- Photocatalytic and Filtration Elimination of Methylene Blue by Nanofibrous Polystyrene Membrane Containing TiO2 Nanotubes
Department of Nanotechnology, Faculty of Chemistry, Urmia University, Urmia, 5756151818, Iran
- TiO2 나노튜브를 함유한 나노섬유 Polystyrene 막을 이용한 Methylene Blue의 광촉매 및 여과 제거 연구
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
In this research, the nanofibrous polystyrene /titanium dioxide nanotube (PS/TiO2 nanotubes) membrane was prepared by the electrospinning method. Titanium dioxide nanotubes were synthesized by the anodizing method and stabilized on the fibrous polystyrene membrane. Then, the effect of different weight percentages of titanium dioxide nanotubes on the performance of the resulting membranes was investigated. The nanoparticles and nano-composites prepared in this research were analyzed using several analyses, including Fourier transform infrared spectroscopy (FTIR) and field emission scanning electron microscopy (FE-SEM), along with X-ray energy diffraction spectroscopy (EDS). The thermal behavior and crystal structure of prepared nano-composite membranes were investigated using (TGA) analysis and X-ray diffraction (XRD), respectively. Afterward, to investigate the prepared structures, analyses such as porosity measurement, water contact angle, swelling, and water flux of the membranes were performed. The photocatalytic property of the prepared membranes in the decomposition of methylene blue was investigated by two methods (static and dynamic). The obtained results demonstrated that the dye removal was accomplished in the best way in the static method (using the membrane as an absorbent) for 48 h. The prepared nano-composite membranes were also used in the dye filtration removal process, and the best result was obtained by using the membrane containing titanium dioxide nanotubes with a concentration, of 0.05 gr/V(50). The swelling degree in the fibrous polystyrene membrane was 135%, while in the membrane containing 0.1% titanium dioxide nanotubes, which is the optimal membrane, was 66%. Hydrophilicity measurements by water contact angle revealed that the presence of TiO2 nanotubes improved by about 10%.
TiO2 nanotubes were synthesized by anodizing method and fixed on the fibrous membrane made by electrospinning technique. The fibrous membrane containing these nanostructures was used for the photocatalytic removal of methylene blue. The results showed that the methylene blue dye was removed from the feed solution through the membrane screening mechanism and photocatalytic degradation activity of nanostructures.

Keywords: nano-composite membrane, electrospinning, titanium dioxide nanotubes, water treatment, photocatalysis.
The authors express their appreciation and thanks to those who were helping during this investigation.
The authors declare that there is no conflict of interest.
More detailed information about swelling, water contact angle and pure water flux tests is given in the Supporting Information file. The materials are available via the Internet at http://journal.polymer-korea.or.kr.
PK_2024_048_01_1_Supporting_Information_template.pdf (320 kb)
Supplementary Information
Fresh water is one of the basic human needs, which is used for drinking and agriculture purposes. Despite the fact that more than two-thirds of the earth’s surface is covered with water, only 1% of these water resources can be used. On the other hand, human activities cause pollution and limit usable water resources. Therefore, the development of new methods for the recovery and treatment of water resources has become a necessity. Water purification includes methods to remove pollutants, and suspended soluble particles in water. Filtration, ion exchange, Electro-Deionization, and reverse osmosis are used in industrial water purification.1
Membrane-based technologies have gained popularity in recent years due to their high separation efficiency, relatively low costs, and ease of operation. The membrane is a semipermeable layer that acts like a barrier; In other words, it only allows certain materials to permeate and removes waste compounds. Membranes made of nanofibers are considered as one of the suitable options in the wastewater treatment process due to their unique properties, such as high surface-to-volume ratio, suitable porosity, flexibility, and chemical activity in nano dimensions.2 Nanofibers are very narrow strands that have a long length compared to their diameter and used in different applications. The electrospinning method is the most common method to produce nanofibers from a wide range of polymeric materials with different diameters.3 In this method, high voltage is used to generate charged current in polymer solution or melt.4 Then the solution is subjected to a high voltage electric field, and with the stretching of the polymer solution due to the electric field, the solvent is evaporated, and filaments with a diameter of less than a micron are produced on the collector. So far, more than 100 types of polymers have been successfully utilized to fabricate fibers in the electrospinning process, including polyacrylonitrile, polyethylene oxide, polyethylene terephthalate, polystyrene, polycarbonate, polysulfone, polyvinyl phenol, cellulose, etc.5
Titanium dioxide is one of the best available semiconductor photocatalysts due to its optical activity, ability to emit light, mechanical strength, and low cost. Titanium dioxide is found as a mineral in magmatic rocks of hydrothermal vents and also in perovskite walls.6
TiO2 nanotubes have gained great importance due to their unique properties, such as ion exchange capability, photocatalytic potential, large surface area, and significant electrical properties. However, its specific capacity is low due to the presence of brittle phases, and due to its wide energy gap, it has poor conductivity.7 One of the methods for the synthesis of TiO2 nanotube is the anodic oxidation method. Thermal and electrochemical methods have been used to modify their structure.8 The production of self-organized TiO2 nanotubes by anodizing titanium in fluoride-containing solutions is a relatively new method that has great potential in producing TiO2 nanotube bundles with high order and controlled size. The effect of titanium dioxide nanoparticles on the performance of nano-composite membranes has been investigated before.9
Some of the studies conducted on membranes containing titanium oxide nanoparticles are given below. Mansourpanah et al.10 explained that a sharp decrease in pure water flux in the PES/PI-modified membrane contains titanium dioxide nanoparticles occurred due to the formation of a dense layer on the membrane surface. Kim et al.11 reported that the composite membrane of the thin polymeric film coated with nanoparticles of titanium dioxide system has good flux stability in reverse osmosis. Yu et al.12 found that the high performance of TiO2-coated polymer membrane has an effect on flux recovery after filtration of 1% bovine serum albumin (BSA) under light irradiation. Hyun Ku et al.13 succeeded in making a photoanode composite of titanium dioxide nanoparticles/nanorods in order to improve the power of solar cell converting. Hang Yan Chen et al.14, created an effective pigment for solar cells. Hong et al.15 investigated the effect of rutile phase titanium dioxide nanorods on the photoelectrode of solar cells.
Despite studies on the performance of titanium dioxide nanoparticles as an additive in membranes, titanium dioxide nanotubes have not been used in the membranes. In addition, nano-composite membranes prepared by electrospinning method are very limited. In the current study, polystyrene was used in the electrospinning process to prepare fibrous membranes, and the synthesized TiO2 nanotubes were stabilized on the fibrous filter. The synthesized nanotubes, and the membranes were characterized and their performance and photocatalytic activity were studied in the degradation of methylene blue dye.
Materials. Polystyrene (Density1.04 (g/cm3), MW =104.15 g/ol) was purchased from Sigma-Aldrich (USA), dimethyl formamide (DMF), ethylene glycol, ammonium fluoride, hydrofluoric acid, and nitric acid were provided form Merck (USA). Pure titanium and platinum metal sheets (China) were used.
Method. Electrolyte Solution Required for Anodizing:
In order to perform anodizing, an electrolytic solution should be made that shows good conductivity at the desired voltage. For this purpose, 0.5 grams of ammonium fluoride powder and 2.5 mL of distilled water were poured into a 100 mL volumetric flask and made up to volume with ethylene glycol.
Treatment of Ti and Pt Metals: In the first step, the metals were cut in dimensions of 5 × 10 cm, and the surface of the metal was first sanded well with 100P to remove the contaminations, and then it was sanded again with soft sandpaper number 420P to make it smooth and bright. Then, the metal was treated with distilled water. Finally, to completely clean the Ti metal and Pt sheet from other impurities, these metals was placed in Sonesh’s solution, and after soaking for a few minutes, the metals were taken out and rinsed with distilled water.
Synthesis of TiO2 Nanotubes by the Anodizing Method: To synthesize TiO2 nanotubes by anodizing method, the desired electrolyte solution must be prepared first. Ti and Pt sheets were used as anode and cathode electrodes, respectively. After preparing the metals and the desired electrolyte solution, the circuit is closed using a power source as follows: Ti metal as the working electrode is connected to the positive pole and Pt metal is connected to the negative pole as an auxiliary electrode, and the potential difference between the two ends of the circuit is brought to 40 volts. Simultaneously with the application of voltage, the electrolyte solution was placed on a magnetic stirrer at room temperature to avoid heterogeneous accumulation of nanotubes during the anodizing process. The process of growth and synthesis of nanotubes continued during the application of voltage for three h. After completing this stage, the circuit was cut off, and the metal was rinsed with distilled water. After performing this step, the anodized metal was placed at room temperature for one day and night to dry. Then, the nanotubes on the metal surface were scratched and separated. This process was repeated until a sufficient amount of TiO2 nanotubes were obtained.
Nanofiber Production Process: Stabilization of TiO2 nanotubes on the fibrous membrane:
Mixtures with different percentages of TiO2 nanotubes were prepared and passed through the membrane using a Buchner funnel. The formulation of the prepared membranes is shown in Table 1.
Characterization: FE-SEM, FTIR, EDX, XRD, and TGA were used to identify the prepared structures. FE-SEM instrument was the Mira3 Fe -Gsem brand made by Czech Republic of the Czech Republic. EDX analysis was used for elemental analysis using a Bruker instrument made in Germany, and it was done with the help of detection, which is installed on SEM-FE. FTIR analysis was done with the NEXUS-670 American Nicolet device for monitoring the sample. This spectroscopy method is based on the absorption of infrared waves by the sample and is a very useful method for examining functional groups. The XRD analysis (Panalytical device from the Netherlands) was used to study the structure of the crystalline structure of materials. In addition, in this article, TGA analysis was used to check the structure and thermal stability using the TA company model Q600 made in the United States. The heating rate was 20 ℃/min, and the scanned temperature range was from ambient temperature to 600 ℃.
Water Contact Angle Test: To measure the water contact angle, several drops of water were placed on the prepared membranes using a micro syringe, and the images of the drops were recorded on the surface using a precise digital camera. These images were surveyed to measure the water contact angle with Image J software.
Swelling Test: In this test, membrane samples are cut in certain dimensions (1×1 cm), and after drying in a vacuum oven, they were accurately weighed. The samples were immersed in distilled water for 24 h and weights again after dewatering. eq. (1) was used to obtain the swelling percentage. In this equation, Wd and Ws were the weight of the membranes before and after immersion in water, respectively.

Porosity Measurement: Porosity measurement in this research is defined as the volume of holes divided by the total volume of the membrane and can be calculated with the help of eq. (2).
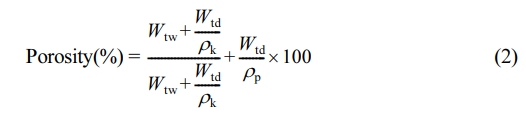
In this equation, the Wtw is the weight of the wet membrane, and the Wtd is the weight of the dry membrane. In the meantime, ρk is the density of water, and ρp is the polymer density.
Photocatalytic Properties of Nano-composite Membranes: In this study, two methods were used to evaluate the removal efficiency of methylene blue from water. The first method is done statically and according to the photocatalytic property of the membrane containing TiO2 nanotubes. Therefore, a methylene blue solution (10 ppm) was prepared and maintained in a container for 10 h. The prepared membranes were cut into certain sizes and immersed in a certain volume in the beaker containing the dye solution. Then, the containers were exposed to an ultraviolet light source. After performing this test, at 1, 2, 4, 24, and 48 h, the amount of color degradation and its removal from the solution was measurable by spectroscopic method.
In the second method, the membranes were cut according to the size of the sample site in the nanofiltration cell, and after loading in the cell, the filtration test of 10 ppm methylene blue dye solution was performed under ultraviolet light. The feed and permeate solution were investigated in order to measure the dye concentration, and the amount of dye removal was determined.
Identification of Nano-composite Membrane. In this section, the tests were carried out to determine and approve the chemical structure and morphology of nanotubes and membranes containing these structures. These analyses include FTIR, XRD, FE-SEM, EDX, and TGA.
Infrared Spectroscopy: Figure 1 shows the FT-IR spectra of TiO2 nanotubes, polystyrene, and the final fibrous nano-composite membrane. According to the results in the references,16 the characteristic peaks of TiO2 nanotubes appeared at 1090 and 1220 cm-1, which are related to the asymmetric vibrations of the Ti-O bond and the bending vibrations of chemically absorbed water, respectively. In the spectrum related to polystyrene, the peak at 3035 cm-1 indicated the stretching vibrations of the aromatic C-H bond, and the peaks at 1600, 1492, and 1452 cm-1 were related to the stretching vibrations of the C=C double bond. The appearance of the characteristic peaks of TiO2 nanotubes and polystyrene in the nano-composite membrane sample proved the successful stabilization of nanotubes on the fiber surface.
XRD Analysis: XRD analysis was used to investigate the crystalline structure of TiO2 nanostructures and nano-composite fibrous membranes. The obtained patterns are shown in Figure 2. As can be seen, the characteristic peaks of TiO2 nanotubes have appeared at 2θ = 27, 39, 49, 55, and 63° and are consistent with the results obtained in the reference.17 Polystyrene is an amorphous polymer, and the broad peak appearing in 2θ = 17° proved the disorder of the structure of polystyrene. In the pattern observed in the nano-composite membrane containing TiO2 nanotubes, the characteristic peaks of the nanostructures were clear and proved the successful stabilization of the nanoparticles in the fibrous substrate.
FESEM Analysis: FESEM was used to study the morphology of TiO2 nanotubes and polymeric fibrous membranes. The recorded images are shown in Figure 3. As it is known, the produced TiO2 had a tube-like structure and was hollow. The diameter of the nanotubes was about 300 nm, and they had acceptable uniformity. FESEM images of pure polystyrene and nano-composite fibrous membranes were shown with two different magnifications. The diameter of the fibers in all samples was almost constant and about 1 µm. The surface of the fibers in the sample of pure polystyrene membrane was completely smooth and free of any additional material, while in the samples of nano-composite membranes containing TiO2 nanotubes, fine objects were observed on the surface of the fibers, which were attributed to the added nanofibers. With the increase of additive percentage, the amount of these objects also increased in image D.
Elemental EDAX Analysis: To further emphasize the successful stabilization of TiO2 nanotubes on the surface of fibrous membrane, EDAX elemental analysis was carried out on the pure polystyrene and nano-composite fibrous membrane samples, and the results are shown in the form of a graph and a table of element percentages in Figure 4. Only carbon element was detected in the polystyrene fibrous membrane sample, while titanium and oxygen elements were also seen in the sample containing TiO2 nanotubes, which proved the existence of nanostructures in the fibrous membrane.
TGA Analysis: TGA was used to study the thermal stability of the prepared membranes and find out the presence and effect of TiO2 nanostructures on the thermal stability of the samples, and the resulting thermograms are shown in Figure 5. The membrane made of polystyrene fibers showed a single-stage degradation at a temperature of 330 ℃, and the remaining ash at the end of the degradation is almost zero, while in the fibrous membrane containing TiO2 nanotubes, the degradation starts at a lower temperature (250 ℃) and at the end of the degradation, nearly 10% of ash remained. The faster degradation of the sample can be due to the catalytic role of TiO2 nanotubes, and the residual ash is related to the amount of used additive.18
Swelling Test: In order to investigate the porosity and hydrophilicity of the membrane, a swelling test was designed and implemented. In this test, the amount of water absorbed by the membrane was determined at a certain time, and this parameter was related to the structural properties of the membrane. The results of the analysis are given in Figure 6. The highest amount of absorbed water is related to the sample of pure polystyrene membrane, which seems unusual considering the hydrophobic nature of this polymer. In this sample, the absorbed water has increased due to the porous structure of the fibrous membrane. In nano-composite samples, due to the stabilization of TiO2 nanotubes on the fibers, the porosity decreased, and as a result, the amount of absorbed water decreased. In the nano-composite samples, with the increase in the percentage of stabilized nanotubes, the absorbed water continued to decrease, and in sample 4, it reached the lowest value. Swelling data were presented in Table S1.
Porosity Measurement: The porosity of the fibrous membranes was also measured based on the swelling degree, and as shown in Figure 7, the porosity of the nano-composite membranes was lower than that of the pure polystyrene membrane, and the porosity decreased with the increase of TiO2 nanotubes. This decrease in porosity, along with the high specific surface area in fibers, can help to increase the filtration efficiency of membranes.
Water Contact Angle Measurement: The interaction of a membrane surface with water molecules is a very important parameter that determines the surface hydrophilicity. The water contact angle reflects the degree of this interaction, and the small contact angle is a measure of the hydrophilicity of the surface. The measured angles for the fibrous membranes are shown in Figure 8. The images were provided in Figure S1. As can be seen, the polystyrene membrane had a higher water contact angle, and, in the nano-composite membranes, the contact angle gradually decreased with the increase of nanotubes. This decrease in contact angle showed the increase in hydrophilicity of the nano-composite membrane surface.
Measurement of Pure Water Flux in the Membranes: The pure water flux test was used to investigate the filtration behavior of fibrous membranes. This test was performed for the membranes in the condition without applying pressure, and 0.5 bar pressure, and the results are shown in Figure 9. In measuring the water flux without applying pressure, membranes M1 and M2 showed a flux of 3500 and 500 kg/m2/h, while no flux was observed in the other membranes. These membranes were subjected to pressure (0.5 bar) for 1 minute, and after wetting with water, they were evaluated again in the condition without applying pressure. In this condition, all the membranes had pure water flux. The results of this test are shown as pale blue columns in Figure 9(a). It was observed that the flux of membrane M1 decreased, while the other membranes had an increase in flux. The decrease in the flux of M1 was due to the compaction of the membrane structure, while the increase in the flux of the other membranes was due to the initial wetting of the membranes under pressure. On the other hand, the flux of the membranes has decreased with the increase in the percentage of nanotubes, which can be justified due to the decrease in the porosity of the membranes. In the measurement; of the flux at 0.5 bar pressure, the results are depicted in Figure 9(b); the reduction of the flux with the increase in the percentage of TiO2 nanotubes was clearly seen. The related data were provided in Table S2.
Nano Composite Membrane Performance Evaluation: In the first method (static method), the membrane samples were used as adsorbents under the UV radiation source inside the methylene blue solution, and the amount of removed dye was measured in 48 h. The removal results are shown in Figure 10(a). The dye removal in this test can be due to the absorption ability of membranes and nanotubes as well as the photocatalytic activity of these nanostructures. Considering that the surface of the used membranes was constant in all the samples, the observed changes were attributed to the photocatalytic behavior. As can be seen, the amount of dye removal in all nano-composite samples was higher than the polystyrene membrane without nanotubes, and it reached its maximum value in sample M4, which contained the highest number of nanotubes. On the other hand, with the passage of time, the removal efficiency has improved, and considering the constant absorption capacity of the samples, the increase in efficiency was related to the dye degradation process. In another test, the filtration process of methylene blue solution was performed under UV radiation using the manufactured membranes, and the results were reported as the percentage of dye removal in Figure 10(b). M3 and M4 membranes, which had the highest number of nanotubes, had the highest efficiency in dye removal. Considering the lower porosity of these membranes as well as the photocatalytic activity of nanotubes, the observed results could be justified. On the other hand, the results reported in this test were related to the first moment of the solution passing through the membrane, and by comparing these values with the efficiency observed in the static test, the performance improvement was visible.

|
Figure 1 FTIR spectra of TiO2 nanotubes, polystyrene and nanocomposite fibrous membranes. |

|
Figure 2 XRD patterns of polystyrene and nanocomposite fibrous membranes and TiO2 nanotubes. |

|
Figure 3 FESEM images of TiO2 nanotubes (A) polystyrene fibrous membrane (M1), nano-composite fibrous membrane with 0.02 wt% TiO2 nanotubes (M2), and nano-composite fibrous membrane with 0.1 wt% TiO2 nanotubes (M4) with two different magnifications. |

|
Figure 4 DAX elemental analysis of (a) polystyrene fibrous membrane (M1); (b) nano-composite fibrous membrane (M4). |

|
Figure 5 Thermal gravimetric analysis of polystyrene fibrous membrane (M1); and nano-composite fibrous membrane (M4). |

|
Figure 6 Swelling degree of fibrous membranes. |

|
Figure 7 Porosity of fibrous membranes. |

|
Figure 8 Water contact angle of fibrous membranes. |

|
Figure 9 (a) PWF of fibrous membrane without applying pressure; (b) PWF of fibrous membranes under 0.5 bar pressure. |

|
Figure 10 (a) Dye removal (degradation) ratio by photocatalytic activity of membranes; (b) dye removal ratio of membranes in filtration and photocatalytic degradation process. |
In this study, a new nano-composite membrane was prepared to remove a soluble dye like methylene blue. The membranes were fabricated by the electrospinning method. Nano-composite fibrous membranes were evaluated with different techniques. The results showed that the TiO2 nanotubes were properly fixed on the fibers. This immobilization has increased the hydrophilic nature of the membrane and improved the separation property by blocking the pores. In addition to improving the efficiency of the membrane in methylene blue dye filtration, the photocatalytic activity of the nano-composite membrane containing nanotubes was proved.
- 1. World Health Organization. Progress on Drinking Water and Sanitation, 2014. https://data.unicef.org/resources/progress-on-drinking-water-and-sanitation-2014-report. (accessed May 1, 2014)
-

- 2. Xue, Q.; Pan, X.; Li, X.; Zhang, J.; Guo, Q. Effective Enhancement of Gas Separation Performance in Mixed Matrix Membranes Using Core/shell Structured Multi-walled Carbon Nanotube/graphene Oxide Nanoribbons. Nanotechnology 2017, 28, 065702.
-

- 3. Huang, Z. M.; Zhang, Y. Z.; Kotaki, M. A Review on Polymer Nanofibers by Electrospinning and Their Applications in Nano-composites. Compos. Sci. Technol. 2003, 63, 2223-2253.
-

- 4. Buchko, C. J.; Chen, L. C.; Shen, Y.; Martin, B. C. Processing and Microstructural Characterization of Porous Biocompatible Protein Polymer Thin Films. Polymer 1999, 40, 7397-7407.
-

- 5. Zahmatkeshan, M.; Adel, M.; Bahrami, S.; Esmaeili, F. Polymerbased Nanofibers: Preparation, Fabrication, and Applications. In Handbook of Nanofibers; Springer: New York, 2019; pp 215-261.
-

- 6. Van Driel, B.; Kooyman, P. J.; Van den Berg, K. J. A Quick Assessment of the Photocatalytic Activity of TiO2 Pigments-From Lab to Conservation Studio! Microchem. J. 2016, 126, 162-171.
-

- 7. Su, Y.; Zhang, X.; Han, S.; Lei, L. Preparation of Highly Efficient Photoelectrode of N–F-codoped TiO2 Nanotubes. J. Photochem. Photobiol. A, 2008, 194, 152-160.
-

- 8. Tahmasebpoor, R.; Babalou, A. A.; Shahrouzi, J. R. Theoretical and Experimental Studies on the Anodic Oxidation Process for the Synthesis of Self-ordering TiO2 Nanotubes: Effect of TiO2 Nanotube Lengths on Photocatalytic Activity. J. Environ. Chem. Eng. 2017, 5, 1227-1237.
-

- 9. Lee, K.; Hahn, R.; Altomare, M.; Selli, E. Intrinsic Au Decoration of Growing TiO2 Nanotubes and Formation of a High-Efficiency Photocatalyst for H2 Production. Adv. Mater. 2013, 25, 6133-6137.
-

- 10. Khan, S. U. M.; Al-Shahry, W. B.; Ingler Jr. Efficient Photochemical Water Splitting by a Chemically Modified n-TiO2. Science 2002, 297, 2243-2245.
-

- 11. Kwak, S. Y.; Kim, S. H.; Kim, S. S. Hybrid Organic/inorganic Reverse Osmosis (RO) Membrane for Bactericidal Anti-fouling. 1. Preparation and Characterization of TiO2 Nanoparticle Self-assembled Aromatic Polyamide Thin-film-composite (TFC) Membrane. Environ. Sci. Technol. 2001, 35, 2388-2394.
-

- 12. Yu, S. J.; Sembelante, G. U.; Lu, S. C.; Damodar, R. A. Evaluation of the Antifouling and Photocatalytic Properties of Poly(vinylidene fluoride) Plasma-grafted Poly(acrylic acid) Membrane with Self-assembled TiO2. J. Hazardous Mater. 2012, 237, 10-19.
-

- 13. Javed, H. M. A.; Que, W.; Ahmad, M. R.; Ali, K.; IrfanAhmad, M.; Haq, A. U.; Shaema, S. K. Perspective of Nanomaterials in the Performance of Solar Cells. In Solar Cells; Springer: New York, 2020; pp 25-54.
-

- 14. Chen, H. Y.; Zhang, T. L.; Fan, J.; Kuang, D. B.; Su, C. Y. Electrospun Hierarchical TiO2 Nanorods with High Porosity for Efficient Dye-sensitized Solar Cells. ACS Appl. Mater. Interfaces 2013, 5, 9205-9211.
-

- 15. Dhandole, L. K.; Mahadik, M. A.; Kim, S. G.; Chung, H. S.; Seo, Y. S.; Cho, M.; Ryu, J. H.; Jang, J. S. Boosting Photocatalytic Performance of Inactive Rutile TiO2 Nanorods Under Solar Light Irradiation: Synergistic Effect of Acid Treatment and Metal Oxide Co-catalysts. ACS Appl. Mater. Interfaces 2017, 9, 23602-23613.
-

- 16. Praveen, P.; Viruthagiri, G.; Mugundan, S.; Shanmugam, N. Structural, Optical and Morphological Analyses of Pristine Titanium Di-Oxide Nanoparticles-synthesized via Sol-gel Route. Spectrochim. Acta, Part A, 2014, 117, 622-629.
-

- 17. Sagadevan, S. Synthesis and Electrical Properties of TiO2 Nanoparticles Using a Wet Chemical Technique‖ American J. Nanosci. Nanotechnol. 2013, 1, 27-30.
-

- 18. Madani, M.; Sharifi-Sanjani, N.; Hasan-Kaviar, A.; Choghazardi, M.; Faridi-Majidi, R.; Hamouda, A. S. PS/TiO2 (polystyrene/titanium dioxide) Composite Nanofibers with Higher Surface-to-volume Ratio Prepared by Electrospinning: Morphology and Thermal Properties. Polym. Eng. Sci. 2013, 53, 2407-2412.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2024; 48(1): 1-9
Published online Jan 25, 2024
- 10.7317/pk.2024.48.1.1
- Received on Jun 12, 2023
- Revised on Oct 3, 2023
- Accepted on Oct 16, 2023
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Conflict of Interest
- Supporting Information
Introduction
Experimental
Results and Discussion
Conclusion
- References
Shared
 Correspondence to
Correspondence to
- Mehdi Mahmoudian
-
Department of Nanotechnology, Faculty of Chemistry, Urmia University, Urmia, 5756151818, Iran
- E-mail: m.mahmoudian@urmia.ac.ir
- ORCID:
0000-0002-9949-8579











 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.