact of conductive filler, thus hindering electron conduction. Conductive modifiers such as ionic liquids (ILs) have unique advantages in improving the conductivity of composites. The IL is an ionic system composed of organic cations and organic anions that are liquid at room temperature.
Due to the diversity of anions and cations, ILs is endowed with a series of excellent properties such as high conductivity, good stability, good solubility, high viscosity, and low vapor pressure. Subramaniam et al. modified MWCNTs with ionic liquid 1-butyl 3-methylimidazolium bisfluoromethylsulfonimide salt ([BMIM]TF2N). BMIM modified CNTs through the π-π interaction, which improved the dispersion of CNTs in neoprene rubber and thus improved the conductivity of composite materials. Jiji Abraham et al.
modified MWCNTs with ionic liquid 1-benzyl-3-methylimidazolium chloride ([B2MIM]Cl) to prepare CNTs/styrene butadiene rubber composite with electromagnetic shielding effect. When the filling amount of modified CNTs was 10 phr, the shielding effect of the composite material reached 35 dB.
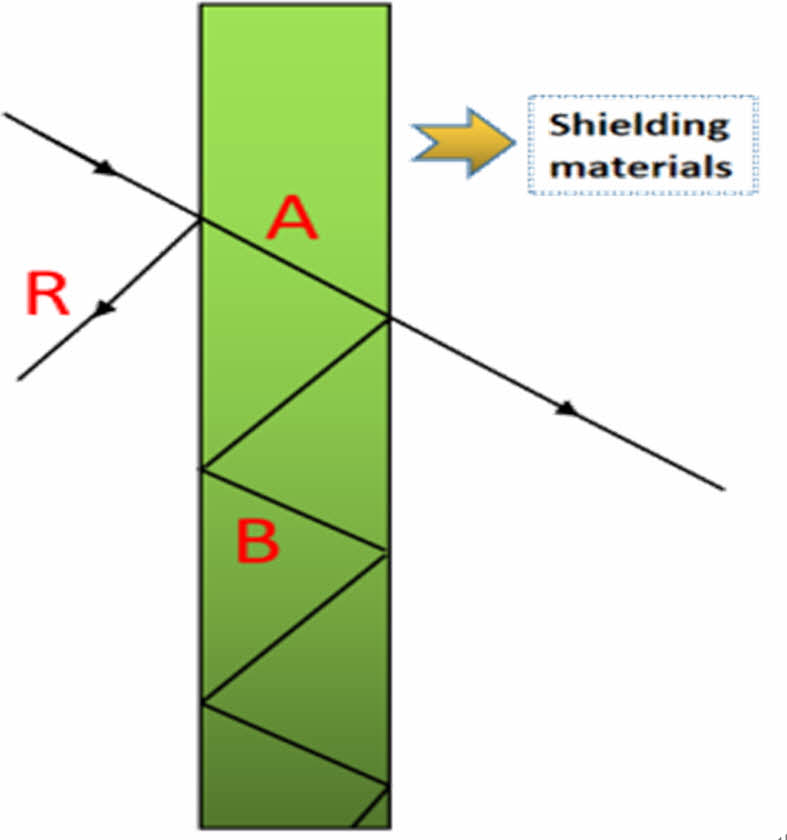
Electromagnetic shielding refers to the transmission of electromagnetic wave barrier or loss. The electromagnetic shielding performance of materials is usually characterized by shielding efficiency, shielding efficiency (SE) = reflection loss (SER) + absorption loss (SEA) + multiple reflection loss (SEB). Shielding materials usually require high conductivity or permeability. According to the loss mechanism, it can be divided into three categories : absorption loss A is dominant, Reflection loss R is dominant or Absorption loss R is combined with reflection loss B. The electromagnetic shielding mechanism of composite materials is shown in Figure 1.
|
Figure 1 Electromagnetic shielding mechanism diagram of composite materials. |
Materials. The experimental materials used in this experiment are shown in Table 1.
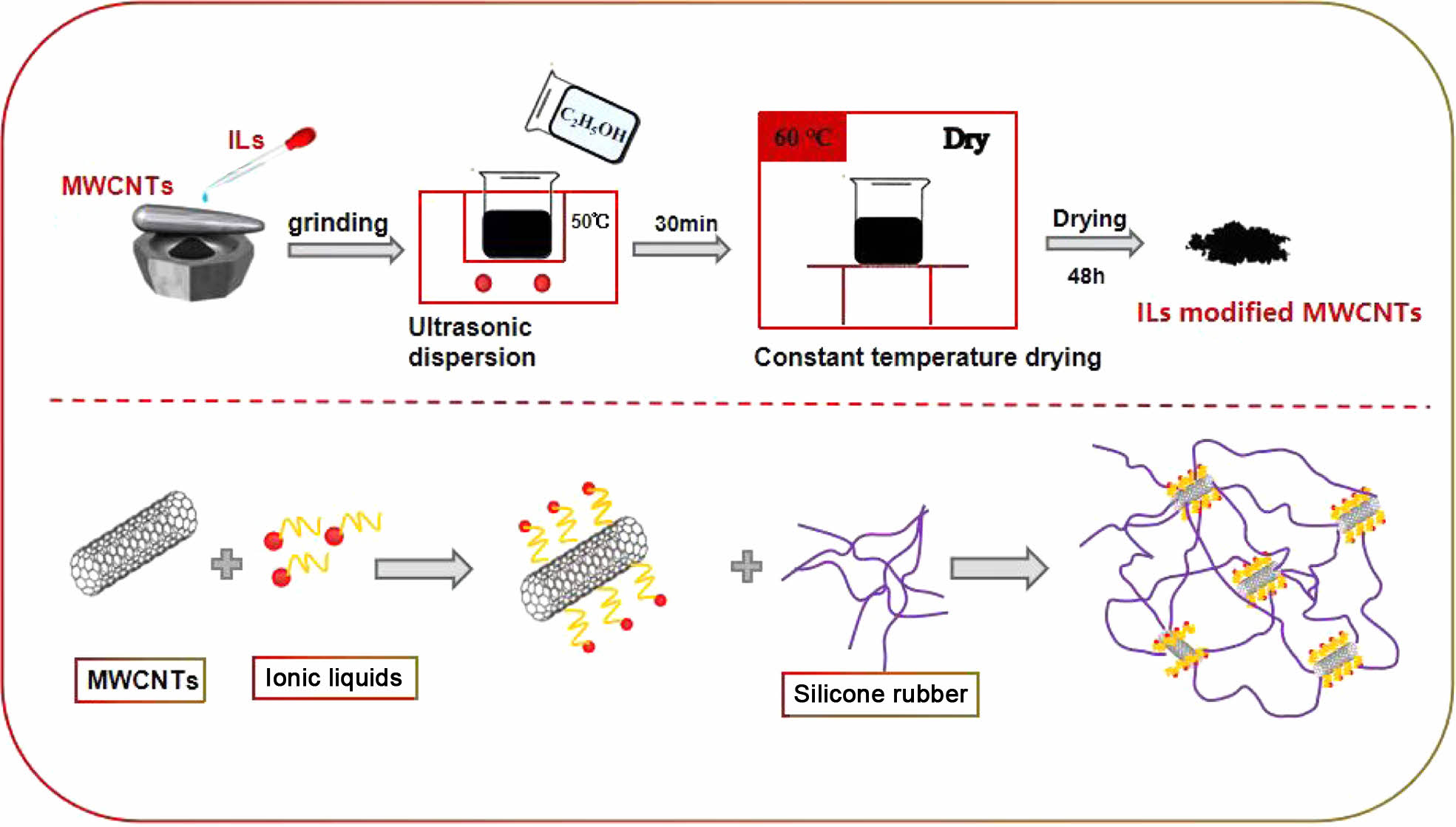
Preparation of CNTs/ILs Composites. The modification of ionic liquid and CNTs is to grind in agate mortar for 30 minutes to get a black paste mixture. The mixture is ultrasonic in ethanol solution for 30 minutes, and then vacuum drying at 50 ℃ for 48 hours to finally get the modified CNTs. The sample is named ImLn, where I and L are the ionic liquid and CNTs respectively, and M and N are the number of CNTs and the ratio of ionic liquid to CNTs respectively. For example, I0L1 represents an unmodified CNT; I1L1 represents a modified CNT with a 1:1 ratio of ionic liquid to CNT. I5L10 indicates that the proportion of ionic liquid to CNTs is 5:1 and the number of modified CNTs is 10. The modification process of CNTs is shown in Figure 2.
Preparation of CNTs/ILs/silicone Rubber Composites. The silicone rubber was mixed in the mixing machine and packing shall be carried out in accordance with the certain proportion, this process is to first make silicone rubber completely package on double roller roller, partial join quantitative packing, and then stay mixing mill completely after eating is expected to add double minimum curing agent, mixing 30 minutes, and then the final will be mixed evenly mixed gum out of silicone. Initial vulcanization is in the plate vulcanization machine 170 ℃, 10 MPa under the condition of vulcanization for 10 minutes. The second stage of vulcanization is carried out in the digital display drying oven, the process requires two hours of secondary vulcanization treatment at 200 ℃.
Instruments and Equipment. The experimental equipment and instruments used in this experiment are shown in Table 2.
|
Figure 2 Modification process of CNTs. |
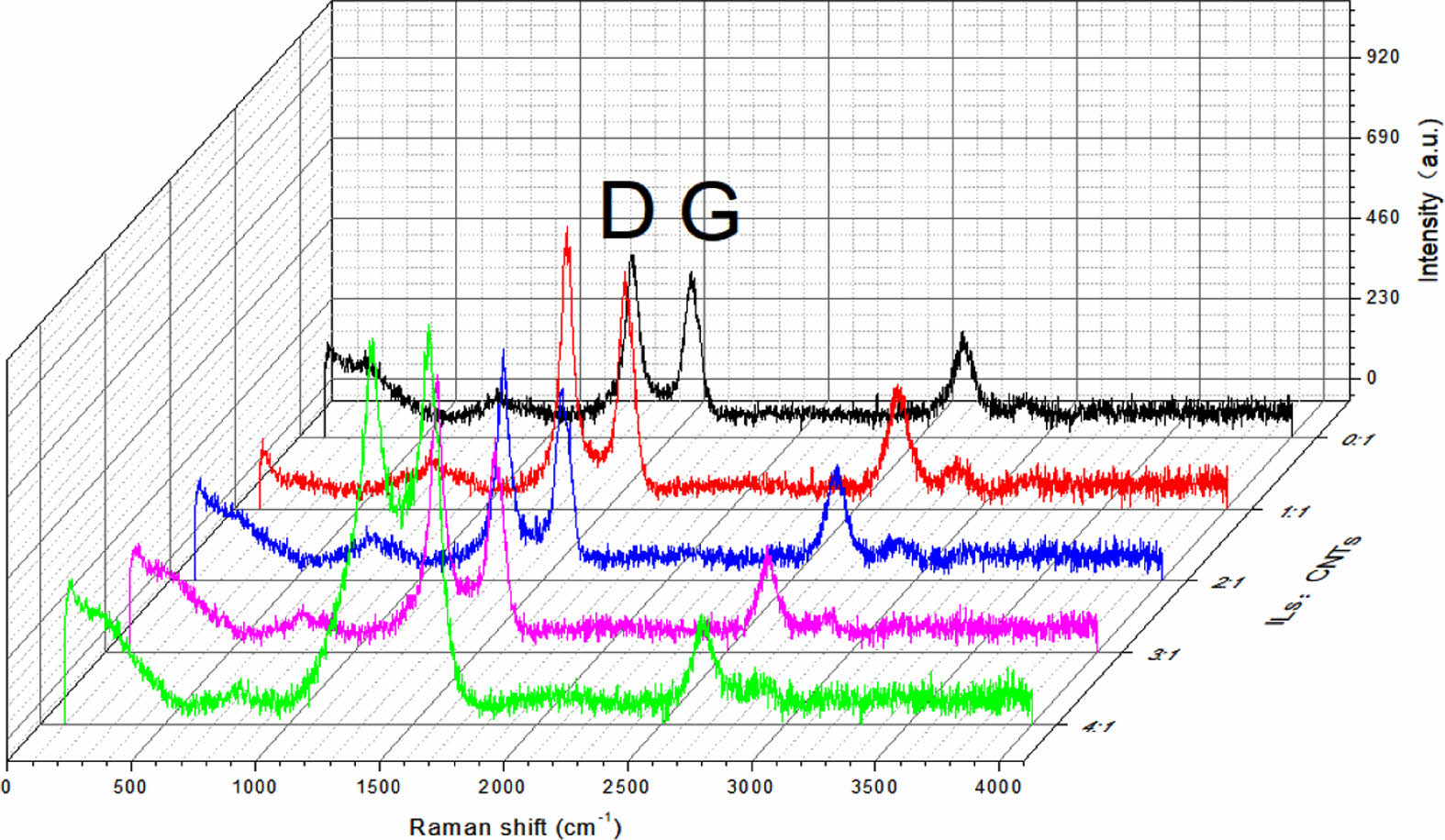
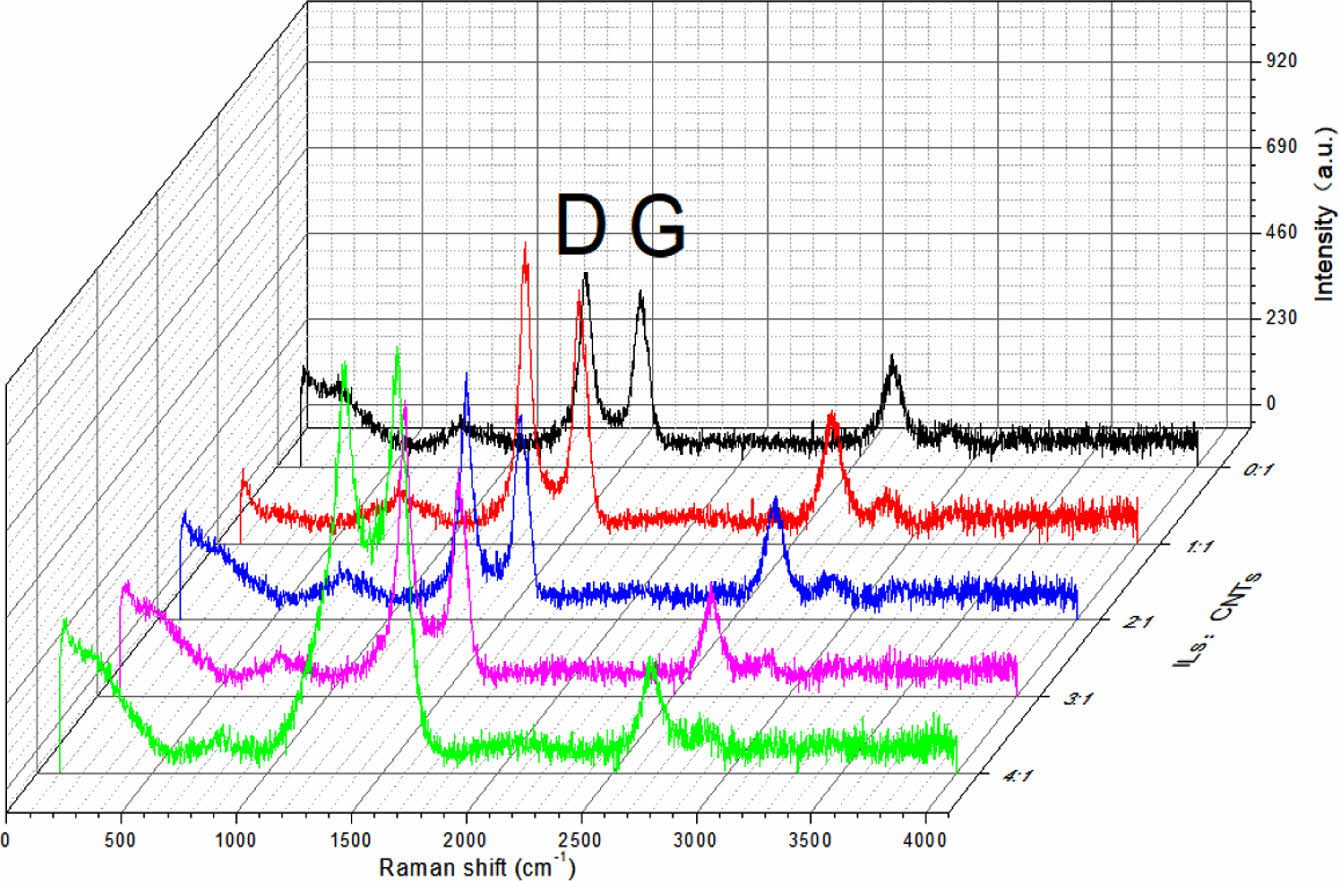
Raman Spectra of Composites. Raman spectra of CNTs before and after modification are shown in Figure 3. Similar to graphite, two strong peaks can be observed. The peak value around 1600 cm-1 comes from tangential vibration of carbon atoms, which is called G peak. The peak at about 1300 cm-1 is known as the D-peak, which is due to significant defects or disturbances in the nanostructure. For the modified CNTs, the D and G peaks shift to some extent, but no new peak is observed as opposed to that of the unmodified CNTs. The upward shift of the spectra is due to the cationic π bond interaction between the ionic liquid and the CNTs or the π-π interaction between the multi-wall of the CNTs. In summary, the modification method is a physical modification, and there is no chemical damage to CNTs.
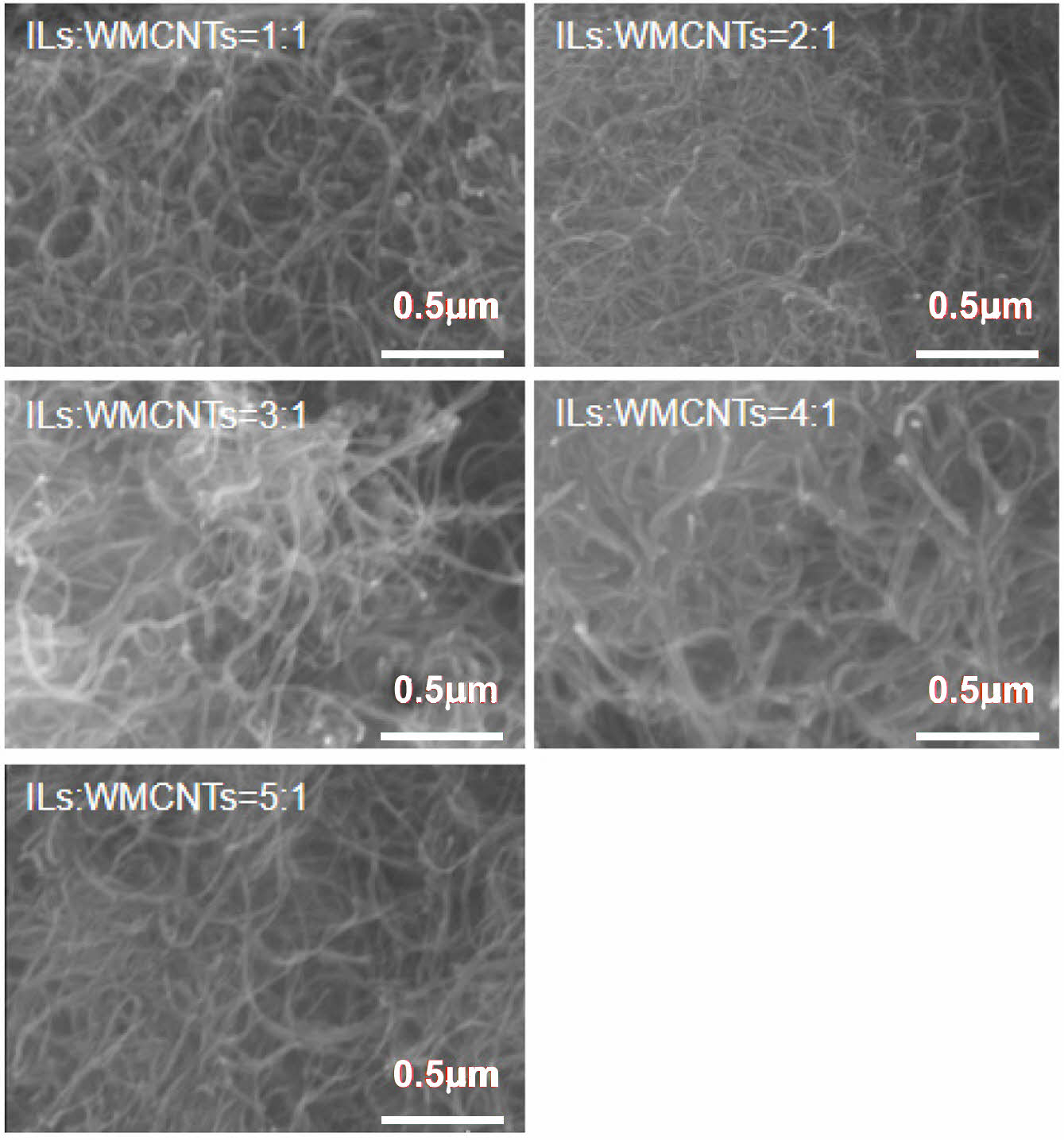
Scanning Electron Microscopy of Composites. As shown in Figure 4, when the ratio of ionic liquid to CNTs is 1:1, the dispersion of CNTs is poor and obvious entanglement occurs, because the amount of ionic liquid is insufficient to completely disperse CNTs. When the ratio of ionic liquid to CNTs is 4:1, the dispersion of CNTs is obviously improved and entanglement is reduced. This is because the modification of CNTs by ionic liquid can effectively reduce the van der Waals force between CNTs, reduce the entanglement phenomenon of CNTs, and improve the dispersion of CNTs. Under the electron microscope, it can be clearly seen that with the increase of the proportion of ionic liquid, the dispersion of CNTs is enhanced, and the improvement of the dispersion of CNTs is the main reason for the good electrical conductivity of composites.
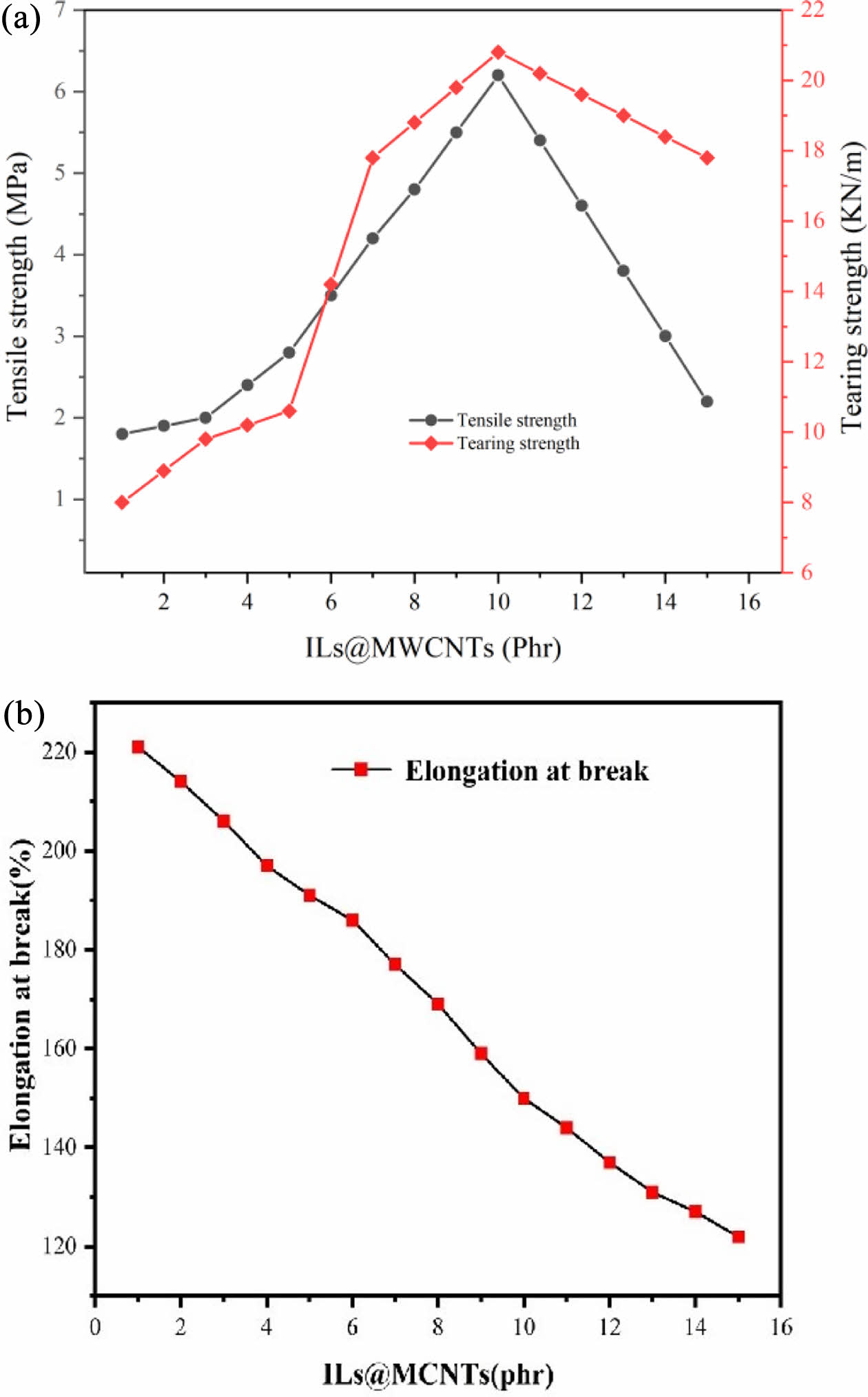
Mechanical Properties of Composites. As shown in Figure 5, when the ratio of ionic liquid to CNTs is 1:1 (1 mmol ionic liquid modifies one CNT), it can be seen that the tensile strength increases from 1.92 MPa to 6.17 MPa and then decreases to 2.23 MPa. At 2 phr (phr: parts per hundred rubber), 3, 5, and 7 phr, CNTs/silicone rubber composites have the reinforcing effect, and reach the maximum tensile strength (6.1 MPa) and the maximum tearing strength (21 N/m) at 10 phr. The elongation at break of the composite is related to the filling amount of CNTs. When the filling amount of CNTs increases from 1 phr to 15 phr, the elongation at break of the composite decreases from 221% to 122%. The analysis shows that the silicone rubber molecules are connected to many active sites on the CNTs, which can help the external force to better transfer to the CNTs. The effective transfer of load can effectively improve the tensile strength of composite materials, mainly because it prevents rubber cracks caused by stress concentration.11-12 Due to the poor dispersibility of CNTs, increasing the amount of CNTs will lead to agglomeration, which will form a stress concentration point in the CNTs/silicone rubber composite and eventually lead to fracture. To sum up, the number of copies using modified CNTs has an obvious impact on the mechanical properties of the composites, which fill the amount of 10 phr, preparation of the composite material has the highest tensile strength and tearing strength, which shows that modified CNTs and the fusion of the silicone rubber matrix, further promotes the filler dispersion. Therefore, we took 10 parts of modified CNTs as the optimal level.
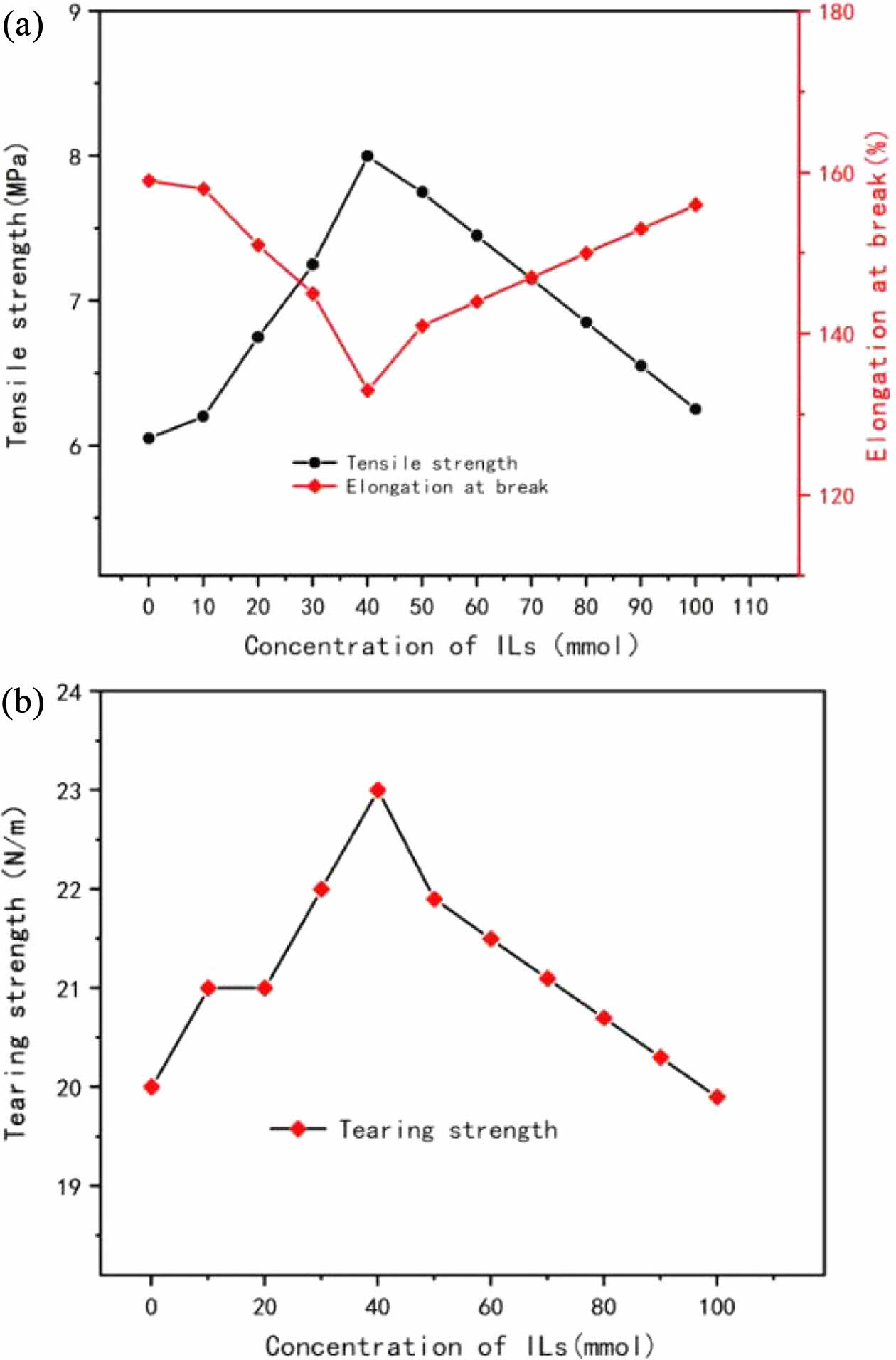
As shown in Figure 6, when the filling amount of CNTs is 10 phr, the tensile strength increases from 6.03 MPa to 8.21 MPa and then decreases to 6.24 MPa with the increase of ionic liquid usage. When the amount of ionic liquid was 40 mmol, the tensile strength of CNTs/silicone rubber composite reached the maximum 8.21 MPa, and 10, 20, 30, 40, 50 and 100 mmol all played a reinforcing role. The elongation at break decreased from 159% to 133% and then increased to 156%. The amount of ionic liquid has a significant impact on the fracture elongation of the composite material. Analysis suggests that when the amount of ionic liquid is less than 40 mmol, the CNT-rubber interfacial bonding is poor, leading to a decrease in the fracture elongation of the composite material. As the amount of ionic liquid increases, it plays a plasticizing role in enhancing the dispersion of CNTs in the silicone rubber matrix, thereby reducing the aggregation of CNTs and improving their reinforcing effect on the silicone rubber matrix. When the amount of ionic liquid is 0-40 mmol, the tensile strength of CNTs/silicone rubber composites increases continuously. This is because the modification effect of ionic liquid on CNTs improves the dispersion of CNTs in rubber matrix. Thus, the mechanical properties of CNTs/silicone rubber composites were improved. However, when the usage tendency of 40-100 mL, with the increase of ionic liquid usage, the dispersion of the CNTs increased, but the tensile strength of the composite material didn't get the corresponding ascension, this is due to excessive amounts of ionic liquid had the effect of plasticizer in the silicone rubber matrix influence the mechanical properties of CNT/silicone rubber composites.13 So I4L10 is the optimal level.
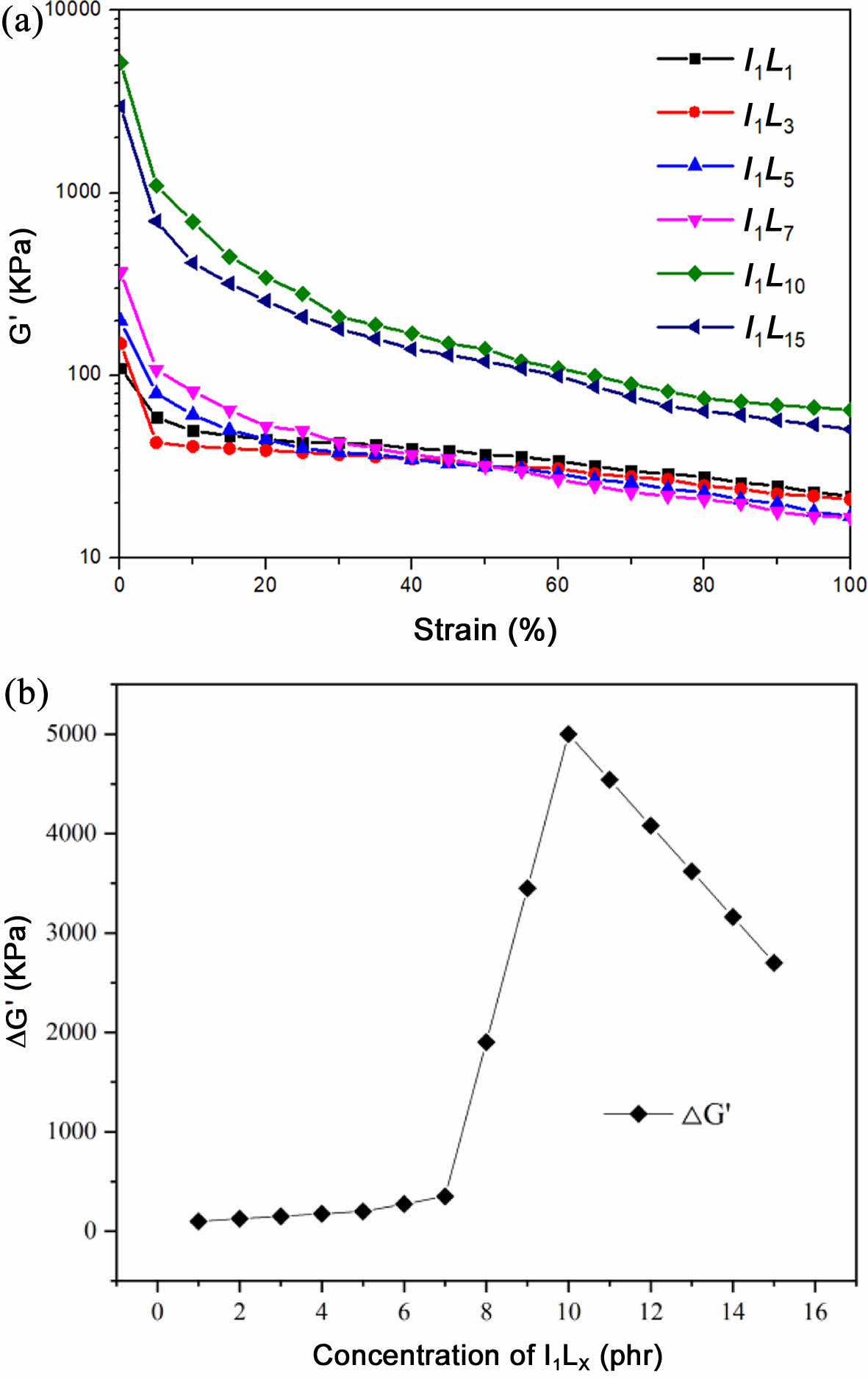
Dynamic Rheological Properties of Composites. The dynamic modulus of rubber decreases with increasing strain, a phenomenon known as the Payne effect. Payne effect is mainly used to characterize the interaction between filler and filler in rubber composites. The stronger the filler network structure is, the more obvious the effect will be.14-15 G' represents the destruction and reconstruction of filler network. As can be seen from Figure 7, when the filling amount of CNTs is 1-10 phr, the initial energy storage modulus is positively correlated with the filling amount of CNTs. As the filling amount of CNTs continues to increase, the initial energy storage modulus has a downward trend. When the strain is less than 10%, the energy storage modulus decreases rapidly, resulting in the destruction of the packing network structure. At this time, the destruction speed of the packing network is much faster than the reconstruction speed. However, when the damage of filler network reaches a certain degree, the energy storage modulus G' is no longer related to the strain, and G' tends to a constant value. The strength of the packing mesh can be shown by the decay value of the storage modulus. When the filling amount of CNTs is increased, the structure of the filler network is also enhanced. When the filling amount of CNTs is 10 phr, the packing network structure is the strongest, indicating that the dispersion of CNTs in the silicone rubber matrix is the best.16-18
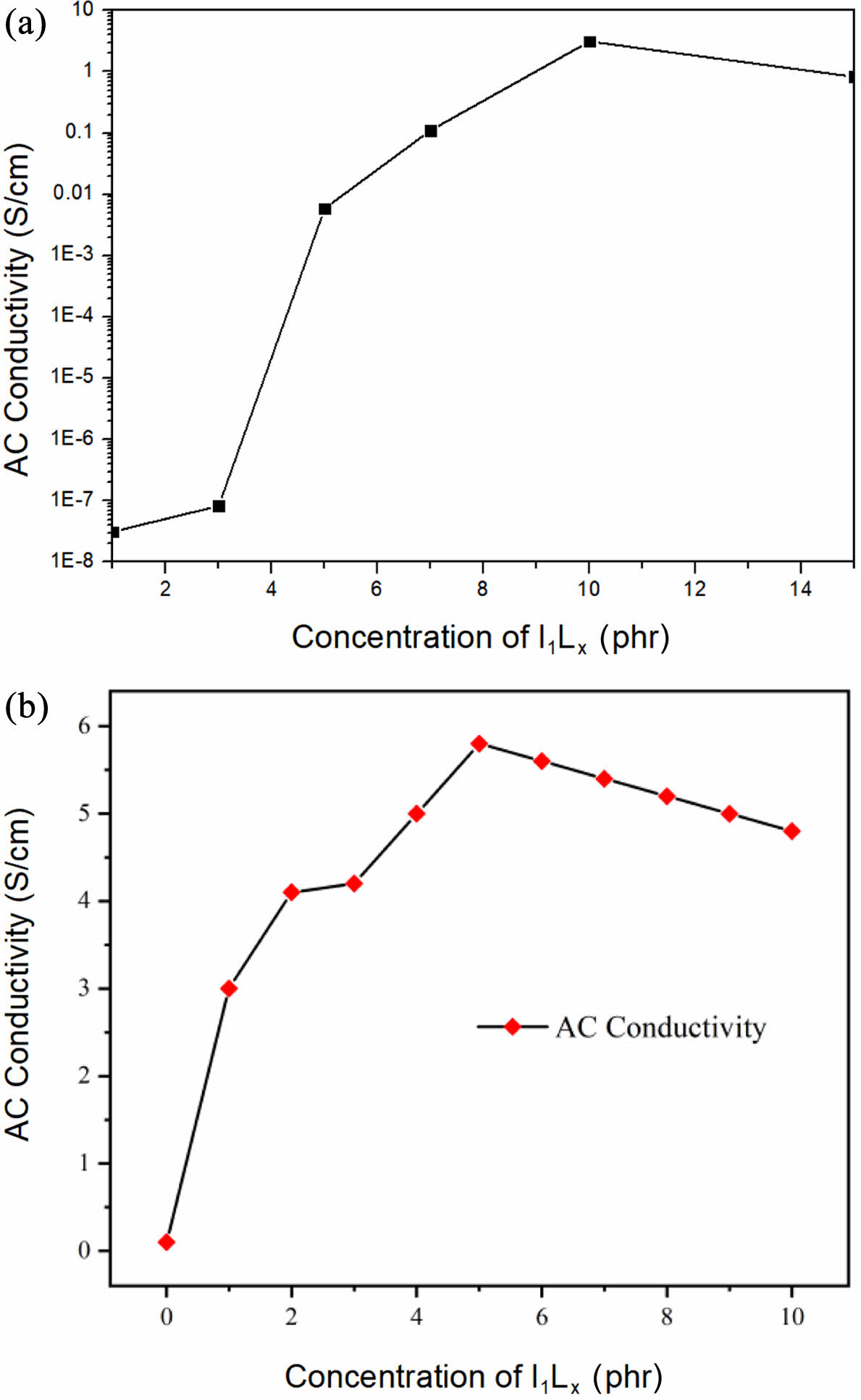
Conductivity Test of Composites. CNTs can be thought of as wires through which electrons can be transferred freely. The insulating rubber matrix has conductivity beyond the critical concentration of CNT tubes, which is called the percolation threshold. As can be seen from Figure 8, when the mass fraction of conductive filler is 1-5 phr, CNTs are isolated and dispersed in the insulating matrix, resulting in the increase of the distance between CNTs and the slow conduction of electrons. Furthermore, the conductivity of the matrix determines the conductivity of the composite material. When the mass fraction of CNTs is 7 phr, the percolation network is initially formed, and the conduction can be completed by direct contact or electronic transition between the tubes, leading to a rapid increase in the conductivity of the composite material, which is the phenomenon of percolation. At this time, the critical mass fraction of filler is also known as the threshold of conductive percolation. Conducting more than to seepage threshold also represents the formation of conductive network, at this time, even if the increase in the amount of CNTs, also almost had no effect on conductivity of ascension, the conductive fillers and polymer matrix type of conductive composites were decided more than threshold, but distribution of conductive filler in matrix and matrix of the state of aggregation structure for more than 45 there is influence on the size of the threshold, in contrast, The conductivity of I5L10 sample has been improved obviously. The results show that the CNTs fully modified by ionic liquid form a good conductive network in rubber matrix. In addition, as the ionic liquid/CNT ratio further increases from 1:1 to 5:1, a large number of CNT percolation paths are formed, and electrons can move freely in the whole frequency range. Therefore, a significant increase in electrical conductivity can be observed in 5:1 (I5L10) composites. When the ratio of ionic liquid continues to increase to 10:1 (I10L10), the conductivity of the composite is not significantly improved. This is because at I5L10, the ionic liquid has optimized the modification of CNTs, and increasing the proportion of ionic liquid in the modified CNTs cannot further improve the dispersion of CNTs. Therefore, we chose I5L10 as the optimal formula.
Electromagnetic Shielding Performance Test of Composites. Electromagnetic waves are made up of two parts: electric and magnetic fields, which are perpendicular to each other and vary periodically. Various studies have been carried out on electromagnetic shielding effect in the field of nanocomposites, in which electrical conductivity plays an important role in shielding efficiency. In order to improve the electrical conductivity of the composite, conductive nanofiller is usually added into the insulating matrix to improve the electrical conductivity of the composite. The overall electromagnetic shielding performance is determined by three mechanisms, namely, reflection loss on the surface of shielding material, absorption loss of electromagnetic wave by shielding material, and internal multiple reflection loss of different interface radiation.19
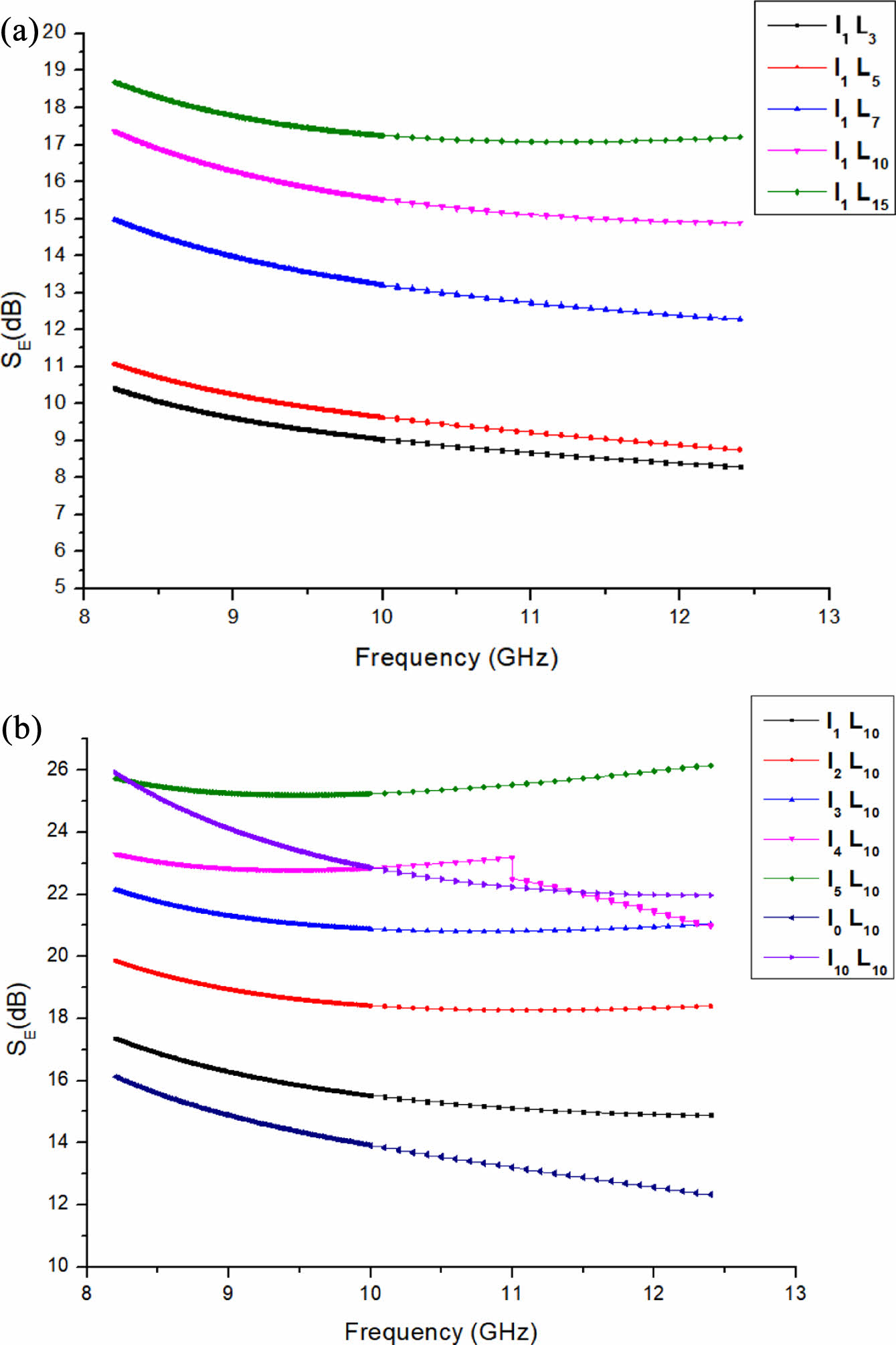
In rubber composites, the cross-linking network of conductive particles is the basic requirement of reflecting electromagnetic waves, and the electrical conductivity of composites is the main reason to produce excellent electromagnetic shielding efficiency. The electromagnetic shielding performance of composites depends largely on the conductivity, dispersion and aspect ratio of the conductive filler in the polymer matrix. Among them, the conductivity and connectivity of CNTs play a crucial role in realizing electromagnetic shielding reflection.20-22 The research focus of this paper is to use ionic liquid modified CNTs in rubber matrix uniform dispersion to establish conductive network to achieve electromagnetic wave reflection. As shown in Figure 9, with the increase of mass fraction of CNTs, material of electromagnetic shielding performance, this is because the quality as the CNTs number reaches 10, the conductive composite materials more than seepage threshold can be transcended, and improve the material’s conductivity, eventually making composite materials of electromagnetic shielding performance is improved. The shielding efficiency of the composite containing 10 modified CNTs was determined to be 26 dB in the frequency range of 8 to 12 GHz. The fine network structure of CNTs promotes the conduction of more free electrons, which ultimately helps the composite to reflect electromagnetic waves. In addition, the thin MWCNT network can also promote multiple scattering in the network due to its large specific surface area.23
In order to study the shielding mechanism, the total shielding efficiency is further decomposed into reflection loss (SER) and absorption loss (SEA), and the electromagnetic shielding efficiency is calculated by the following formula.
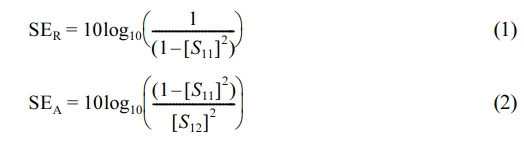
where SER and SEA represent reflective electromagnetic shielding efficiency and absorption electromagnetic shielding efficiency respectively. S11 and S12 are called input reflection coefficient and reverse transmission coefficient respectively and are usually measured by vector network analyzer.
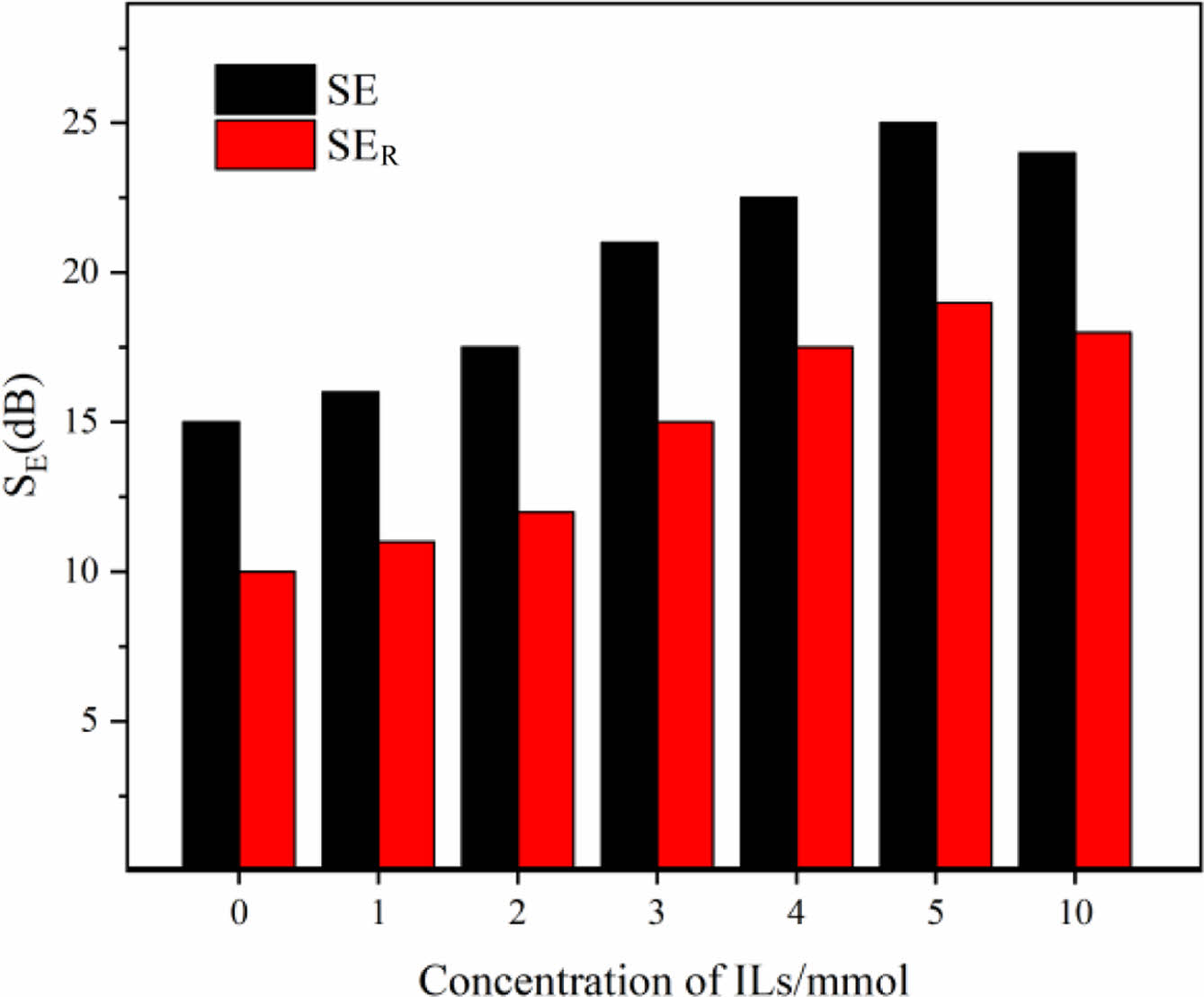
As shown in Figure 10, the electromagnetic shielding efficiency caused by absorption and reflection (SEA and SER) increases with the increase of the mass fraction of ionic liquid. It can be seen from the experimental data that the contribution of reflection loss to electromagnetic attenuation is greater than that of absorption loss due to the increase of electrical conductivity. To sum up, the shielding of electromagnetic waves by electromagnetic shielding silicone rubber is mainly completed by reflection loss.
|
Figure 3 Raman spectra of the pristine and modified MWCNTs. |
|
Figure 4 SEM of functionalization-MWCNTs (F-MWCNTs). |
|
Figure 5 Effect of F-MWCNTs filling on the mechanical properties of composites: (a) tensile strength and tearing strength; (b) elongation at break. |
|
Figure 6 Effect of ILs filling on the mechanical properties of composites (MWCNTs = 10 phr): (a) tensile strength & elongation at break; (b) tearing strength. |
|
Figure 7 Effects of filling amount of F-MWCNTs on the storage modulus and the storage modulus attenuation of composites: (a) storage modulus; (b) decay of energy storage modulus. |
|
Figure 8 Effects of filling amount of F-MWCNTs on the conductivity of composites: (a) different loading amounts; (b) different ratios. |
|
Figure 9 Effects of filling amount of F-MWCNTs on the electromagnetic shielding effect of composites: (a) different number of components; (b) different proportions. |
|
Figure 10 Effect of reflection and absorption loss on electromagnetic shielding effect of silicon rubber composites (9 GHz). |
In this paper, well-dispersed functionalized ionic liquids were coated on the surface of MWCNTs by cation-π bonds. The use of ILs improved the dispersion of CNTs in the silicone rubber matrix and reduced the agglomeration of CNTs, thus improving the reinforcing effect of CNTs on the silicone rubber matrix. The tensile strength of the composite material was significantly affected by different amounts of ionic liquid. When the amount of ionic liquid was 40 mmol, the tensile strength of the composite material was the highest, which indicated that the modified CNTs were well fused with the silicone rubber matrix and further promoted the dispersion of the filler. In addition, due to the good dispersion and the generation of CNT conductive network, the CNT/silicone rubber composites have high electrical conductivity and stable electromagnetic shielding effectiveness in a wide frequency range. The conductivity and electromagnetic shielding effectiveness of CNT/silicone rubber composites can be adjusted by changing the filling amount of conductive fillers. The maximum shielding effectiveness was 26 dB for a 3 mm thick shielded (8 GHz frequency) sample with 10 phr CNTs.
- 1. Coleman, J. N.; Khan, U.; Blau, W. J.; Gun'Ko, Y. K. Small But Strong: a Review of the Mechanical Properties of Carbon Nanotube-polymer Composites. Carbon, 2006, 44, 1624-1652.
-

- 2. Bauhofer, W.; Kovacs, J. Z. A Review and Analysis of Electrical Percolation in Carbon Nanotube Polymer Composites. Composites Science and Technology, 2009, 69, 1486-1498
-

- 3. Wen, M.; Sun, X.; Su, L.; Shen, J.; Li, J.; Guo, S. The Electrical Conductivity of Carbon Nanotube/carbon Black/polypropylene Composites Prepared Through Multistage Stretching Extrusion. Polymer, 2012, 53, 1602-1610.
-

- 4. Dou Yanli, Gu Haijing, Sun Shixiang, Yao Weiguo, Guan Dongbo. Synthesis of a Grape-like Conductive Carbon Black/Ag Hybrid as the Conductive Filler for Soft Silicone Rubber. RSC advances, 2021, 12, 1184-1193.
-

- 5. Gao, H.; Liu, H.; Song, C.; Hu, G. Infusion of Graphene in Natural Rubber Matrix to Prepare Conductive Rubber by Ultrasound-assisted Supercritical CO2 Method. Chemical Engineering Journal, 2019, 368, 1013-1021.
-

- 6. Jiang, Y.; Gan, L.; Yuen, M. C. Improvenment of Carbon Nanotubes Dispersion by Chitosan Salt and its Application in Silicone Rubber. Compos. Sci. Technol. 2013, 86, 129-134.
-

- 7. Jiang, Y.; Gan, L.; Yuen, M. C.; Jiang, S.; Luo, N. M. Carbon Nanotubes Based High Temperature Vulcanized Silicone Rubber Nanocomoposite with Excellent Elasticity and Electrical Properties. Compos. Part A: Appl. Sci. Manuf. 2014, 66, 135-141.
-

- 8. Hallett, J. P.; Welton, T. ChemInformabstract: Room-tem-perature Ionic Liquids:solvents for Synthesis and Cataly-sis. Part 2. Chem. Reviews, 2011, 43, 2071-2083.
-

- 9. Kalaivani Subramaniam, Amit Das, Gert Heinrich. Development of Conducting Polychloroprene Rubber Using Imidazolium Based Ionic Liquid Modifified Multi-walled Carbon Nanotubes. Compos. Sci. Technol. 2011, 71, 1441-1449.
-

- 10. Jiji, A.; Hong, S.; Dong, W. P.; Sang. E. S. Water-borne Graphene-derived Conductive SBR Prepared by Latex Heterocoagulation. Macromol. Res. 2010, 18, 558-565.
-

- 11. Zhao, A.; Shi, X. Y.; Sun, S. H.; Zhang, H. M.; Zuo, M.; Song, Y. H.; Zheng, Q. Insights Into the Payne Effect of Carbon Black Filled Styrene-butadiene Rubber Compounds. Chin. J. Polym. Sci. 2021, 39, 81-90.
-

- 12. Zhongjia, X.; Yihu, S.; Qiang, Z.. Payne Effect of Carbon Black Filled Natural Rubber Compounds and Their Carbon Black Gels. Polymer, 2019, 185, 121953-121953.
-

- 13. Nanotechnology - Carbon Nanotubes; Reports from University of Tehran Add New Data to Findings in Carbon Nanotubes (A computational approach to evaluate the nonlinear and noisy DC electrical response in carbon nanotube/polymer nanocomposites near the percolation threshold). Chemicals & Chemistry, 2020.
- 14. Chung, D. D. L. Materials for Electromagnetic Interference Shielding. Mater. Chem. Physics, 2020, 255, 123587.
-

- 15. Yun, T.; Kim, H.; Iqbal, A.; Cho, Y. S.; Lee, G. S.; Kim, M.-K.; Kim, S. J.; Kim, D.; Gogotsi, Y.; Kim, S. O.; Koo, C. M.; Electromagnetic Shielding of Monolayer MXene Assemblies. Adv. Mater. 2020, 32, 1906769.
-

- 16. Arun Kumar, D. S.; Puneeth Kumar, T. R.; Krishnamoorthy, K.; Devadas Bhat, P.; Rahman, M. R. Flexible Electromagnetic Shielding Material Using Multi-Walled Carbon Nanotube Coated Cotton Fabric, in IEEE Transactions on Components, Packaging and Manufacturing Technology, 2022, 12, 479-488.
-

- 17. Kirner, J.; Qin, Y.; Zhang, L.; Jansen, A.; Lu, W. Optimization of Graphite–SiO blend electrodes for lithium-ion batteries: Stable cycling enabled by single-walled carbon nanotube conductive additive. J. Power Sources, 2020, 450, 227711-227711.
-

- 18. Wu, T.; Mei, X.; Liang, L.; Peng, X.; Wang, G.; Zhang, S. Structure-function Integrated Poly(aryl ether ketone)-grafted MWCNTs/poly(ether ether ketone) Composites With Low Percolation Threshold of Both Conductivity and Electromagnetic Shielding. Compos. Sci. Technol. 2022, 217, 109032.
-

- 19. Tang, L.; Wang, X.; Ji, P. Molecular Dynamics Simulation of Mechanical and Friction Properties of CNTs / NBR Composites. Lubrication Engineering, 2022, 47, 21-26.
-

- 20. Zhu, W.; Chen, Z.; Liang, J.; Su, D. A Laminated Carbon Nanotubes/silicon Boron Carbonitride Film for High-efficiency Electromagnetic Interference Shielding with Oxidation Resistance. Carbon, 2022, 197, 65-75.
-

- 21. Abraham, J.; Xavier, P.; Bose, S.; George, S. C.; Kalarikkal, N.; Thomas, S. Investigation Into Dielectric Behaviour and Electromagnetic Interference Shielding Effectiveness of Conducting Styrene Butadiene Rubber Composites Containing Ionic Liquid Modified MWCNT. Polymer, 2017, 112, 102-115.
-

- 22. Tamore, M. S.; Ratna, D.; Mishra, S.; Shimpi, N. G. Effect of Functionalized Multi-walled Carbon Nanotubes on Physicomechanical Properties of Silicone Rubber Nanocomposites. J. Compos. Mater. 2019, 53, 3157-3168.
-

- 23. Lai, W.; Wang, Y.; He, J. Effects of Carbonyl Iron Powder (CIP) Content on the Electromagnetic Wave Absorption and Mechanical Properties of CIP/ABS Composites. Polymers, 2020, 12, 1694.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2024; 48(2): 195-203
Published online Mar 25, 2024
- 10.7317/pk.2024.48.2.195
- Received on Nov 6, 2023
- Revised on Dec 27, 2023
- Accepted on Dec 29, 2023
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Conflict of Interest
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Guangyi Lin
-
College of Electromechanical Engineering, Qingdao University of Science and Technology,Qingdao 266061, P.R. China
- E-mail: 1029393842@qq.com
- ORCID:
0000-0001-8797-8607










 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.