- Mechanical Properties and Biocompatibility of Electrospun Poly(ε-caprolactone)/Gelatin Scaffolds Loaded with Cellulose Fiber
Department of Biomedical Engineering, Daelim University, Anyang 13916, Korea
- 전기방사법으로 제조한 PCL/젤라틴/셀룰로오스 지지체의 기계적 특성 및 생체적합성
대림대학교 보건의료공학과
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
The cellulose fiber (CF)-loaded PCL/gelatin (P/g) scaffolds were electrospun to investigate the effect of CF content on the strength, moisture vapor transmission rate (MVTR), water uptake capacity (WUC), and cytotoxicity of the P/g/CF scaffolds. The fiber diameter gradually increased from 462 to 869 nm with increasing the CF content from 0 to 4 wt% due to the increase in viscosity from 811 to 1121 cP. The strength increased from 2.2±0.3 to 4.8±0.8 MPa as the CF content increased from 0 to 2 wt%, and decreased with additional CF doping. FTIR results revealed that PCL crystallinity decreased with increasing CF content due to H bonds between cellulose and gelatin. The highest MVTR (CF=1 wt%) and WUC (CF=2 wt%) values are observed. Excellent cell viability and proliferation were observed in P/g/CF scaffolds regardless of CF content. It can be concluded that P/g scaffolds loaded with 2% CF are highly suitable as wound dressings.
18 wt% PCL/젤라틴(P/g)에 셀룰로오스 섬유(CF)를 첨가하여 P/g/CF 지지체를 전기방사하고, CF 농도에 따른 인장강도, 투습도, 수분흡수도, 세포독성 및 증식 특성을 조사하였다. CF 농도가 0에서 4 wt%로 증가함에 따라, 점도는 811에서 1121 cP로 증가하였고 섬유 직경은 462에서 869 nm로 증가하였다. 2 wt% CF가 첨가된 P/g/CF에서 4.8±0.8 MPa 최대 인장강도값이 관찰되었지만, CF 농도가 증가함에 따라 강도값은 감소하였다. FTIR 결과, CF농도가 증가함에 따라 지지체 내부의 CF와 젤라틴 간의 수소결합에 의하여 PCL 결정도가 감소하였다. 최적의 투습도와 물흡수도는 CF농도가 각각 1%와 2%일 때 관찰되었다. 세포생존률과 세포 증식은 CF농도에 상관없이 P/g/CF지지체에서 유사하였다. 실험결과, 상처드레싱재로 적합한 최적의 특성을 가진 지지체는 CF농도가 2 wt%인 P/g/CF에서 관찰되었다.
The cellulose fiber (CF)-loaded PCL/gelatin (P/g) scaffolds were electrospun using a common solvent (a mixture of acetic acid, ethyl acetate, and distilled water). Among the P/g/CF scaffolds, the P/g scaffolds loaded with 2 wt% CF are highly regarded as biomaterials for wound dressings because of their excellent mechanical strength, moisture vapor transmission rate, water uptake capacity, cell viability and proliferation.
Keywords: poly(ε-caprolactone), gelatin, cellulose fiber, electrospinning, scaffold, wound dressing.
This work was supported by a grant (code # 2022-022) from Gyeonggi-do research-centered R&D support project funded by Gyeonggi Province.
The authors declare that there is no conflict of interest.
Biopolymer scaffolds composed of synthetic and natural polymers play an important role in wound dressings due to their properly interconnected pore networks, structural integrity, moisture vapor transmission rate (MVTR), water uptake capacity (WUC), biocompatibility, and adequate biodegradability.1-4 The development of electrospun hybrid polymer combinations is limited because of the limited number of common solvents that can be used to dissolve both natural and synthetic polymers.1-4 Nanofibrous gelatin/poly(ε-caprolactone) (g/P) scaffolds with specific ratios of gelatin and PCL exhibit appropriate mechanical properties and biological functions similar to natural tissues in cell attachment and proliferation.4 Fluorinated alcohols (hexafluoro-2-propanol and trifluoroethanol) are mainly used as solvents, but polymers are rapidly decomposed, expensive, and toxic.2 Non-toxic aqueous solvents (acetic acid and formic acid) have emerged as the solvent of choice for dissolving and electrospinning natural polymers such as chitosan and gelatin.1,2 The P/g precursor solution was prepared by mixing PCL in chloroform/methanol and gelatin in acetic acid.2 P/g composite scaffolds can be prepared by using an inexpensive and less toxic diluted acetic acid and ethyl acetate common solvent system.2-5
To evaluate the effect of P/g weight ratio (9/1 to 5/5) on mechanical properties, porosity, and biocompatibility, a g/P scaffold was used using a common solvent of diluted acetic acid and ethyl acetate at 16 wt% polymer concentration. P/g scaffolds containing 30 to 40% gelatin have been reported to be optimal for wettability, bioactivity, and controlled degradation in bone tissue engineering applications.3 Porous P/g scaffolds were electrospun at 18 wt% polymer concentration using a common solvent of diluted acetic acid and ethyl acetate.4 Among the scaffolds, 7/3 and 6/4 P/g scaffolds were shown to be suitable as wound dressings due to their excellent MVTR, water uptake capacity, cell viability, proliferation, adhesion, and mechanical strength. Recently, cellulose fibers (CF, 1-3 wt%) were incorporated into a 10 wt% P/g blend using a solvent system consisting of acetic acid, formic acid and ethanol to enhance the fracture energy and reduce the degradation rate of P/g scaffolds.5,6 The combination of biopolymers and CFs can simulate natural tissues in chemical, mechanical, and biological terms, and consequently may introduce the most promising materials for wound dressings. In this study, the effect of CF content (0-4 wt%) on the mechanical properties and biocompatibility of the P/g/CF scaffolds synthesized by electrospinning CF-loaded 7/3 P/g scaffolds using acetic acid, ethyl acetate, and distilled water common solvents was investigated.
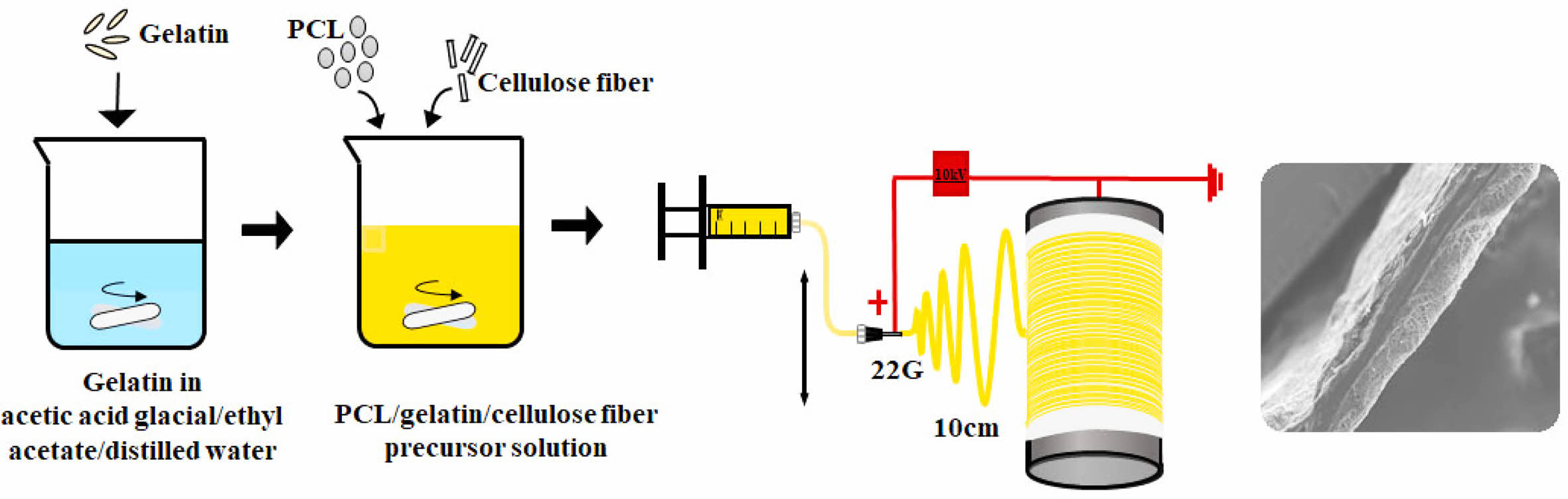
Materials. PCL ((CH2CH(OH))n, Mw: 80000), gelatin from porcine skin and CF were purchased from Sigma-Aldrich. Acetic acid (99.5%), ethyl acetate (99.5%), and NaOH (98%) were purchased from Samchun Pure Chemical Co., Ltd., Korea and used as they were received. A mixture of acetic acid, ethyl acetate, and distilled (DI) water was used as a common solvent throughout the study.4 The experimental procedure for a P/g precursor solution with a P/g weight ratio of 7/3 has been described elsewhere.4 CFs soaked in ethanol were stirred using ultrasound for at least 30 min. Then, dried CF (1-4 wt%) was added to the 7/3 P/g solution and degassed for 10 min to obtain a homogeneous P/g/CF precursor solution.
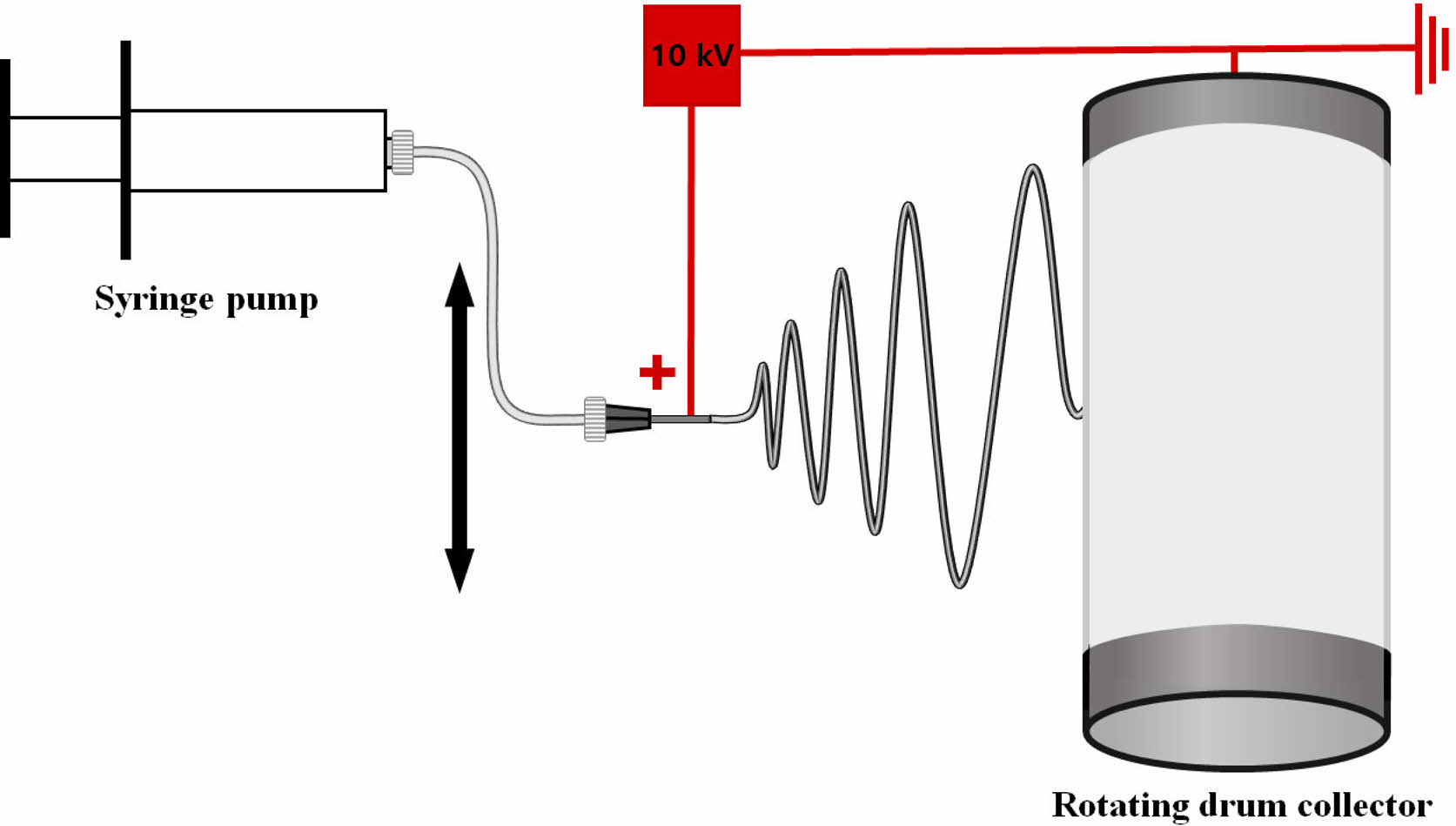
Electrospinning. The electrospinning device consisted of a syringe pump (KDS-200, Stoelting Co., USA), a 22 gauge BD metal needle, a grounded collector, and a high-voltage power supply (ES30P-5W, Gamma High Voltage Research Inc., USA) equipped with current and voltage digital meters.4,7-9 The solution was placed in a 5 mL BD luer-lock syringe attached to a syringe pump and fed into a 22 gauge metal needle at a flow rate of 1 mL/h. A rotating metal drum with a diameter of 9 cm and a length of 20 cm was used to collect the membranes at a voltage of 10kV and a distance of 10 cm using.4 The rotational speed of the mandrel-type collector and the transverse speed of the needle were 250 rpm and 60 cm/min, respectively, as shown in Figure 1.4,7,8 Scaffolds with a thickness of 0.3 mm were prepared. The as-spun scaffolds were placed in a vacuum oven at 40 oC to remove residual solvent. They were neutralized with 0.1 N NaOH solution and then washed three times with DI water. The scaffolds were then sterilized by exposing them to ethylene oxide (EO) gas for 4 h with an EO sterilizer (HS-4313EO, Hanshin Medical, Korea).4,10
Characterization. Solution viscosity and electrical conductivity were measured at room temperature using a viscometer (DV 1M, Brookfield, USA) with spindle NO. SC4-31 at 20 rpm and an electrical conductometer (912, Metrohm, Switzerland), respectively.4,7,8,10-12 The morphology of the P/g/CF scaffold was examined using a scanning electron microscope (SEM, S-3000H, Hitachi, Japan) and an optical stereomicroscope (SV-55, Sometech, Korea). Fiber diameter was then determined by using an optical microscope equipped with iSolution Lite image software.4,10-12 Fourier transform infrared spectroscopy (FTIR, Spectrum Two, PerkinElmer, UK) was used to analyze the chemical structure of the scaffold.10-14 The mechanical properties of the scaffolds were investigated at room temperature using an Instron 5564 with a 1000 N force cell at a crosshead speed of 10 mm/min.4,7,8 Samples were prepared according to ASTM D-638 (type V) in the shape of a dumbbell. All experiments were performed in triplicate. The data were expressed as the mean±standard deviation, and statistical significance was set at p<0.05.4
MVTR and WUC of P/g/CF Scaffolds. TheMVTR of the permeable scaffold is determined according to EN 13726-2:2002-Part 2.4,15,16 After fixing on one flange with a flat plate, 20 mL of distilled water was filled.4,15 A sample with a diameter of 55 mm was attached to the opposite side of the flange.4 Sample container was stored for 24 h at 37 oC and 20% humidity in a thermo-hygrostat. The liquid that forms inside the wound dressing turns into a vapor state and is transported to the atmosphere. The moisture vapor permeation helps prevent wound infection and thus aids in wound healing. MVTR is then determined.4,15,16 The WUC of the scaffold is also examined.4,6
Cytotoxicity and Cell Proliferation. The extract test method is based on the International Organization for Standardization (ISO 10993-5) for the evaluation of cytotoxic potential (L-929 mouse fibroblast cell (NCTC Clone 929, ATCC, USA)) against P/g/CF scaffolds.4,7,8,10-14 Detailed experimental procedures are summarized elsewhere.4,10-14
|
Figure 1 A schematic diagram of electrospinning apparatus. |
An effective blend system was investigated by varying the CF content of the electrospun 7/3 P/g scaffolds loaded with CF at 18% polymer concentration. SEM images of the surfaces of P/g/CF scaffolds with different CF content are shown in Figure 2. Fiber diameter was determined by measuring between 70 and 100 fibers. As the CF content increased from 0 to 4 wt%, the fiber diameter gradually increased from 462 nm to 499 nm, 558 nm, 735 nm, and 869 nm, as depicted in Figure 3. The precursor solution exhibited a CF concentration dependent increase in viscosity (811 to 1121 cP) and conductivity (42.2±0.4 to 61.5±0.7 mS/cm), as displayed in Figure 4. Gradual increase in viscosity due to CF addition may be attributed to increase in fiber diameter.4,5
Tensile strength of P/g/CF scaffolds are illustrated in Figure 5. As the CF content increased from 0 to 1 wt%, the strain increased from 4.2 to 5.4. It then started to decrease from 5.4 to 4.6 and 2.5 with additional CF doping (2-3 wt%). Tensile strength increased dramatically from 2.2±0.3 to 3.9±0.5 MPa and 4.8±0.8 MPa with increasing CF content from 0 to 1 and 2 wt%, but decreased to 2.1±0.5 MPa with further CF doping (3 wt%). This can be detrimental to PCL crystallinity due to the non-uniform distribution of CF fillers and strong hydrogen bonding between gelatin and CF.5 Gourdarzi et al.5 reported that the highest strength of 3.24±0.22 MPa was observed for the P/g/CF scaffold containing 2 wt% CF, which is in good agreement with our results. However, our results (4.8±0.8 MPa) are slightly higher due to different sample preparation procedures. The degassing process was supplemented to obtain a homogeneous precursor solution. Optimized strength and strain were observed for P/g scaffold loaded with 2 wt% CF.
FTIR has been used to investigate the presence and possible interactions of polymers and nanomaterials in composite.4,5 FTIR spectra of gelatin, PCL, CF, and P/g/CF scaffolds are shown in Figure 6. The amide (N-H stretching) peak of gelatin at 3300 cm-1 was severely attenuated after CF loading on P/g scaffolds. The PCL peaks located at 2946 cm-1 and 2868 cm-1, corresponding to symmetrical and asymmetrical methylene groups, shifted slightly to 2943 cm-1 and 2863 cm-1, respectively. The sharp intensities of the amide bands (1645 cm-1 and 1538 cm-1) of the P/g scaffold indicate the good miscibility of the two polymers (PCL (-COO) and gelatin (-NH2)).4 The peak intensity and shape of gelatin, C=O stretching at 1645 cm-1 (amide I) and -NH bending at 1538 cm-1 (amide II), weakened and broadened dramatically after CF addition.5 Shifting and broadening of these peaks can be an example of the presence of two polymers in a blend and possible interactions between them.5 The CF-loaded P/g scaffolds favored hydrogen (H) bonds between cellulose (hydroxyl (-OH) and methylol (NH2·CH2OH) groups) and gelatin (carboxyl (-COOH) and amine groups (-NH2)) within the scaffold, detrimental to PCL crystallinity.2,5 This observation indicates that higher amounts of CF may be harmful to PCL crystallinity due to the favorable interaction between CF and gelatin. The decreased strength of the P/g/CF scaffold may be due to the decrease in PCL crystallinity, as verified in Figure 5.
MVTR is an important criterion for wound dressing requiring proper healing.4,6,15,16 Low MVTR deposits produce exudates and delay the wound healing process, making the wound more susceptible to infection.4 On the other hand, high MVTR increases dehydration at the wound site, which prevents the skin from being maintained for a long time in an adequately moist environment, leading to scarring.4,6 It is recommended that the MVTR of wound dressing be in the range of ~500 to ~2000 g/10 cm2·24 h for proper skin regeneration.6 The MVTR and WUC values of P/g/CF scaffolds are shown in Figure 7. The MVTR values of the scaffolds initially increased from 2240 to 2466 g/10 cm2·24 h as the CF content increased from 0 to 1%. Thereafter, as the CF content increased from 1 to 2 wt%, it slightly decreased from 2466 to 2450 g/10 cm2·24 h. However, it rapidly decreased from 2450 to 1986 g/10 cm2·24 h above 3 wt%. All scaffolds prepared in this study could be considered as suitable biomaterials for infected skin due to their MVTR values.6 WUC showed the capacity of the scaffolds to absorb wound exudate.4,6,16,17 The P/g/CF scaffolds exhibited WUCs of 588, 677, 716, 503, and 517% with increasing CF content from 0 to 1, 2, 3, and 4 wt%, respectively. Although the highest MVTR (CF=1 wt%) and WUC (CF=2 wt%) values are observed for the P/g/CF scaffolds, all scaffolds can be applied to wound dressings due to their appropriate MVTR and WUC values.4,6
Cytotoxicity of P/g/CF scaffolds with varying CF content ranging from 0 to 4% determines whether the scaffold will have toxic effects on living cells.10-20 Regardless of the CF content, the test extracts showed no evidence of causing cell lysis or toxicity, as displayed in Figure 8. P/g/CF scaffolds containing CF content of 0, 1, 2, 3, and 4 wt% showed cell viability of 130, 121, 114, 135, and 136%, respectively, compared to the negative control, as measured at a wavelength of 415 nm by using the microplate absorbance spectrophotometer.10-14 As shown in Figure 9, the results of proliferation of L-929 cells on P/g/CF scaffolds suggested that L-929 cells adhered well to P/g/CF scaffolds and continued to proliferate over time. All P/g/CF scaffolds can be considered suitable as wound dressings because they are non-cytotoxic and have excellent cell adhesion and proliferation under the conditions of this study. As a result of the experiment, the P/g scaffold loaded with 2 wt% CF was determined as the optimal membrane due to the mechanical properties, physical properties, and biocompatibility.

|
Figure 2 SEM images of P/g/CF scaffolds with different CF concentrations of (a) 0; (b) 1; (c) 2; (d) 3; (e) 4 wt%. Note that the P/ g ratio is 7/3, the scale bar is 5 m and the inset bar graph is a measure of the fiber diameter. |

|
Figure 3 The variation of fiber diameter as a function of CF concentration in P/g/CF scaffold. |

|
Figure 4 The variation of viscosity and electrical conductivity as a function of CF content in P/g/CF scaffold. |

|
Figure 5 Tensile strength of various P/g/CF scaffolds. |

|
Figure 6 FTIR spectra of gelatin, PCL, cellulose fiber, and various P/g/CF scaffolds. |

|
Figure 7 Water uptake capacity and MVTR values of various P/g/ CF scaffolds. |

|
Figure 8 Photographs of cell morphologies: (a) negative control; (b) positive control; P/g/CF scaffolds containing (c) 0%; (d) 1%; (e) 2%; (f) 3%; (g) 4% CF content. Scale bar is 100 μm. |

|
Figure 9 Proliferation of L-929 cells on negative control and P/g/ CF scaffolds over time. Note that the P/g ratio is fixed at 7/3. |
7/3 P/g scaffolds loaded with CF at 18 wt% polymer concentration were prepared using common solvents to evaluate the effect of CF content on mechanical properties, MVTR, WUC, and biocompatibility of P/g/CF scaffolds. The precursor solution showed a CF concentration dependent increase in viscosity and conductivity. Fiber diameter increased gradually with increasing CF content due to increased viscosity. The tensile strength increased from 2.2±0.3 to 4.8±0.8 MPa as the CF content increased from 0 to 2 wt%, and decreased with additional CF doping due to lower PCL crystallinity as confirmed by FTIR observations. Decreased PCL crystallinity may be due to favorable H bonds between cellulose and gelatin inside the scaffold. The highest MVTR (CF=1 wt%) and WUC (CF=2 wt%) values were observed for the P/g/CF scaffolds. Non-cytotoxicity and cell proliferation were observed in all P/g/CF scaffolds regardless of CF content. Among the P/g/CF scaffolds, it can be concluded that the P/g scaffolds loaded with 2 wt% CF are highly regarded as biomaterials for wound dressings due to their mechanical strength, MVTR, WUC, cell viability and proliferation.
- 1. Ghasemi-Mobarakeh, L.; Prabhakaran, M. P.; Morshed, M.; Nasr-Esfahani, M.; Ramakrishna, S. Electrospun Poly(ε-caprolactone)/gelatin Nanofibrous Scaffolds for Nerve Tissue Engineering. Biomater. 2008, 29, 4532-4539.
-

- 2. Gautam, S.; Dinda, A. K.; Mishra, N. C. Fabrication and Characterization of PCL/gelatin Composite Nanofibrous Scaffold for Tissue Engineering Applications by Electrospinning Method. Mater. Sci. Eng. C 2013, 33, 1228-1235.
-

- 3. Binulai, N. S.; Natarajan, A.; Menon, D.; Bhaskaran, V. K.; Mony, U.; Nair, S. V. PCL-gelatin Composite Nanofibers Electrospun Using Diluted Acetic Acid-Ethyl Acetate Solvent System for Stem Cell-based Bone Tissue Engineering. J. Biomater. Sci. 2014, 25, 325-340.
-

- 4. Song, Y.; Kim, B.; Yang, D. H.; Lee, D. Y. Poly(ε-caprolactone)/gelatin Scaffolds for Wound Dressing. Appl. Nanosci. 2022, doi.org/10.1007/s13204-021-02265-w.
-

- 5. Goudarzi, Z. M.; Behzad, T.; Ghasemi-Mobarakeh, L.; Kharaziha, M.; Enayati, M. S. Structural and Mechanical Properties of Fibrous Poly(caprolactone)/gelatin Nanocomposite Incorporated with Cellulose Nanofibers. Polym. Bull. 2020, 77, 717-740.
-

- 6. Salehi, M.; Niyakan, M.; Ehterami, A.; Haghi-Daredeh, S.; Nazarnezhad, S.; Abbaszadeh-Goudarzi, G.; Vaez, A.; Hashemi, S. F.; Rezaei, N.; Mousavi, S. R. Porous Electrospun Poly(ε-caprolactone)/gelatin Nanofibrous Mat Containing Cinnamon for Wound Healing Application, in vitro and in vivo Study. Biomed. Eng. Lett. 2020, 10, 149-161.
-

- 7. Oh, G.; Rho, J.; Lee, D. Y.; Lee, M.; Kim, Y. Synthesis and Characterization of Electrospun PU/PCL Hybrid Scaffolds. Macromol. Res. 2018, 26, 48-53.
-

- 8. Seol, B.; Shin, J.; Oh, G.; Lee, D. Y.; Lee, M. Characteristics of PU/PEG Hybrid Scaffolds Prepared by Electrospinning. J. Biomed. Eng. Res. 2017, 38, 248-255.
- 9. Ke, R.; Yi, W.; Tao, S.; Wen, Y.; Hongyu, Z. Electrospun PCL/gelatin Composite Nanofiber Structures for Effective Guided Bone Regeneration Membranes. Mater. Sci. Eng. C 2017, 78, 324-332.
-

- 10. Shin, J.; Lee, D. Y.; Kim, B.; Yoon, J. I. Effect of Polyethylene Glycol Molecular Weight on Cell Growth Behavior of Polyvinyl Alcohol/Carboxymethyl Cellulose/Polyethylene Glycol Hydrogel. J. Appl. Polym. Sci. 2020, 137, 49568.
-

- 11. Shin, J.; Lee, D. Y.; Yoon, J. I. Effect of CMC Concentration on Cell Growth Behavior of PVA/CMC Hydrogel. Macromol. Res. 2020, 28, 813-819.
-

- 12. Shin, J.; Jeong, H.; Lee, D. Y. Synthesis of PVA/NaCMC Hydrogels Crosslinked by Cyclic Freezing/Thawing and Subsequent Gamma-ray Irradiation and Their Properties. J. Biomed. Eng. Res. 2018, 39, 161-167.
- 13. Son, S.; Choi, J. E.; Cho, H.; Kang, D. J.; Lee, D. Y.; Kim, J. T.; Jang, J. W. Synthesis and Characterization of Porous Poly(ε-caprolactone)/Silica Nanocomposites. Polym. Korea 2015, 39, 323-328.
-

- 14. Kim, S.; Lim, H.; Kim, S.; Lee, D. Y. Effect of PVA Concentration on Strength and Cell Growth Behavior of PVA/gelatin Hydrogels for Wound Dressing. J. Biomed. Eng. Res. 2020, 41, 1-7.
- 15. Kuppan, P.; Sethuraman, S.; Krishnan, U. M. PCL and PCL-gelatin Nanofibers as Esophageal Tissue Scaffolds: Optimization, Characterization and Cell-matrix Interactions. J. Biomed. Nanotechnol. 2013, 9, 1540-1555.
-

- 16. Chellamani, K. P.; Sundaramoorthy, P.; Suresham, T. Wound Dressing Made Out of Polyvinyl alcohol/Chitosan Nanomembranes. J. Acad. Indus. Res. 2012, 1, 342-347.
- 17. Lee, S. M.; Park, I. K.; Kim, Y. S.; Kim, H. J.; Moon, H.; Mueller, S.; Jeong, Y. Physical, Morphological, and Wound Healing Properties of a Polyurethane Foam-Film Dressing. Biomater. Res. 2016, 20, 15.
-

- 18. Jeong, H.; Rho, J.; Shin, J.; Lee, D. Y.; Hwang, T.; Kim, K. J. Mechanical Properties and Cytotoxicity of PLA/PCL Scaffolds. Biomed. Eng. Lett. 2018, 8, 267-271.
-

- 19. Kim, D.; Lee, M.; Lee, D. Y.; Han, J. Mechanical Properties, Phase Stability, and Biocompatibility of (Y,Nb)-TZP/Al2O3 Composite Abutments for Dental Implant. J. Biomed. Mater. Res. 2000, 53, 438-443.
-

- 20. Longhao, J.; Park, K.; Yoon, Y.; Kim, H. S.; Kim, H. Y.; Choi, J. W.; Lee, D. Y.; Chun, H. J.; Yang, D. H. Visible Light-cured Antibacterial Collagen Hydrogel Containing Water-Solubilized Triclosan for Improved Wound Healing. Mater. 2021, 14, 2270.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2022; 46(6): 837-842
Published online Nov 25, 2022
- 10.7317/pk.2022.46.6.837
- Received on Aug 15, 2022
- Revised on Sep 16, 2022
- Accepted on Sep 20, 2022
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Conflict of Interest
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Deuk Yong Lee
-
Department of Biomedical Engineering, Daelim University, Anyang 13916, Korea
- E-mail: duke1208@gmail.com
- ORCID:
0000-0003-1674-412X










 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.