- Study on the Application of Canola Oil and Cottonseed Oil in Solution Polymerized Styrene-butadiene Rubber
Yuan Jing†
 , Chunwei Zhang, Su Zhang*
, Chunwei Zhang, Su Zhang*  , Guangyi Lin*,† , Huidong Cao, Jiahui Wen, Zonglong Wang, Yuanyuan Niu**, and Zetao Lin**
, Guangyi Lin*,† , Huidong Cao, Jiahui Wen, Zonglong Wang, Yuanyuan Niu**, and Zetao Lin** College of Civil Engineering, Qingdao University of Technology, Qingdao, 266033, P.R. China
*College of Electromechanical Engineering, Qingdao University of Science and Technology, Qingdao 266061, P.R. China
**Qingdao University of Science and Technology Guangrao Rubber Industry Research Institute, Dongying, 257029, P.R. China- 중합된 스티렌 부타디엔 고무 용액에서 카놀라유와 면실유 응용에 관한 연구
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
The application of vegetable oil-based softeners canola oil and cottonseed oil in Solution polymerized styrene-butadiene rubber (SSBR) system composites was investigated. The results showed that when the aromatic oil was replaced by 5 parts of vegetable oil-based softeners in equal amounts in the base formulation of SSBR system, the working performance of the rubber was better, the tear strength and elongation at break were improved and the tensile strength was reduced; the vulcanization time of the rubber prepared with vegetable oil-based softeners was significantly reduced compared with that of aromatic oil; the glass transition temperature of the rubber was significantly reduced and the cold resistance and snow performance of the rubber were improved.
The application of vegetable oil-based softeners canola oil and cottonseed oil in soluble styrene butadiene rubber system composites was investigated. The results showed that when the aromatic oil was replaced by 5 parts of vegetable oil-based softeners in equal amounts in the base formulation of solution polymerized styrene-butadiene rubber (SSBR) system, the working performance of the rubber was better, the tear strength and elongation at break were improved and the tensile strength was reduced; the vulcanization time of the rubber prepared with vegetable oil-based softeners was significantly reduced compared with that of aromatic oil; the glass transition temperature of the rubber was significantly reduced and the cold resistance and snow performance of the rubber were improved.
Keywords: canola oil, cottonseed oil, vulcanization time.
This work was supported by the Ocean Science and Technology of Collaborative Innovation Center Project (22-05-CXZX-04-04-28) and the Key Research and Development Plan of Shandong Province (Major Scientific and Technological Innovation Project, 2020CXGC010312).
The authors declare that there is no conflict of interest.
Rubber softeners are generally a type of softening agent added to rubber formulations in the rubber processing process. These softeners themselves, as small molecule compounds, can improve the interfacial interaction between filler and rubber after entering the rubber matrix, reducing intermolecular forces and increasing the compatibility between rubber and filler. It also helps to control the preparation process of rubber products, allowing them to be prepared using various methods and equipment, reducing energy consumption in the production process and ensuring process safety.1-2 The use of rubber softeners can improve the physical and mechanical properties of rubber composites to a certain extent: increase the toughness of rubber, improve the tear resistance of rubber; reduce the hardness and tensile strength of rubber, improve the elongation at break of rubber; reduce the glass transition temperature to improve the low temperature resistance of rubber, reduce the heat generation of rubber, improve wear resistance, etc., which greatly expands the functionality and application potential of rubber products.3 Most of the traditional softeners are small molecule compounds based on petroleum resources, with the increasing depletion of petroleum resources and environmental pollution, there is an urgent need to find a new, environmentally friendly softeners to replace aromatic oils, naphthenic oils and other petrochemical-based softeners. Vegetable oil-based softeners as an environmentally friendly, renewable resources, with a wide range of sources, low cost and other advantages according to its raw materials can be seen in the vegetable oil-based softeners basically do not contain polycyclic aromatic hydrocarbons, in the production and use of rubber composites, will not produce substances harmful to the environment and health, is a green and environmentally friendly softeners. In addition, its origin from nature can be quickly degraded in nature, according to the United States environmental protection department of the relevant tests can be seen in nature, vegetable oil can be completely degraded in 7 days, the environment and human impact is minimal.4-8
Vegetable oil has good compatibility, permeability, and filling effects on SSBR. ① Component compatibility: The fatty acids and other organic components in vegetable oil can be compatible with the polymer chains of SSBR. This compatibility promotes dispersion and diffusion between the two, allowing vegetable oil to penetrate into the SSBR. ② The low molecular weight components of vegetable oil can penetrate into the polymer chains of SSBR, disrupting the intermolecular forces between rubber molecules and reducing its cohesion. This increases the mobility of the SSBR polymer chains and makes it softer. ③ Vegetable oil can fill the gaps and pores in SSBR, increasing the volume of the rubber. This filling effect reduces the hardness of the rubber and increases its flexibility. Therefore, theoretically, vegetable oil is a feasible option as a softening agent for rubber.
At present, there is less research on the application of vegetable oil-based softeners in rubber. In this paper, the working performance, vulcanization characteristics, physical properties, glass transition temperature of rubber materials prepared by comparing canola oil, cottonseed oil and aromatic oil are studied, and through the characterization of microscopic morphology, the effect of two vegetable oil-based softeners of canola oil and cottonseed oil on the performance of soluble styrene butadiene rubber is investigated, so as to provide a reference for the application of vegetable oil-based softeners in rubber materials This study will provide a reference for the application of vegetable oil-based softeners in rubber materials.
Raw Materials. Solution-polymerized styrene-butadiene rubber (SSBR): T2000, China, Sinopec Shanghai Gaoqiao Petrochemical Co. Ltd.; Carbon black N330: industrial grade, China, Shanghai Cabot Chemical Co. Ltd.; White carbon black: TH-628, China, Shandong Wanhua Tiande New Materials Co. Ltd.; rubber oil: aromatic oil (aniline point) 36 ℃; flash point: 300 ℃; density (20 ℃) 0.8495 g/cm3), China, Dongying Richers Technology Co. Ltd.; canola oil, cottonseed oil: Yingsida (Shandong) New Materials Co. Ltd.; all other materials are commercially available.
Experimental Formulation. The experimental formulations (parts by mass) were: SSBR 100; carbon black N330 30; silica 20; stearic acid 2; antioxidant 2.5; zinc oxide 4; microcrystalline wax 1; accelerator 1.3; accelerator D 0.3; sulfur 2; canola oil, cottonseed oil or aromatic oil) 5.
Instruments and Equipment. XK-160 Type Kneader: China, Dalian Hua Han Rubber & Plastic Machinery Co., Ltd; XSMG500 Type Rubber & Plastic Testing & Refining Machine: China, Shanghai Kechuang Rubber & Plastic Machinery Equipment Co. 3000AU, scanning electron microscope (SEM), differential scanning calorimeter (DSC) Ltd., Wallace International Hardness Tester, DIN abrasion tester: China, Taiwan High-Tech Testing Instruments Co, MR-CDS3500 nuclear magnetic resonance cross-linking density measurement instrument: German IIC Co.
Specimen Preparation. The first step is to cut the SSBR into thin strips after plasticizing; the second step is to preheat the mixing machine at 80r/min (the preheating temperature is 90 ℃); the third step is to put the thin strips of plasticizing rubber into the mixing machine, add the small material when mixing 1 min, add half of carbon black when mixing 2 min, add the remaining carbon black when mixing 3 min, add the softening agent when mixing 4 min, and mix at 5.5 The rubber is discharged at 5.5 min.
Vulcanized rubber preparation: open refiner roller preheating to 30 ℃, open refiner wrapping roller 1 min after adding sulfur accelerator 2 min, left and right cutter 5 times, playing triangle package 5 times, under the film, parking 12 h; mixed rubber vulcanization temperature of 150 ℃, vulcanization time of 1.3 ¡¿ positive vulcanization time (tc90), the preparation of vulcanized rubber.
Analysis and Testing. Working performance: The Menny viscosity of all rubber materials was tested by Menny Viscometer M-3000AU. Testing method: Round rubber specimens with diameter about 45 mm and thickness about 3 mm were cut from the rubber without air bubbles, one of them was punched with a circular hole of diameter about 8 mm in the center, parked for about 2 h and placed on the rotor of Menney viscometer with testing temperature of 100 ℃ and testing time of 5 min.
Vulcanization characteristics: The MG2000GAN rotorless rheometer was used to test the vulcanization characteristics of all rubber materials. Testing method: a round rubber specimen with diameter of 35 mm, thickness of 4-5 mm and mass of about 6.5 g was cut from the bubble-free rubber material, and after the temperature of the upper and lower cavities of the rotorless vulcanizer reached 150 ℃ and stabilized, the specimen was put into the test for 60 min.
Extraction test for oil migration: Use petroleum ether as a solvent to extract the free plasticizers from vulcanized rubber. Cut several 1x1 mm cubes from the vulcanized rubber, weigh 5 g of the sample. Place the sample in 100 mL of petroleum ether and seal it. Keep it in a water bath at 20 ℃ and 100 r/min for 24 hours. Remove the sample, dry it, and weigh it again. Calculate the difference in weight before and after the test.
NMR cross-linking density measurement: The cross-linking density of natural rubber was measured using the MR-CDS3500 nuclear magnetic resonance (NMR) cross-linking density measurement instrument manufactured by IIC from Germany. The measurements were conducted at a temperature of 60 ℃, a frequency of 15 MHz, and a magnetic induction strength of 315 A/m. The signal was then analyzed using the built-in analysis software of the instrument to obtain the total cross-linking density (XLD).
Tensile stress-strain: Tensometer 2000 tensile machine was used to test the mechanical properties of all rubber materials. Testing method: The rubber specimens were cut into 5 dumbbell-shaped specimens and 3 right-angle-shaped specimens, and the specimens were selected to test the thickness at three positions, and the average value was input into the universal testing machine and tested under the condition of tensile rate of 500 mm/min.
Shore A hardness: The hardness of all the rubber materials was tested by using Wallace International Hardness Tester. Testing method: The pressure needle is at least 12 mm away from the edge of the vulcanized rubber specimen smoothly press the pressure foot onto the specimen, so that the pressure needle is pressed vertically into the specimen until the pressure foot and the specimen are completely in contact when the reading is taken within 1s.
DIN abrasion: The abrasion of all glues was tested by using Wallace International hardness tester. Testing method: A cylindrical specimen of 16 mm in diameter and 12 mm in thickness was prepared using a standard mold to start the test.
Glass transition temperature: The glass transition temperature of all the glues was tested by DSC. Testing method: Each group took one vulcanization sheet (size 112x83x2 mm) of each sample, cut the vulcanization sheet in 5 positions to take 5 specimens, wipe the surface of the specimens with alcohol, spray a layer of graphite evenly, wash the ear ball and blow dry the graphite layer, put the specimens into the tester respectively, and wait for the test to be completed.
Scanning electron microscope (SEM): All adhesives prepared with different softeners were examined under a field emission scanning electron microscope (FESEM) FSU8020 (Hitachi, Japan) All adhesives prepared with different softeners were examined under a field emission scanning electron microscope (FESEM) FSU8020 (Hitachi, Japan) operated under an accelerated voltage of 5 kV.
Working Performance. The effects of canola oil, cottonseed oil and aromatic oil on the working properties of solubilized styrene butadiene rubber are shown in Table 1.
From Table 1, it can be seen that the Menney viscosity of rubber with canola oil and cottonseed oil tends to be the same and lower than that of rubber with aromatic oil. Menny viscosity reflects the good or bad processing performance and molecular weight of rubber. With high Menny viscosity, the rubber is not easy to be mixed evenly, and its molecular weight is high and wide distribution range, and its processing performance is poor. Therefore, compared with the rubber prepared by aromatic oil, the rubber prepared by canola oil and cotton seed oil has better processability.
SSBR is non-polar rubber, for non-polar rubber, softeners on rubber plasticization mechanism is through the softeners on rubber penetration and swelling effect, softeners molecules into the molecular chain between the rubber molecular spacing increases, as shown in Figure 1, intermolecular forces are reduced, chain segment mobility is enhanced, plasticity increased. The effect of softener on rubber is mainly related to solubility, according to the principle of similar compatibility, the closer the solubility parameters of softener molecules and solubilized SBR molecules, the easier it is for the softener molecules to enter between the molecular chains of solubilized SBR, the better the softening effect on the rubber.
The composites prepared from canola oil and cottonseed oil have lower meniscus viscosity than those prepared from aromatic oil, which may be caused by the following reasons: ① The aromatic oil contains polar components, which affects its swelling effect on the solubilized SBR, and the softening molecules do not easily enter the rubber molecular chain. ② The main chemical components of canola oil and cottonseed oil are triglycerides. The structure of their molecular formula shown in Figure 2 shows that canola oil and cottonseed oil contain longer carbon chains and more double bonds in the molecular chains. Their structural characteristics lead to the excellent flexibility of vegetable oil molecular chains, which are easier to penetrate into SSBR molecular chains than aromatic oils, causing full swelling, weakening the interaction force between SSBR rubber molecular chains, and playing a better softening effect.9
Vulcanization Characteristics. As shown in Table 2, MH is the maximum torque, ML is the minimum torque, MH-ML is the difference between the maximum and minimum torque, the larger the difference the higher the crosslink density of the rubber. The MH-ML of aromatic oil at 151 ℃ is slightly larger than that of the rubber prepared from canola oil and cottonseed oil, mainly because of the presence of polar functional groups in the aromatic oil component, which are easy to form cross-linkage with the soluble styrene butadiene rubber molecules and the high cross-linkage density of the rubber; the positive vulcanization time (tc90) of cottonseed oil and canola oil at 151 ℃ is significantly less than that of the rubber prepared with aromatic oil, indicating that the vulcanization speed of rubber composite materials prepared with cottonseed oil and rapeseed oil is faster than that of rubber composite materials prepared with aromatic oils. The main reason is that canola oil and cottonseed oil can not only increase the activity of accelerator, but also there is a certain amount of stearic acid in the two vegetable oils, and stearic acid and zinc oxide vulcanize at high temperature to produce zinc stearate, which plays a synergistic activation and promotion role.10-12
Oil Migration. Petroleum ether effectively extracts the free softening agents in rubber. As shown in Table 3, the quality degradation of sulfur vulcanized rubber prepared using aromatic oil measured by the mass difference method is slightly lower after extraction with petroleum ether compared to that prepared using rapeseed oil and cottonseed oil. This indicates that rapeseed oil and cottonseed oil exhibit a higher migration of oil within the polybutadiene system. The migration of oil within rubber causes swelling, which impacts the mechanical properties of the rubber.
Cross-linking Density. According to Table 4, it can be observed that the XLD (NMR cross-linking density) of sulfur-vulcanized rubber prepared with aromatic hydrocarbon oil is higher than that of rubber prepared with rapeseed oil and cottonseed oil. This is attributed to the formation of a conjugated system between carbon atoms in the aromatic ring structure, resulting in the sharing of electrons and the formation of ¥ð bonds. This conjugated system increases the number of carbon-carbon (C-C) bonds, leading to a relatively higher quantity of C-C bonds. During the vulcanization process, some sulfur atoms react with the C-C bonds in rubber molecules to form cross-linking structures. The XLD of sulfur-vulcanized rubber prepared with cottonseed oil is higher than that of rubber prepared with rapeseed oil, possibly due to the higher proportion of polyunsaturated fatty acids such as palmitic acid and linoleic acid in cottonseed oil. As a result, cottonseed oil may contain more C-C bonds and form a greater number of cross-linking structures.This corroborates the trend that the measured values of aromatic oil in Table 2 are higher than those of cottonseed oil and rapeseed oil for the preparation of sulfurized rubber MH-ML.
Mechanical Properties. From Figure 3(a), it can be observed that the tensile strength of sulfur vulcanized rubber prepared with aromatic oil is slightly lower than that prepared with rapeseed oil and cottonseed oil.It is analyzed that although the crosslink density of the composites prepared by adding aromatic oil is higher than that of the composites prepared by the two vegetable oils, the good softening effect of the two vegetable oils enhances the flexibility of the rubber molecular chains and promotes the dispersion effect of the fillers such as carbon black and silica, which has an effect on the tensile strength of the vulcanized rubber.
From Figure 3(b), the results showed that the tensile elongation at break of sulfur vulcanized rubber prepared with rapeseed oil and cottonseed oil was 16.3% and 20.4% higher, respectively, compared to that of rubber with aromatic oil added. This is mainly because the elongation at break of rubber composites is mainly related to the flexibility of macromolecules, and the composites prepared by adding cottonseed oil and canola oil enhance the flexibility of rubber macromolecules due to their better swelling and softening effects.13
From Figure 3(c), it can be seen that the 300% elongation stress of the composites prepared by adding canola oil and cottonseed oil tends to be the same, which is lower than that of the material prepared by aromatic oil. 300% elongation stress mainly reflects the ability of the vulcanized network structure to resist deformation under the action of external forces, which is mainly related to the molecular weight and the magnitude of intermolecular forces. The composites prepared by adding aromatic hydrocarbon oil can increase the adhesion of the adhesive material due to the aromatic hydrocarbon in the aromatic oil, resulting in high intermolecular force, which enhances the tensile stress of the composites.This corroborates the trend that the measured values of aromatic oil in Table 2 are higher than those of cottonseed oil and rapeseed oil for the preparation of sulfurized rubber MH-ML.
It can be seen from Figure 3(d) that the tear strength of vulcanizates prepared by adding canola oil is 1.6% and 8% higher than that of composites prepared by adding cottonseed oil and aromatic oil, respectively. The tear strength of vulcanized rubber is related to the uniformity of dispersion of fillers such as carbon black and silica, and the more uniform the filler dispersion, the higher the tear strength. The tear strength of the vulcanized rubber prepared by two vegetable oils was higher than that of the vulcanized rubber prepared by aromatic oil, and the mechanism was that the good softening effect of the two vegetable oils enhanced the flexibility of the rubber molecular chains and promoted the dispersion effect of the fillers such as carbon black and silica.14
From Figure 3(e), it can be seen that the hardness of vulcanized rubber prepared by adding canola oil and cottonseed oil is the same, and the hardness of vulcanized rubber prepared by adding aromatic oil is 3.7% higher than it. This coincides with the elongation at break data pointing to the same. The main reason for this is that the two vegetable oils enhanced the toughness of the rubber molecular chains and reduced the hardness.
From Figure 3(f), it can be seen that the abrasion of the vulcanized rubber prepared by the two vegetable oils is lower than that of the vulcanized rubber prepared by the aromatic oil, which has better abrasion resistance. The main reason is that the two vegetable oils are more conducive to the uniform dispersion of fillers, which enhances their wear resistance.
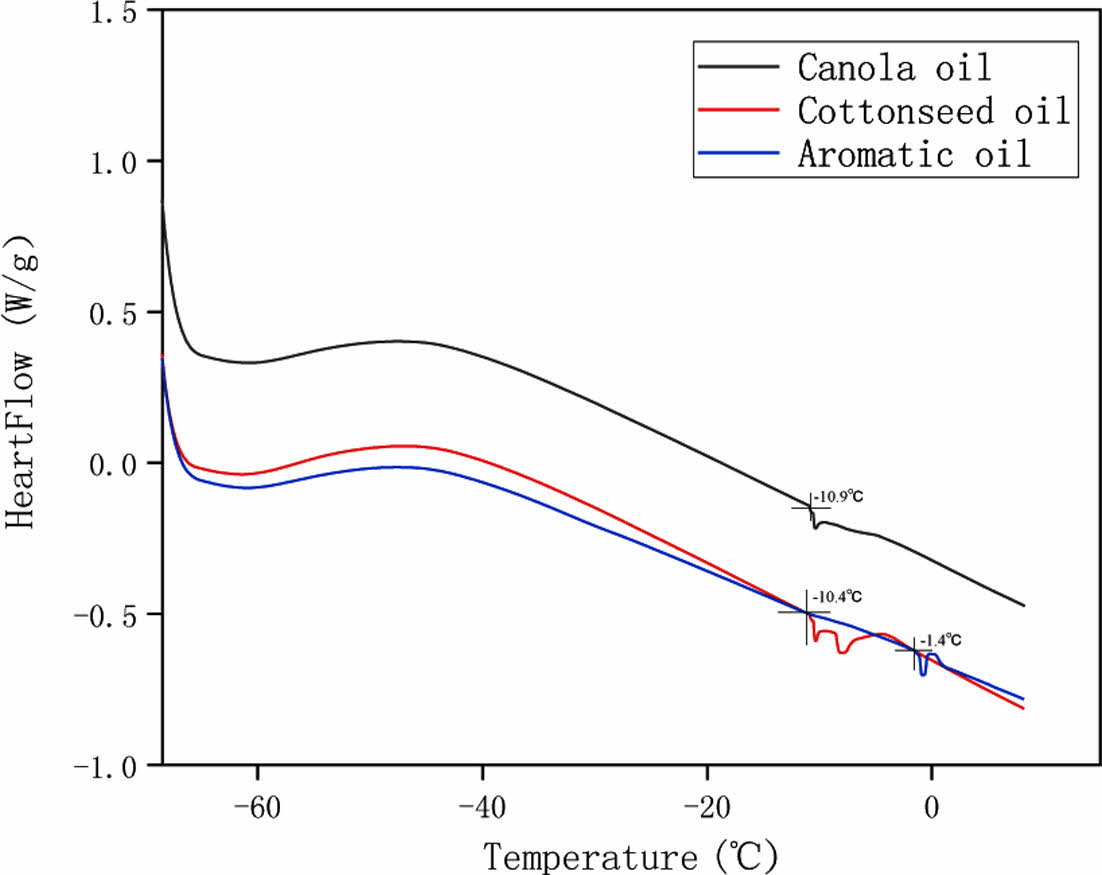
Glass Transition Temperature. As can be seen from Figure 4, the glass transition temperature of the composites prepared by adding canola oil and cottonseed oil is close to and significantly lower than that of the composites prepared by aromatic oil, with a difference of about 10 ℃. This indicates that the rubber prepared by adding castor oil has excellent frost resistance and is suitable for tire production in special application scenarios such as snow tires.
The glass transition temperature is related to molecular flexibility, molecular chain spacing, and cross-linking. The greater the molecular flexibility, the lower the glass transition temperature, the greater the molecular chain spacing, the lower the glass transition temperature, the more the polymer molecules are cross-linked, the more the free volume is reduced, the molecular chain movement is blocked, and the glass transition stability rises.
As mentioned before, two vegetable oils are more conducive to penetrate into the rubber molecular chains due to their swelling effect and structural flexibility, which increases the molecular chain spacing, reduces the molecular chain-related forces, and enhances the flexibility of rubber molecular chains.
Micromorphology. The microscopic morphology of vulcanized rubber prepared from canola oil, cottonseed oil and aromatic oil is shown in Figure 5.
Figure 5 shows the scanning electron micrographs of the rubber prepared by different softeners, from which it can be seen that the dispersion of the fillers of the rubber prepared by adding canola oil and cottonseed oil is more uniform, and the phenomenon of filler aggregation appears in the rubber prepared by adding aromatic oil. This is consistent with the Menny viscosity and physical properties pointing to a better softening effect of the plant-based softeners on the rubber and better activation of the rubber molecular chains, which makes the rubber molecular chains more flexible and conducive to the dispersion of the fillers.15-16

|
Figure 1 Mechanism of plasticizing effect of softeners on rubber. |

|
Figure 2 Chemical molecular formula of triglyceride. |

|
Figure 3 Effect of different softeners on the physical and mechanical properties of the rubber: (a) tensile strength; (b) elon-gation at break; (c) 300% elongation stress; (d) tearing strength; (e) shore hardness; (f) DIN wear. |

|
Figure 4 Effect of different softeners on the glass transition temperature of the adhesive. |

|
Figure 5 SEM images of (a) preparation of SSBR composite materials using canola oil; (b) preparation of SSBR composite materials using Cottonseed oil; (c) preparation of SSBR composite materials using Aromatic oil. |
|
Table 2 Effect of Adding Different Softeners on Vulcanization Characteristics of Vulcanized Rubber |

|
Table 3 Quality Degradation in the Preparation of Sulfur Vulcanized Rubber using Various Softening Agents via Petroleum Ether Extraction |

|
Table 4 NMR Cross-linking Density of Sulfur Vulcanized Rubber Prepared with Different Plasticizers |

In this paper, the vulcanization speed of the rubber prepared by adding canola oil and cottonseed oil is significantly higher than that of the rubber prepared by aromatic oil.
The tensile strength of vulcanized rubber prepared by adding canola oil and cottonseed oil was slightly lower than that of vulcanized rubber prepared by aromatic oil; the addition of rapeseed oil and cottonseed oil to sulfur vulcanized rubber resulted in a tensile elongation at break that was respectively 16.3% and 20.4% higher compared to sulfur vulcanized rubber prepared with aromatic oil; the tear strength of vulcanized rubber prepared by adding canola oil was 1.6% and 8% higher than that of vulcanized rubber prepared by cottonseed oil and aromatic oil, respectively. The hardness of vulcanized rubber prepared with canola oil and cottonseed oil was the same, and it was 3.7% lower than that of vulcanized rubber prepared with aromatic oil.
The glass transition temperature of vulcanized rubber prepared by adding canola oil and cottonseed oil is about 10 ℃ lower than that of vulcanized rubber prepared by cyclic aromatic oil, which is more suitable for application in low temperature environment, and can ensure the low temperature performance of rubber more effectively and improve the low temperature snow performance of rubber in comparison with existing formulations.
Vegetable oil is a renewable resource derived from natural sources, which is more environmentally friendly and has better low-temperature performance compared to aromatic hydrocarbon oils. There are many potential applications for substituting vegetable oil for aromatic hydrocarbon oils. Apart from its use in snow tires, vegetable oil can be used in the medical device manufacturing industry as a rubber softener. Additionally, due to its lower toxicity and better biocompatibility, vegetable oil can be used in the production of rubber products such as medical tubing and surgical gloves.
- 1. Rodrigues Corrêa, K. C.; da S.; Lima, J. C.; de Fett Neto, A. G. Pine Oleoresin: Tapping Green Chemicals, Biofuels, Food Protection, and Carbon Sequestration from Multipurpose Trees. Food & Energy Security 2012, 1, 81-93.
-

- 2. Bocqué, M.; Voirin, C.; Lapinte, V.; Caillol, S.; Robin, J.-J. Petro-based and Bio-based Plasticizers: Chemical Structures to Plasticizing Properties. J. Polym. Sci. Part A: Polyme. Chem. 2016, 54, 11-33.
-

- 3. Kim, S.-H.; Kim, S.-B.; Cha, S.-H. Preparation and Characterization of Biopolyurethane Film with a Novel Cross-linkable Biopolyol Based on Cardanol. Polym. Korea2018, 42, 736-746.
-

- 4. Kim, J.-K.; Huh, K. M. Synthesis of Biomass-based Reactive Plasticizer and Its Effects on the Processing Properties of Poly(vinyl chloride). Polym. Korea 2021, 45, 380-389.
-

- 5. Kim, J.-K.; Kang, E.; Huh, K. M. Synthesis of a Coconut Oil-based Bioplasticizer and Its Effects on the Rheological and Fusion Properties of Poly(vinyl chloride). Polym. Korea 2019, 43, 778-786.
-

- 6. Rodrigues Corrêa, K. C.; da S.; Lima, J. C.; de, Fett Neto, A. G. Pine Oleoresin: Tapping Green Chemicals, Biofuels, Food Protection, and Carbon Sequestration from Multipurpose Trees. Food. Energy Security 2012, 1, 81-93.
-

- 7. Barnes, T. M.; Greive, K. A. Topical Pine Tar: History, Properties and Use as a Treatment for Common Skin Conditions. Australasian J. Dermatology 2016, 58, 80-85.
-

- 8. Jr, A. L. Basics of Compounding with Tars. Int. J. Pharm. Compoun. 2013, 17, 400-410.
- 9. Song, P.; Cheng, X.; Qu, X.; Peng, Z.; Wang, S. Research Progress on Application of Biomass Softener in Regenerated Rubber. Special Rubber Products, 2017, 38, 58-64.
- 10. Abdulhakim, M.; Siriwat, S.; Hayeemasae, N. Influence of Sulfur/Accelerator Ratio on Tensile Properties and Structural Inhomogeneity of Natural Rubber. Polym. Korea 2020, 44, 519-526.
-

- 11. Ouyang, G.; Wu, L.; Ye, C.; Wang, J.; Dong, T., Effect of Silane Coupling Agent on the Rheological and Mechanical Properties of Alkali-activated Ultrafine Metakaolin Based Geopolymers, Construction and Building Mater. 2021, 290, 123223-123234.
-

- 12. Wu, X.; Zheng, J.; Han, B.; Zhang, L.; Lu, J. Designing Novel Epoxy-terminated Polybutadiene to Construct Chemical Interface Between Nanosilica and Rubbers with Green Nature, Compos. Part B. 2019, 178, 107451-107464.
-

- 13. Zhang, S.; Han, L.; Bai, H.; Li, C.; Wang, X. Introducing Mechanochemistry Into Rubber Processing: Green-functionalized Cross-linking Network Of Butadiene Elastomer, ACS Sustainable Chem. Eng. 2021, 9, 8053-8058.
-

- 14. Lin, G.; Wang, H.; Qu, G.; Qu, S.; Liang, Z.; Yu, K.; Xu, N. N. Properties and Process Optimization of Nanocomposites with Organically Modified Montmorillonite as Environment-friendly Flocculants for Natural Latex. Mater. Exp. 2020, 12, 2108-2121.
- 15. Coleman, J. N.; Khan, U.; Blau, W. J.; Gun'Ko, Y. K. Small But Strong: a Review of the Mechanical Properties of Carbon Nanotube-polymer Composites. Carbon 2006, 44, 1624-1652.
-

- 16. Kalaivani Subramaniam, Amit Das, Gert Heinrich. Development of Conducting Polychloroprene Rubber Using Imidazolium Based Ionic Liquid Modifified Multi-walled Carbon Nanotubes.Compos. Sci. Technol. 2011, 71, 1441-1449.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2023; 47(6): 729-736
Published online Nov 25, 2023
- 10.7317/pk.2023.47.6.729
- Received on Jun 18, 2023
- Revised on Aug 7, 2023
- Accepted on Aug 9, 2023
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Conflict of Interest
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Yuan Jing, Guangyi Lin*
-
College of Civil Engineering, Qingdao University of Technology, Qingdao, 266033, P.R. China
*College of Electromechanical Engineering, Qingdao University of Science and Technology, Qingdao 266061, P.R. China - E-mail: 2777273463@qq.com, 02479@qust.edu.cn
- ORCID:
0009-0002-1249-221X, 0000-0001-8797-8607










 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.