- Enhancing the Photostability of Poly(Vinyl Chloride) (PVC) Through the Incorporation of Cerium and Samarium Oxide
Ali Jabbar A. Al-Sarray†
 , Basma Esam Jasim*, Nibras Abdul-Ameer Aboud*, and Fatimah Jumaah Moaen
, Basma Esam Jasim*, Nibras Abdul-Ameer Aboud*, and Fatimah Jumaah MoaenPolymer Research Unit, College of Science, Mustansiriyah University, 10052 Iraq
*Department of Chemical Industrial, Institute of Technology/Baghdad, Middle Technical University, 10074 Iraq- 폴리비닐 클로라이드의 광안정성 향상을 위한 세륨 및 사마륨 산화물 도입
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
This study focuses on enhancing the photostability of poly(vinyl chloride) (PVC) by incorporating it with a nanocomposite (CeO2/Sm2O3) synthesized through a chemical method. The nanocomposite’s characteristics, including the crystallite size (21.23 nm), grain size (27 nm by field emission scanning electron microscope, 23 nm by transmission electron microscope), and X-ray diffractometer analysis, were investigated. Thin PVC films with and without the nanocomposite were fabricated and analyzed for optical and morphological properties using Fourier-transform infrared spectroscopy and atomic force microscopy. Photostability was assessed through indices (ICO, IPO, IOH), weight loss measurements after UV radiation exposure. Results indicate a noticeable reduction in PVC film decomposition, showcasing the nanocomposites' potential as effective light stabilizers. AFM and microscopic analyses further support the stabilizing efficiency. This research contributes valuable insights into utilizing CeO2/Sm2O3 nanocomposites for enhancing PVC photostability.
The manuscript explores improving the photostability of poly(vinyl chloride) (PVC) by incorporating cerium and samarium oxide nanocomposites. The nanocomposite, synthesized through a chemical method, effectively reduces PVC film decomposition after UV radiation exposure. This research provides valuable insights into using CeO2/Sm2O3 nanocomposites as potential light stabilizers for PVC, offering an alternative to traditional stabilizers with broader industrial applications.
Keywords: poly(vinyl chloride), CeO2/Sm2O3 nanocomposite, roughness, photo degradation, photostability.
The authors would like to thank Mustansiriyah University (www.uomustansiriyah.edu.iq) Baghdad-Iraq for supporting the present work.
The authors declare that there is no conflict of interest.
Poly(vinyl chloride) (PVC) is one of the widely used and adapted thermoplastic materials due to insulating the electricity, resisting the weathering, possessing excellent aesthetic quality, and being durable.1
Being the building block of producing medical and sport equipments, clothes, construction materials, packaging, and electronic devices, PVC is one of the highly demanded materials from the industrial perspective in order to meet the increasing global requirement.2-4 However, the inherent thermal instability of PVC, stemming from structural weaknesses within the polymer chain, such as allyl chloride, tertiary chloride, and carbonyl allyl chloride, poses challenges during thermal processing.5-7
Exposing PVC to high temperature and ultraviolet radiation accompanied by environmental oxygen, causes chemical and physical alternations, including dehydrochlorination process, which are undesirable.8,9 Releasing hydrochloric acid (HCl) induces the formation of polyene, hydroxy, and carbonyl residues,10 which initiates the formation of free radical that accelerate the degradation process of PVC.11,12 To address this issue, thermal stabilizers like layered hydrotalcite compounds, liquid mixed metals, metal soaps, and organic tin/lead salts have been historically employed to prevent thermal degradation during PVC production.13 The environmental concerns associated with lead-based and organotin compound stabilizers have prompted significant research into developing non-toxic alternatives.14,15
In recent years, rare earth compounds have gained attention as potential PVC thermal stabilizers, with examples including lanthanum cyanurate,16 lanthanum-pentaerythritol alkoxide,17 lanthanum histidine,16 and lanthanum sulfadiazine.3 These stabilizers have exhibited the ability to modify PVC conformation, inhibit dehydrochlorination, and replace unstable chlorine in PVC chains, ultimately impeding the chain reaction and catalysis by HCl, thereby stabilizing the material.14,15
Among these materials, cerium oxide nanoparticles (CeO2) have emerged as promising candidates for PVC stabilization due to their compatibility with the binary transition state.18
CeO2 stands out as a versatile material with multifaceted utility, characterized by its excellent thermal stability at elevated temperatures, abundance of oxygen vacancies on its surface, substantial specific area facilitating Faradaic processes, superior mechanical strength, and enhanced electron transmission.19,20 Furthermore, when combined with metals, either as composites or through doping, CeO2’s properties are further enhanced, including its oxygen storage capacity.19 This versatile compound, thanks to its 4f1 valence state and presence of Ce3+ and Ce4+ oxidation states, also exhibits exceptional recyclability, high retention rates, cost-effectiveness, reduced toxicity, regulated pore size, a favorable environmental profile, improved catalytic properties, and rapid redox reactivity.21
Similarly, samarium (Sm), a member of the lanthanide group, offers a range of oxidation states, enabling its use in a wide spectrum of applications, including optical lasers, supercapacitors, solar cells, biochemical sensors, nanoelectronics, and photocatalysts.22,23 The synergistic effects of binary metal oxides render them more advantageous in terms of specific capacity and electrochemical characteristics compared to their single-metal counterparts. Various synthesis methods, such as co-precipitation, hydrothermal, sol-gel, chemical vapor deposition, and electro-deposition, have been employed to produce nanoparticles, with hydrothermal techniques being particularly versatile and cost-effective, yielding a variety of nanostructures.21
Nevertheless, research into rare earth stabilizers has been relatively limited, constrained by challenging manufacturing processes, low yield, and inconsistent performance.24-26 Therefore, a comprehensive understanding of rare earth thermal stabilizers is essential to leverage the potential benefits of rare earth materials effectively. The aim of the present work is to investigate the stabilization ability of cerium oxide and samarium oxide nanoparticles when integrated with PVC.
Instruments. The experimental work was conducted utilizing the following equipment and instruments: a Companion BS-11, a KERN-ABS digital scale, a CARY 100 Conc UV–visible spectrometer, a Philips XL series 30 Field Emission Scanning Electron Microscope (FE-SEM), a Shimadzu 8400 Fourier-transform infrared spectrometer (FTIR), and a Shimadzu-XRD 6000 X-ray diffractometer.
Materials. Cerium nitrate hexahydrate (Ce(NO3)3·6H2O), samarium (III) nitrate hexahydrate (Sm(NO3)3·6H2O), urea (CH4N2O), and tetrahydrofuran (THF) were procured from Merck, Ltd. whereas PVC with about 233000 g/mol of average molecular weight and 800 degree of polymerization purchased from Petkim Petrokimya (Turkey) and were used without further purification.
Preparation of CeO2/Sm2O3 Nanocomposites. The nanocomposite was synthesized via a chemical process involving the salts Sm(NO3)3·6H2O and Ce(NO3)3·6H2O. An aqueous solution of Ce(NO3)3·6H2O, Sm(NO3)3·6H2O, and urea was prepared at a concentration of 0.5 mol. Sodium hydroxide (NaOH) solution was added dropwise at room temperature and stirred for 3 hours. Subsequently, the mixture was concentrated, washed multiple times with deionized water, and then subjected to drying. The produced composite was heated to 80 ℃, which caused it to participate, and then calcined at 500 ℃ for 3 hours, producing a yellowish-white colored powder.
Preparation of Thin Film Nanocomposites of PVC and CeO2/Sm2O3. The preparation of the thin films of PVC with CeO2/Sm2O3 nanocomposites was carried out in two stages. In the first stage, a pure PVC thin film was prepared by mixing 1 g of PVC with 100 mL of THF to form a 1% weight mixture, which was then stirred and heated to 75 ℃ before being cast into a glass mold. In the second stage, 0.05 g of the CeO2/Sm2O3 nanocomposite material was incorporated into the PVC mixture, thoroughly mixed using a stirrer, and subsequently subjected to ultrasonication for 30 min to remove air bubbles and aid in the dissolution of the nanocomposite material. The resulting nanocomposite films were then cast into 40 µm glass templates and allowed to dry under vacuum conditions in circular glass molds. The films were exposed to UV light at 6.0×10-9 ein·dm-3·S-1 with a λmax of 313 nm for 50-300 h, with occasional turning for even exposure.
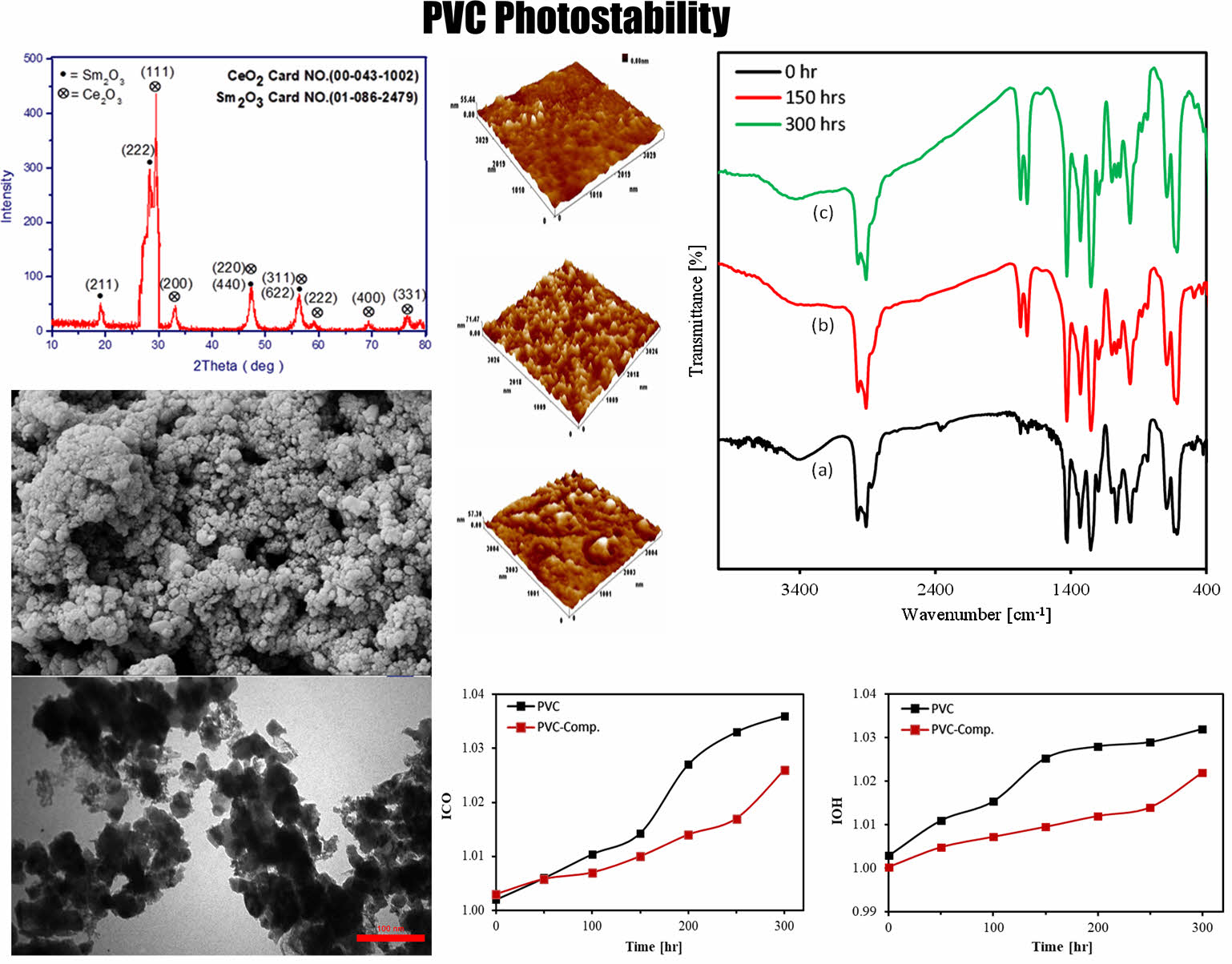
X-ray Diffraction Patterns of the CeO2/Sm2O3 Nanocomposite. The XRD analysis revealed the presence of CeO2 and Sm2O3 with a single-phase present in the prepared sample (Figure 1). The crystallinity of CeO2 was notably high, as indicated by the distinct diffraction peaks observed. The deposited films exhibited a polycrystalline structure with a cubic composition characteristic of fluorite CeO2. Specifically, common peaks were observed at 2θ angles of 29.51°, 33.13°, 59.16°, 69.39°, and 76.52°, corresponding to the (111), (200), (222), (400), and (331) orientation planes, respectively. Additionally, peaks at 2θ angles of 19.19° and 28.25° were observed, corresponding to the (211) and (222) orientation planes for Sm2O3. Notably, there were overlapping peaks at 47.36°, corresponding to (220) CeO2 and (440) Sm2O3, as well as at 56.05°, which belonged to ((311) CeO2 and (440) Sm2O3).
To determine the average crystalline size (D) of the synthesized CeO2/Sm2O3 nanoparticles, Scherrer’s equation was applied based on (222) and (111) lattice plane peaks27,28
as in the following:

Here, with k as the Scherrer constant (k = 0.9), λ as the constant X-ray wavelength (λ = 0.1540 nm), β representing the full width at half maximum in radians, and θ as the Bragg angle, the average crystalline size (D) of the synthesized nanoparticles was calculated to be 21.23 nm.
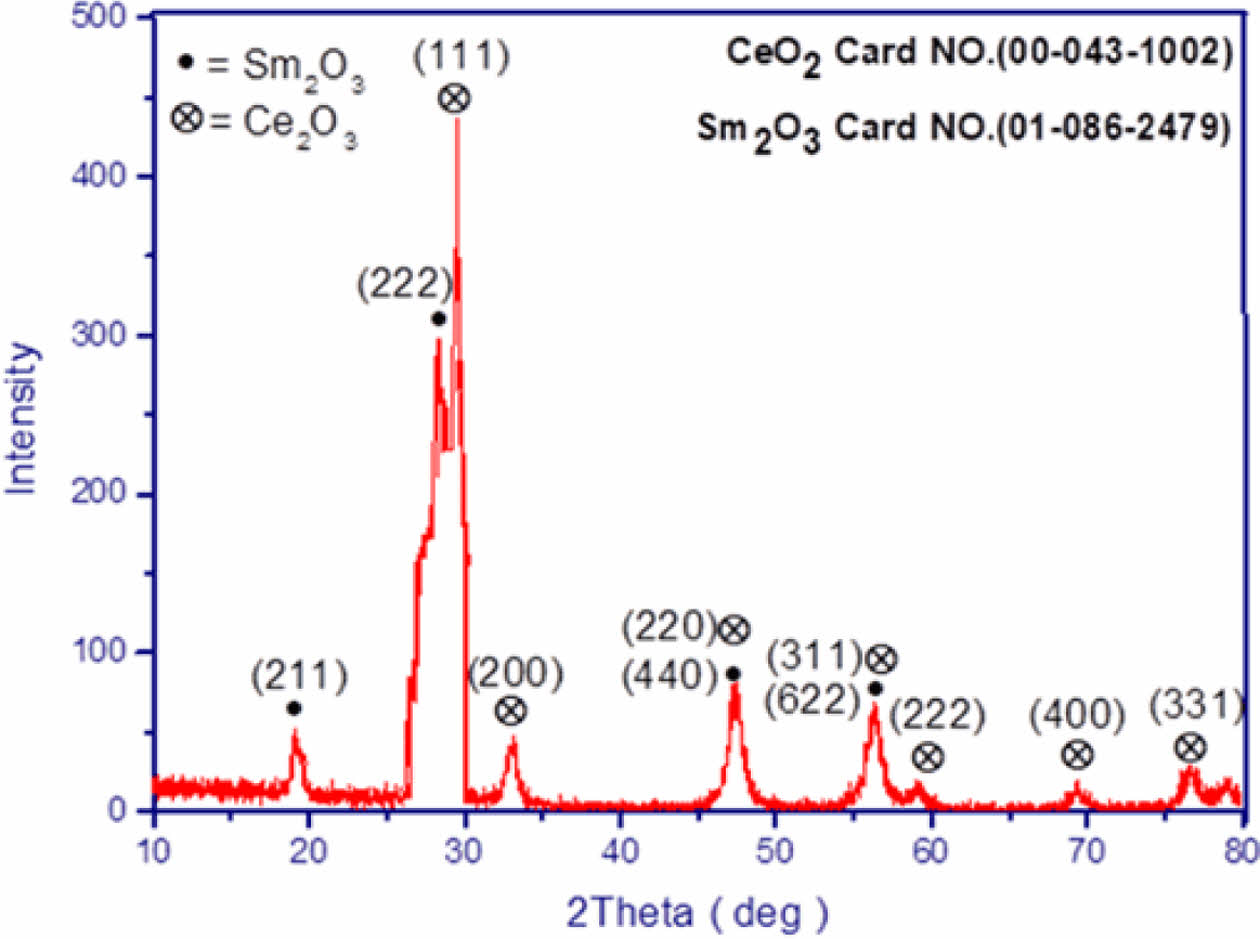
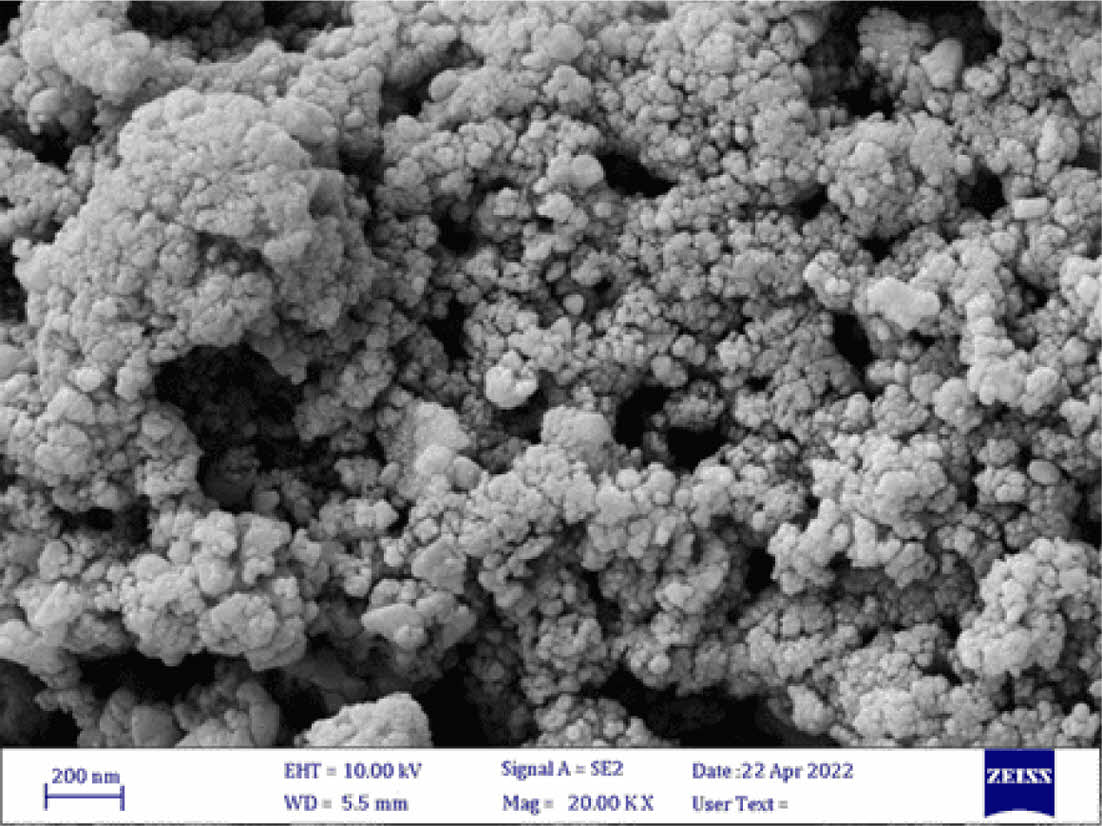
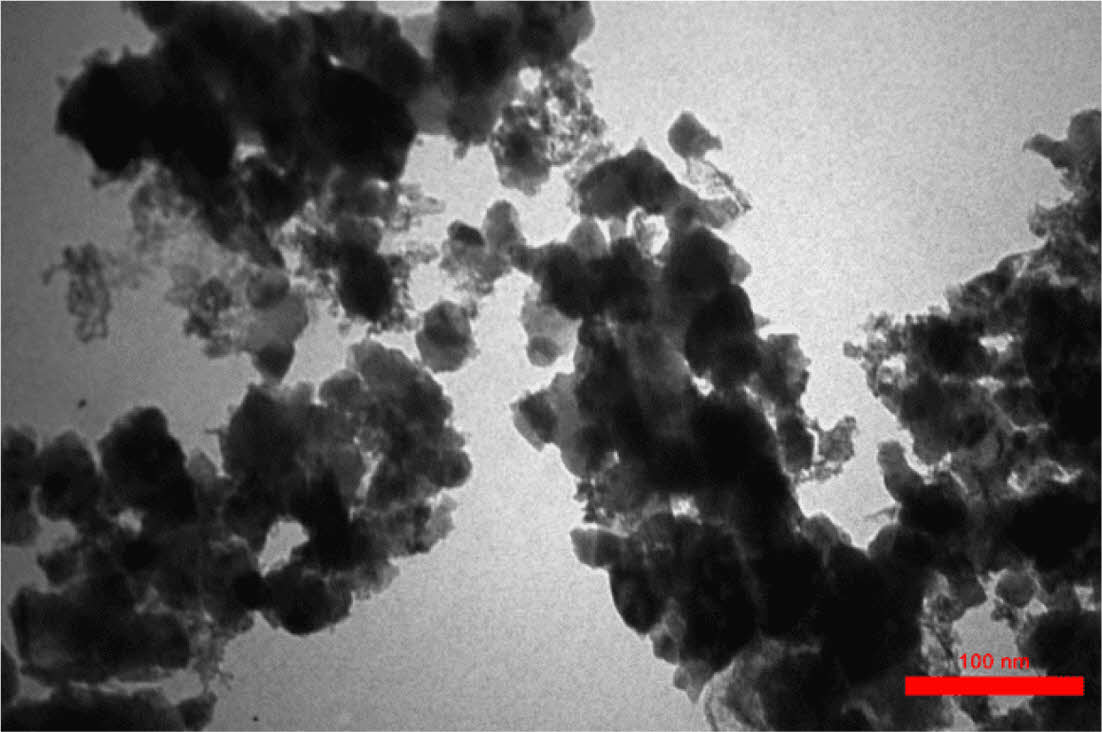
Determination of Grain Size in CeO2/Sm2O3 Nanoparticle. Scanning electron microscopy (SEM) is a cutting-edge technique utilized for the analysis of surface morphology and the elucidation of the nanocomposite structure by employing an electron beam on the sample’s surface. In Figure 2, the FESEM analysis of the nanomaterial surface is presented. The image vividly displays discrete clusters of nanoparticles, each exhibiting varying dimensions at the nanometer scale, with an average size of approximately 27 nm. Furthermore, the TEM analysis, as showcased in Figure 3, not only enhances the visualization of these nanostructures but also furnishes definitive confirmation of the presence of nanoparticles, revealing an average size of 23 nm. Figure 4
Photochemical Investigation of PVC and PVC-Nanocomposite Films via Infrared Spectroscopy
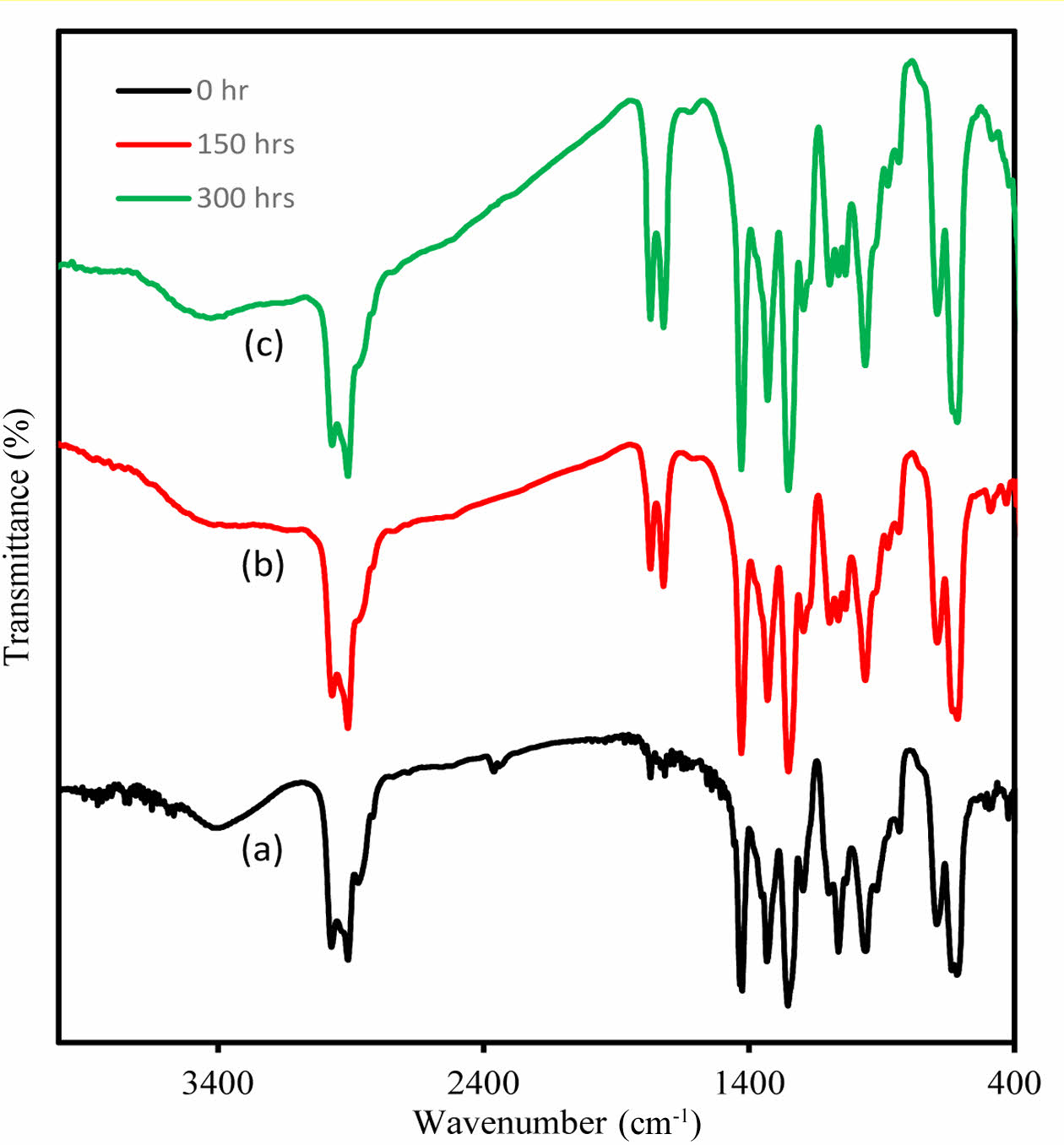
. Pure and composite PVC exhibit clear changes in its infrared spectrum when subjected to high-energy UV light at a wavelength of 365 nm (Figure 5). To understand these changes, films of additive-free PVC, with a thickness of 40 µm, were irradiated for varying durations: 0 h, 50 h, 100 h, 200 h, and 300 h. The emergence of specific bands in the range of 1720-1775 cm-1 is attributed to the synthesis of carbonyl groups associated with chloroketone and aliphatic ketone functionalities. Additionally, a distinct band related to the polyene group first becomes apparent at approximately 1622 cm-1, while a broad band appeared around 3400 cm-1, signifying the formation of hydroxyl groups upon prolonged irradiation. It is noteworthy that the absorption band associated with hydroxyl groups initially existed at a lower intensity prior to irradiation due to thermal oxidation during the PVC production process. These bands represent types of products generated during the decomposition process of PVC.8
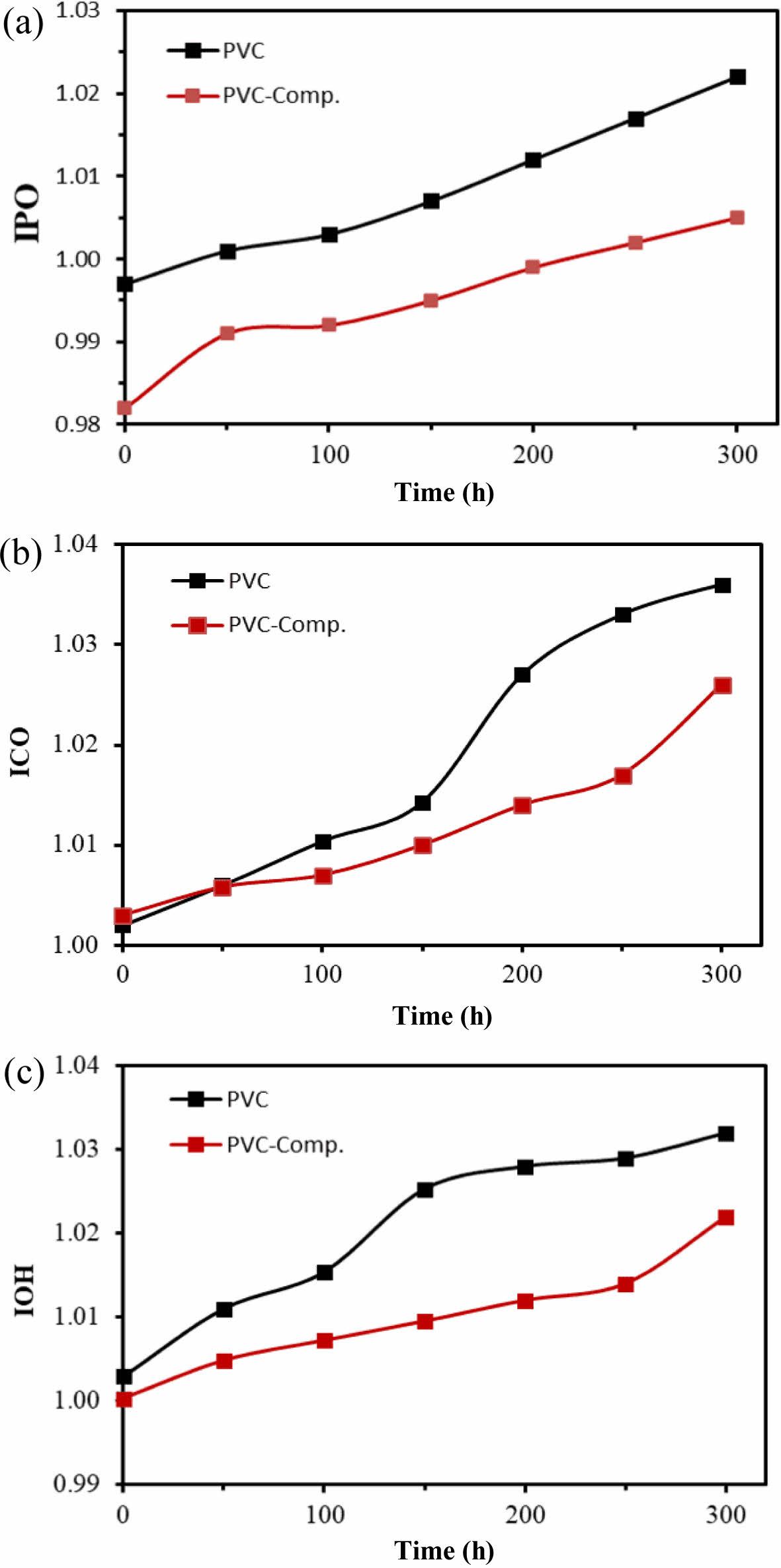
To assess the photostabilization effectiveness of nanocomposites, they were incorporated as optical stabilizers in PVC films, enabling a comparison with films lacking these additives. Infrared spectroscopy was employed to monitor the evolution of carbonyl groups (C=O), polyene groups (C=C), and hydroxyl groups (O-H) as a function of irradiation time during the photolysis of PVC films. This monitoring allowed the determination of coefficients for hydroxyl (IOH), carbonyl (ICO), and polyene (IPO).
The pure PVC and its composite films with a 40 micrometer thickness were observed to be decomposing increasingly as the irradiation progressed with time, resulting in an intensity increase for carbonyl (ICO), polyene (IPO), hydroxyl (IOH) indices. The following equation was used to obtain the mentioned indices.

where Ifun. stand for absorbance of functional groups for IOH, IPO, and ICO. Whereas Ar stands for the absorbance of the reference group, which is the CH2 peak at 1331 cm-1.
The results indicate the effect of the additives' presence in slowing down the increment rate of all peaks of the three cofficient indices in comparison with the absence of the additives in the PVC films. This observation signifies that the additives effectively reduced the decomposition of PVC films, thereby functioning as optical stabilizers. These findings align with prior research, highlighting the role of stabilizers in decreasing the rate of PVC fragmentation.29
Determination of Stabilizing Efficiency through Weight Loss. The effectiveness of stabilization was assessed by measuring the weight loss percentage during the photodegradation of PVC films, both in the absence and presence of additives. Weight loss was quantified using the following equation:30
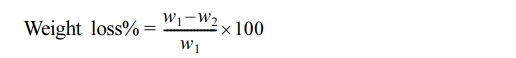
where w1 represents the weight of the original sample before irradiation, while w2 represents the weight of the sample after irradiation.
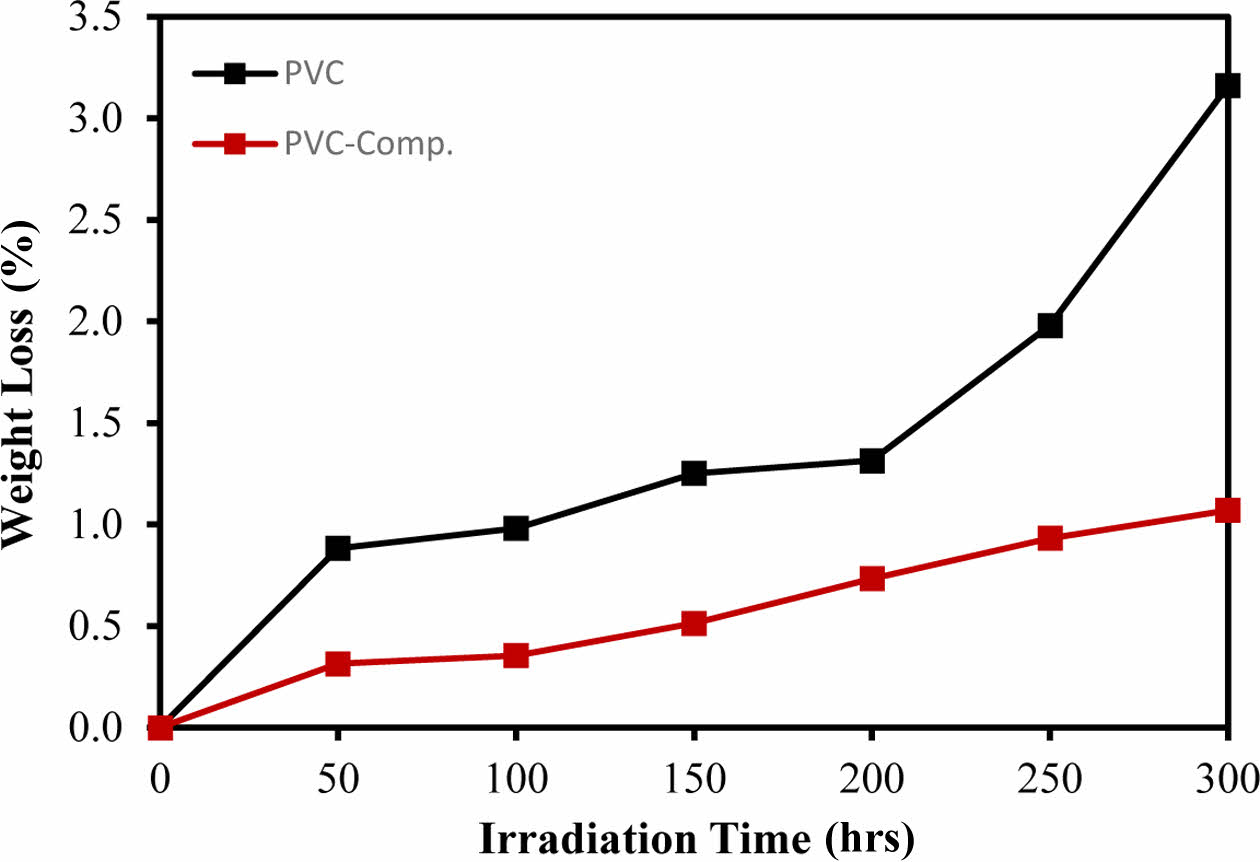
The photodegradation of PVC results in the loss of hydrogen chloride, leading to an increase in weight loss with prolonged irradiation. Therefore, the percentage of weight loss serves as an indicator of irradiation time and can effectively measure the extent of decomposition. This metric provides insights into the stabilizer’s ability to maintain the polymer’s stability and protect it from degradation.31 The pure PVC exhibited the highest weight loss after irradiation for 300 h, as illustrated in Figure 6. The PVC-CeO2/Sm2O3 thin film, however, showed a steady and lowest increase in the weight loss in comparison to the pure PVC.
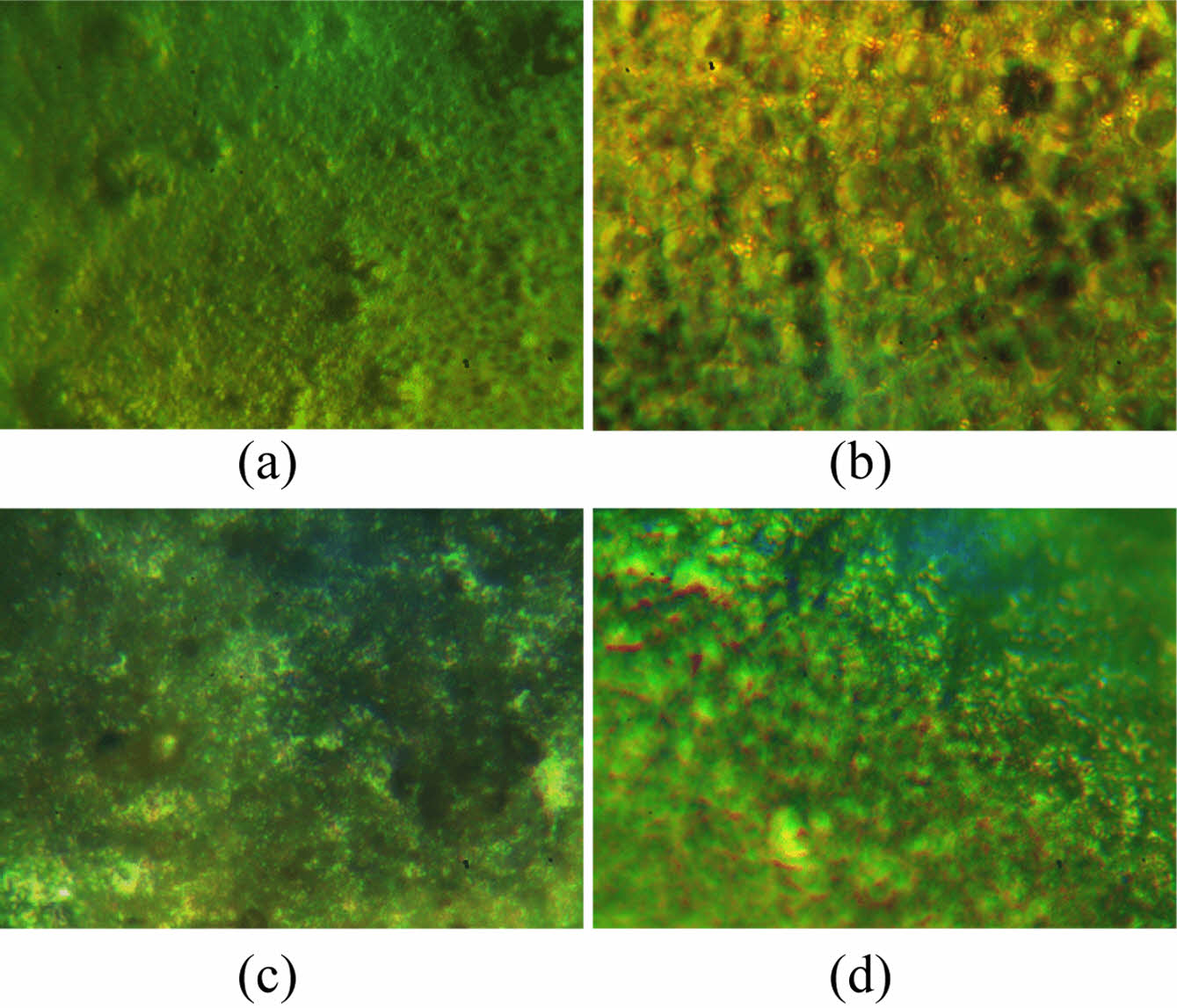
Microscopic Images of PVC and PVC-CeO2/Sm2O3 Thin Film. Microscopic examination of thin films composed of PVC-CeO2/Sm2O3 and pure PVC provided valuable insights into their surface characteristics. Figure 7(a, b) displays the surface morphology of the pure polymer and nanocomposite thin films before irradiation. In contrast, Figure 7(c, d) presents the microscopic images after irradiation. The micrographs reveal that the polymers infused with nanomaterials exhibited minimal changes, indicating the additives' effectiveness in preserving the polymeric films and functioning as optical stabilizers. In contrast, the pure polymer displayed multiple holes on its surface, signifying degradation.32
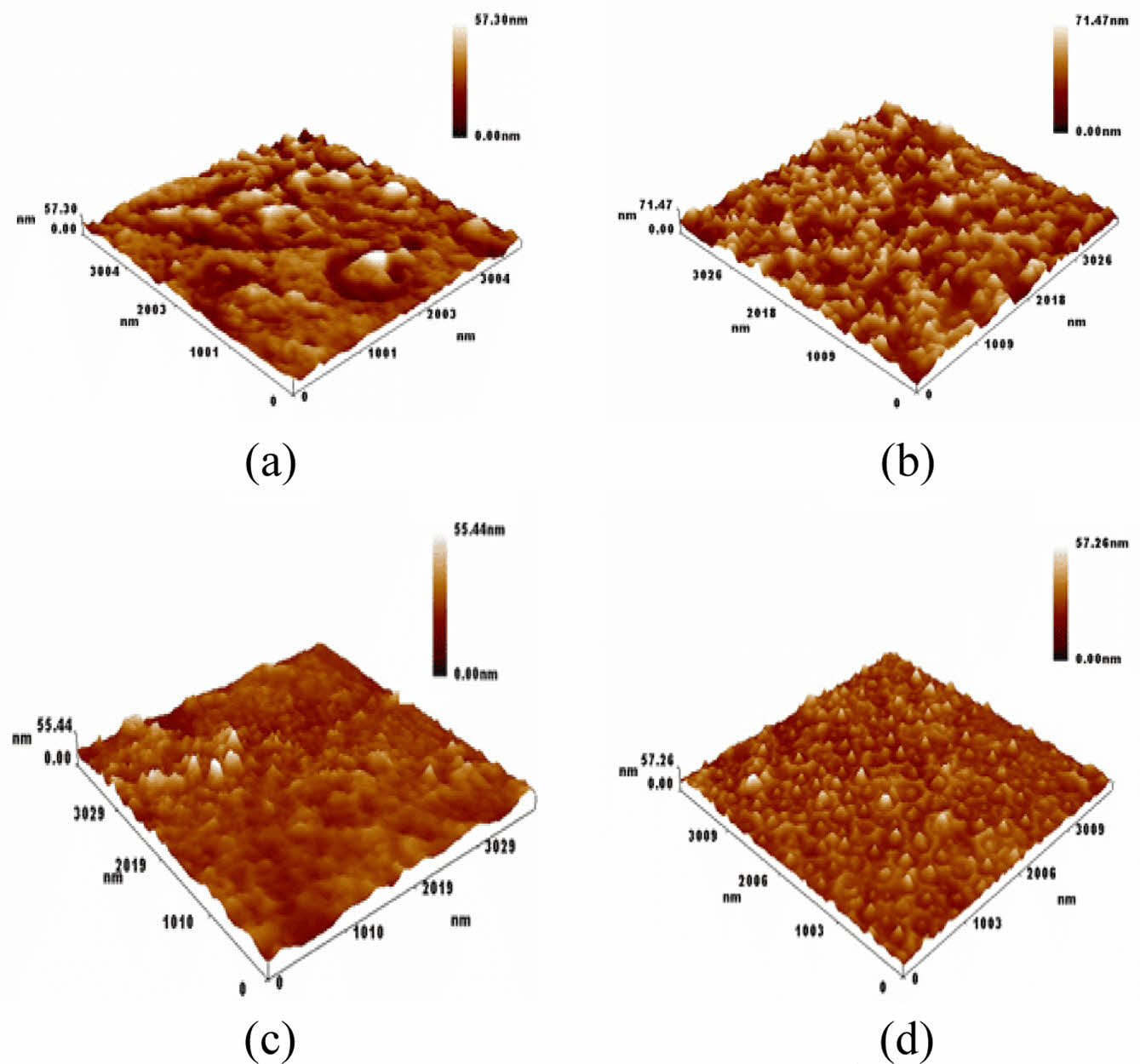
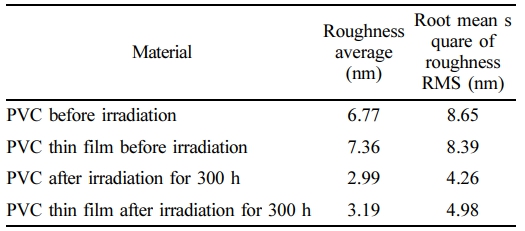
AFM Images for PVC and PVC-Nanocomposite Thin Films. Figure 8(a) illustrates the appearance of pure PVC prior to the irradiation process. The thin film of pure PVC features agglomerates of granules depicted as planar groups, with a measured roughness of 6.77 nm and an root mean square (RMS) value of 8.65 nm. Figure 8(b) presents the aggregated PVC with nano-granules, with white microspheres representing pores. This configuration yields a roughness of 7.36 nm and an RMS of 8.39 nm. Figure 8(c) and (d) portray the PVC and PVC-nanocomposite films after 300 h of radiation. In these images, the PVC matrix is shown to contain pores and grains gathered on the picture plane, with a roughness of 2.99 nm and an RMS of 4.26 nm. Figure 8(d) exhibits densely packed nanopore granules and pores that entirely cover the PVC matrix, resulting in a roughness of 3.19 nm and an RMS of 4.98 nm. These observations underscore the notable roughness of nanocomposite thin films, as detailed in Table 1.
|
Figure 1 XRD patterns of CeO2/Sm2O3 nanoparticle |
|
Figure 2 ESEM image of CeO2/Sm2O3 nanoparticle. |
|
Figure 3 TEM image of CeO2/Sm2O3 nanoparticle. |
|
Figure 4 The index coefficients of (a) polyene (IPO); (b) carbonyl (ICO); (c) hydroxyl index (IOH); (d) after irradiation for 50, 100, 150, 200, 250, and 300 h. |
|
Figure 5 nfrared spectra of (a) PVC-CeO2/Sm2O3 thin film before irradiation; (b) after 150 h; (c) and after 300 h of UV irradiation. |
|
Figure 6 Weight loss of prepared film after irradiation for 50, 100, 150, 200, 250, and 300 h. |
|
Figure 7 Microscopic images of (a) PVC; (b) and PVC-CeO2/Sm2O3 thin film; (c) before irradiation; (d) after irradiation for 300 h. |
|
Figure 8 Microscopic images of (a) PVC; (b) PVC-CeO2/Sm2O3 thin film; (c) before irradiation as well as PVC; (d) PVC-CeO2/Sm2O3 thin film after irradiation for 300 h. |
|
Table 1 The Average Roughness (nm) and RMS (nm) of PVC and PVC Thin Film Before and After Irradiation |
Incorporating nanomaterials, characterized by their high surface area and straightforward synthesis, emerges as a successful strategy for extending polymer longevity and shielding them from solar-induced degradation. Nanomaterials offer an appealing alternative to conventional microscopic additives. This study utilized a commercially available nanomaterial with a relatively large nanoscale size, demonstrating its efficacy in enhancing polymer stability. The results convincingly show that the prepared films remain resilient to environmental factors, such as light and heat, as evidenced by a reduced average weight loss percentage and reinforced by measurements of polyene (IPO), hydroxyl (IOH) and carbonyl (ICO) indices. The addition of nanomaterials proves ideal for long-term polymer stability, protecting against ultraviolet radiation, peroxide decomposition, and radical scavenger mechanisms. This research contributes to the growing understanding of nanomaterials' potential in polymer protection, with implications for various industries reliant on polymer-based materials, from packaging to electronics.
- 1. Wilkes, C. E.; Summers, J. W.; Daniels, C. A.; Berard, M. T. PVC handbook; Hanser: Munich, 2005.
- 2. Gupta, V. K.; Singh, A. K.; Mehtab, S.; Gupta, B. A Cobalt (II)-selective PVC Membrane Based on a Schiff Base Complex of N,N′-bis (salicylidene)-3,4-diaminotoluene. Anal. Chim. Acta 2006, 566, 5-10.
-

- 3. Ye, Q.; Ma, X.; Li, B.; Jin, Z.; Xu, Y.; Fang, C.; Zhou, X.; Ge, Y.; Ye, F. Development and Investigation of Lanthanum Sulfadiazine with Calcium Stearate and Epoxidised Soyabean Oil as Complex Thermal Stabilizers for Stabilizing Poly(vinyl chloride). Polymers 2019, 11, 531.
-

- 4. Jin, D.; Khanal, S.; Zhang, C.; Xu, S. Photodegradation of Polybenzimidazole/polyvinyl Chloride Composites and Polybenzimidazole: Density Functional Theory and Experimental Study. J. Appl. Polym. Sci. 2021, 138, 49693.
-

- 5. Sivalingam, G.; Karthik, R.; Madras, G. Effect of Metal Oxides on Thermal Degradation of Poly(vinyl acetate) and Poly(vinyl chloride) and Their Blends. Ind. Eng. Chem. Res. 2003, 42, 3647-3653.
-

- 6. Taha, T.; Hendawy, N.; El-Rabaie, S.; Esmat, A.; El-Mansy, M. Effect of NiO NPs Doping on the Structure and Optical Properties of PVC Polymer Films. Polym. Bull. 2019, 76, 4769-4784.
-

- 7. Al-Sarray, A. Advancements in Conjugated Polymer Research: Applications in Organic Photovoltaics and Field Effect Transistors. Curr. Chem. Lett. 2024, 13, 207-224.
-

- 8. Lu, T.; Solis-Ramos, E.; Yi, Y.; Kumosa, M. Particle Removal Mechanisms in Synergistic Aging of Polymers and Glass Reinforced Polymer Composites Under Combined UV and Water. Compos. Sci. Technol. 2017, 153, 273-281.
-

- 9. Ma, Y.; Liao, S.; Li, Q.; Guan, Q.; Jia, P.; Zhou, Y. Physical and Chemical Modifications of Poly(vinyl chloride) Materials to Prevent Plasticizer Migration-still on the Run. React. Funct. Polym. 2020, 147, 104458.
-

- 10. Kameda, T.; Ono, M.; Grause, G.; Mizoguchi, T.; Yoshioka, T. Ball Mill-assisted Dechlorination of Flexible and Rigid Poly(vinyl chloride) in NaOH/EG Solution. Ind. Eng. Chem. Res. 2008, 47, 8619-8624.
-

- 11. Braun, D. Poly(vinyl chloride) on the Way From the 19th Century to the 21st Century. J. Polym. Sci. Part A: Polym. Chem. 2004, 42, 578-586.
-

- 12. Keane, M. A. Catalytic Conversion of Waste Plastics: Focus on Waste PVC. J. Chem. Technol. Biotechnol. International Research in Process, Environ. Clean Technol. 2007, 82, 787-795.
-

- 13. Hajibeygi, M.; Maleki, M.; Shabanian, M.; Ducos, F.; Vahabi, H. New Polyvinyl Chloride (PVC) Nanocomposite Consisting of Aromatic Polyamide and Chitosan Modified ZnO Nanoparticles with Enhanced Thermal Stability, Low Heat Release Rate and Improved Mechanical Properties. Appl. Surf. Sci. 2018, 439, 1163-1179.
-

- 14. Cho, S.; Choi, W. Solid-phase Photocatalytic Degradation of PVC–TiO2 Polymer Composites. J. Photochem. Photobiol. A 2001, 143, 221-228.
-

- 15. Wang, M.; Wu, T.; Bu, Q.; Song, X.; Li, M.; Yuan, S. Rare Earth Ce Based Metal Organic Framework as Efficient Synergistic Thermal Stabilizer for PVC: Preparation and Thermal Stabilization Behavior. Thermochim. Acta 2022, 718, 179365.
-

- 16. Li, M.; Jiang, Z.; Liu, Z.; Hu, Y.; Wang, M.; Wang, H. Effect of Lanthanum Cyanurate as Novel Organic Thermal Stabilizers for Polyvinyl Chloride. Polym. Eng. Sci. 2013, 53, 1706-1711.
-

- 17. Xie, L.; Li, D.; Fu, M.; Zhang, J.; Zhang, L.; Zhang, Y.; Zhao, P. Study on Lanthanum-pentaerythritol Alkoxide as a Thermal Stabilizer for Rigid Polyvinyl Chloride. J. Vinyl Addit. Technol. 2017, 23, 55-61.
-

- 18. Jasim, B. E.; Al-Sarray, A. J. and Dadoosh, R. M. Enhanced Alizarin Removal from Aqueous Solutions Using Zinc Oxide/Nickel Oxide Nano-composite. Anal. Sci. Technol. 2024, 37, 39-46.
-

- 19. Liu, J.; Dai, M.; Wang, T.; Sun, P.; Liang, X.; Lu, G.; Shimanoe, K.; Yamazoe, N. Enhanced Gas Sensing Properties of SnO2 Hollow Spheres Decorated with CeO2 Nanoparticles Heterostructure Composite Materials. ACS Appl. Mater. Interfaces 2016, 8, 6669-6677.
-

- 20. Al-Sarray, A. J. A. Molecular and Electronic Properties of Schiff Bases Derived from Different Aniline Derivatives: Density Functional Theory Study. Eurasian Chem. Commun. 2023, 5, 317-326.
-

- 21. Sykora, R. E.; Deakin, L.; Mar, A.; Skanthakumar, S.; Soderholm, L.; Albrecht-Schmitt, T. E. Isolation of Intermediate-Valent Ce (III)/Ce(IV) Hydrolysis Products in the Preparation of Cerium Iodates: Electronic and Structural Aspects of Ce2(IO3)6 (OHx)(x»0 and 0.44). Chem. Mater. 2004, 16, 1343-1349.
-

- 22. Kalinina, M.; Dyuskina, D.; Fedorenko, N. Y.; Shilova, O. Effect of Liquid-Phase Synthesis Method of Nanopowders on Microstructure and Physicochemical Properties of Ceramics in CeO2–Sm2O3 System. Inorg. Mater. Appl. Res. 2022, 13, 501-507.
-

- 23. Dědková, K.; Kuzníková, Ľ.; Pavelek, L.; Matějová, K.; Kupková, J.; Barabaszová, K. Č.; Váňa, R.; Burda, J.; Vlček, J.; Cvejn, D. Daylight Induced Antibacterial Activity of Gadolinium Oxide, Samarium Oxide and Erbium Oxide Nanoparticles and Their Aquatic Toxicity. Mater. Chem. Phys. 2017, 197, 226-235.
-

- 24. Dhamodharan, K.; Singh, A. K. Highly Performed Electrochemical Activities of Hybrid Supercapacitors Based on CeO2-Sm2O3 Nanocomposites. Electrochim. Acta 2023, 466, 143008.
-

- 25. Saraci, F.; Quezada-Novoa, V.; Donnarumma, P. R.; Howarth, A. J. Rare-earth Metal–organic Frameworks: from Structure to Applications. Chem. Soc. Rev. 2020, 49, 7949-7977.
-

- 26. Al-Sarray, A. J.; H. Al-Mousawi, I. M.; H. Al-Noor, T. Acid Activation of Iraqi Bentonite Clay: Its Structural, Dielectric and Electrical Behavior at Various Temperatures. Chem. Methodol. 2022, 6, 331-338.
-

- 27. Chibac-Scutaru, A. L.; Podasca, V.; Dascalu, I. A. and Melinte, V. Exploring the Influence of Synthesis Parameters on the Optical Properties for Various CeO2 NPs. Nanomaterials 2022, 12, 1402.
-

- 28. Joseph, A.; Ayyappan, A.; Subair, T.; Pandibayal, M.; Nair, S.; Ramany, R.; Raama Varma, M. and Thomas, S. Pure and Sm Doped CeO2 Nanoparticles: An Insight Into the Room Temperature Ferromagnetism and Photocatalytic Dye Degradation. Chemistry Select 2023, 8, e202301020.
-

- 29. Al-Tikrity, E. T.; Yaseen, A. A.; Yousif, E.; Ahmed, D. S. and Al-Mashhadani, M. H. Impact on Poly(Vinyl chloride) of Trimethoprim Schiff Bases as Stabilizers. Polym. Polym. Compos. 2022, 30, 09673911221094020.
-

- 30. Jafari, A. J.; Donaldson, J. D. Determination of HCl and VOC Emission from Thermal Degradation of PVC in the Absence and Presence of Copper, Copper (II) Oxide and Copper (II) Chloride. E-J. Chem. 2009, 6, 685-692.
-

- 31. Pospıšil, J. and Nešpurek, S. Photostabilization of Coatings. Mechanisms and Performance. Prog. Polym. Sci. 2000, 25, 1261-1335.
-

- 32. El-Hiti, G. A.; Alotaibi, M. H.; Ahmed, A. A.; Hamad, B. A.; Ahmed, D. S.; Ahmed, A.; Hashim, H.; Yousif, E. The Morphology and Performance of Poly(vinyl chloride) Containing Melamine Schiff Bases Against Ultraviolet Light. Molecules 2019, 24, 803.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2024; 48(2): 188-194
Published online Mar 25, 2024
- 10.7317/pk.2024.48.2.188
- Received on Oct 20, 2023
- Revised on Jan 21, 2024
- Accepted on Jan 21, 2024
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Conflict of Interest
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Ali Jabbar A. Al-Sarray
-
Polymer Research Unit, College of Science, Mustansiriyah University, 10052 Iraq
- E-mail: ali_alsarray@uomustansiriyah.edu.iq
- ORCID:
0000-0001-6484-1763









 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.