- Physical and Physico-mechanical Properties of Elastomers Based on Urethane Oligomers with Terminal Epoxy Groups as Affected by the Structure of Diisocyanates and Diamines
Anna V. Savchuk*,†
 , Aleksey I. Slobodinyuk
, Aleksey I. Slobodinyuk  , Valery Yu. Senichev
, Valery Yu. Senichev  , and Vladimir N. Strelnikov
, and Vladimir N. Strelnikov 
Institute of Technical Chemistry of Ural Branch of the RAS as Affiliated Branch of Perm Federal Research Center of Ural Branch of the RAS Acad., Korolev St. 3, 614013 Perm, Russian Federation
- Diisocyanate와 Diamine의 구조가 미치는 Epoxy 말단을 갖는 우레탄 올리고머 기반 탄성체의 물리적 특성 연구
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
There were investigated physical and physico-mechanical properties of elastomers based on three series of epoxy-terminated oligomers based on differently structured diisocyanates and diamines. The use of 3-isocyanatomethyl-3,5,5-trimethylcyclohexyl isocyanate enabled better strength properties to be obtained owing to the implementation of the strengthening effect. Also, there was elicited a positive influence of an increase in the content of 3-aminomethyl-3,5,5-trimethylcyclohexylamine in a mixture of hardeners on the strength of elastomers; it was associated with enhancing polarity of a polymer chain, therewith giving rise to changes in the phase separation degree.
There were investigated physical and physico-mechanical properties of elastomers based on three series of epoxy-terminated oligomers based on differently structured diisocyanates and diamines. The use of 3-isocyanatomethyl-3,5,5-trimethylcyclohexyl isocyanate enabled better strength properties to be obtained owing to the implementation of the strengthening effect. Also, there was elicited a positive influence of an increase in the content of 3-aminomethyl-3,5,5-trimethylcyclohexylamine in a mixture of hardeners on the strength of elastomers; it was associated with enhancing polarity of a polymer chain, therewith giving rise to changes in the phase separation degree.
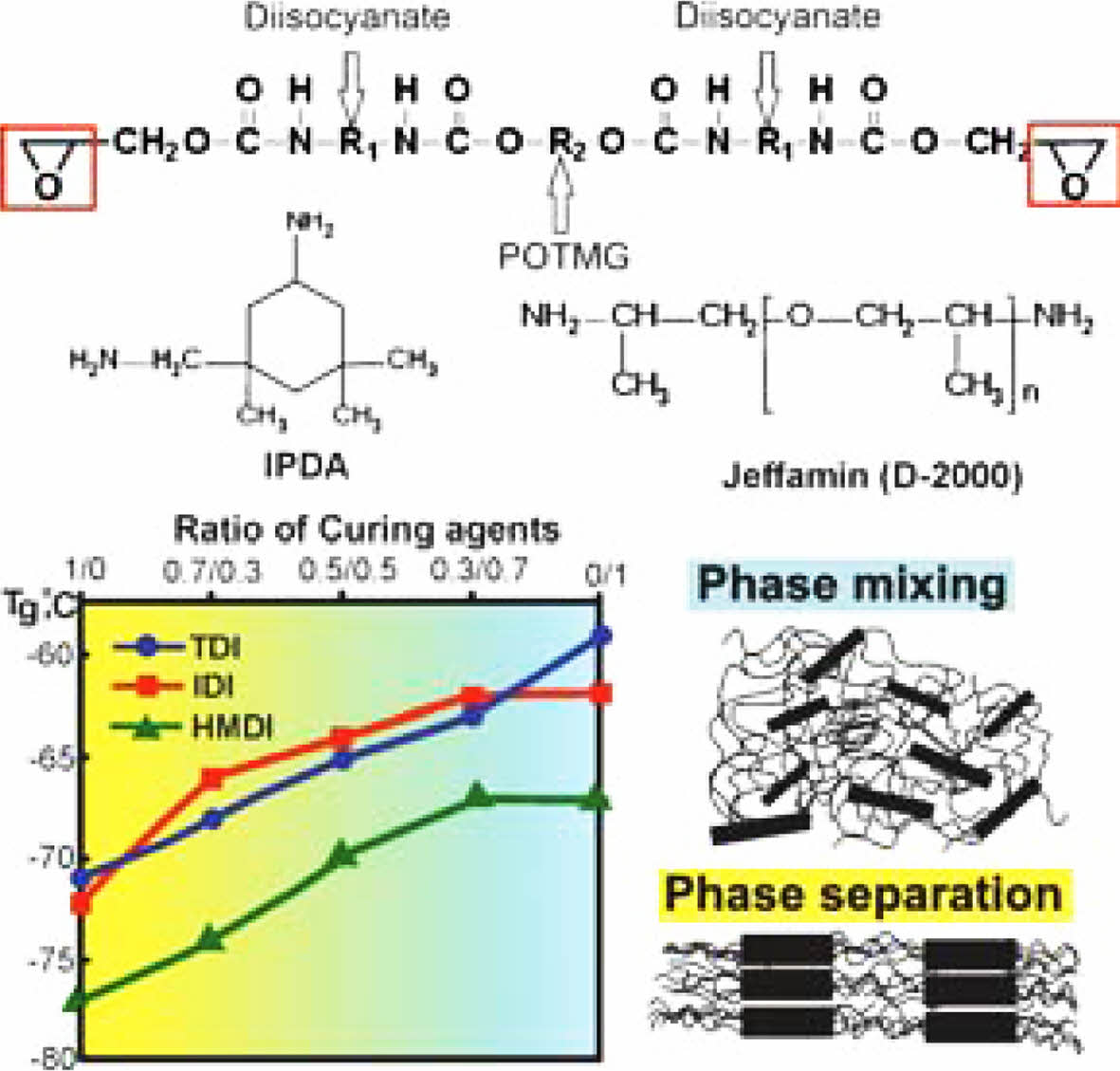
Keywords: hard segments, soft segments, Fourier transform infrared spectrum, swelling, polyurethane, oligoetherurethane diepoxide, network density, glass transition temperature, physical and mechanical properties, phase separation.
The work was carried out within the framework of the State Assignment (theme state registration number 122011900165-2) using the equipment of the Center for Collective Use “Investigations of materials and substances” of Perm Federal Research Center of the Ural Branch of the Russian Academy of Sciences.
The authors declare that there is no conflict of interest.
Polyurethane elastomers have been a wide class of polymeric materials with very useful properties and applicable in various fields of industry.1-4 However, such materials exhibit certain disadvantages associated primarily with poor adhesion to metals and high sensitivity to humidity in production areas. These disadvantages are because of the fact that the traditional curing system of polyurethane elastomers is interconnected with the reaction of hydroxyl or amine groups of a curing agent with free isocyanate groups of urethane prepolymers capable of interacting with atmospheric moisture as well.1-4 The transition to a more stable system for curing polyurethanes can be associated with use of urethane oligomers with functional epoxy groups, particularly oligoetherurethane diepoxides.5-11
The elastomers based on oligoetherurethane diepoxides cured with diamines are polyurethanes of a block structure, the chains of which consist of soft and hard segments (blocks).5 The soft segments consist of fragments of oligoethers, while the hard segments are the product of reactions between isocyanate groups, hydroxyl groups of epoxy alcohol, and amine groups of low molecular weight diamines used as curing agents.5 The difference in the polarity of soft and hard blocks can lead to the phase separation and the subsequent appearance of domains being localized phase formations.12-18 The possibility of phase separation can be affected by such factors as the chemical structure of blocks, their length, average molecular weight, as well as the formation of hydrogen bonds between soft blocks and hard ones.1-11,19-24 The domains of hard blocks can play a positive role in providing enhanced strength and strain properties of elastomers within certain concentration limits.1 Moreover, the highest strength of polyurethanes is realized only for samples with a high degree of the phase separation. The value attained in this case (45-50 MPa) is 7÷10 times higher over that of amorphous elastomers.4,16-18 This fact, however, applies to urethane-containing elastomers, in which the hard blocks are formed on the basis of urethane and urea fragments of polymer chains.3,4,16-18,20 Polymer chains of polyurethane elastomers based on OEUDE contain urethane hydroxyl hard blocks, which is the main difference from usual polyurethanes.5 Despite there being a relatively large number of works devoted to the study in the properties of such elastomers, the correlation of their physical and physic-mechanical properties with the phase- and supramolecular structure was not studied in full measure.4-15,22-28 This circumstance is not in line with the prospects of the practical application of these materials.
Concurrently, these elastomers were found as being potentially important for the development of frost-resistant binders and elastomers. In the course of the previous studies, the use of polyoxytetramethylene glycol as an oligoether with a molecular weight of 1400 g/mol was proven to enable obtaining elastomers with a low glass transition temperature and the absence of crystallization of soft blocks.11,19,20 However, the mentioned works were carried out using a limited number of diisocyanates, and this circumstance did assessing the development potential of this type of binder impossible.
This work is aimed at the study in the peculiarities of changes in the physical and physic-mechanical properties of polyurethane elastomers obtained by curing oligoetherurethane diepoxides, depending on the type of the diisocyanates used and the ratio between the used amine hardeners.
The initial components employed in the synthesis were: hydroxyl-containing oligoether-polyoxytetramethylene glycol (POTMG) with a molecular weight of 1400 g/mol (99.8% purity, Connect Chemicals, Germany), 2,4-Toluene diisocyanate (TDI) (99.98% purity, Covestro, Germany), 3-Isocyanatomethyl-3,5,5-trimethylcyclohexyl isocyanate (IPDI) (98% purity, Acros Organic, Belgium), or 1,6-Hexamethylene diisocyanate (HMDI) (99.9% purity, Alfa Aesar, USA).
Polyurethane elastomers were synthesized in three stages. In the first stage, urethane prepolymers were synthesized from an oligomeric diol and a diisocyanate (Figure 1(a)). In the synthesis, the molar ratio NCO/OH ≈ 2.03 was used. The reaction proceeded in a mixer under stirring for 7 hours at a temperature of 60-70 °C. To analyze the content of free isocyanate groups in obtained prepolymers (FR-10, FR-20, FR-30 in Table 1), the back titration technique was applied in accordance with ASTM D2572-19. By reason of a lower reactivity of aliphatic isocyanate groups,22 tin dibutyl dilaurate (DBDLO) (95% purity, Alfa Aesar, USA) as a catalyst was introduced into a series of FR-20 and FR-30 in an amount of 0.01 wt%.25
The mentioned prepolymers with terminal isocyanate groups reacted with an epoxy-containing alcohol (2,3-epoxypropanol, 98.5% purity, Russian Federation) with molar ratio OH/NCO≈2.03 to obtain the corresponding oligoetherurethane diepoxides (FR-11, FR-21 and FR-31). The synthesis was carried out under stirring at a temperature of 70 °C in the presence of the DBDLO catalyst in an amount of 0.02 wt%. The reaction between the urethane prepolymer and epoxy alcohol (Figure 1(b)) was completed 5 hours later (for FR-11) and 6 hours (for FR-21 and FR- 31). The completion of the urethane formation process was registered as the disappearance of the absorption band of free isocyanate groups at 2275 cm-1.26 The FTIR spectra of the samples were recorded on an IFS66/S Fourier spectrometer (Bruker, Germany). The content of free epoxy groups was determined by the back titration technique according to ISO 3001:1999. The components used in the synthesis of these oligomers are presented in Table 1.
In the third stage, oligoetherurethane diepoxides were cured with an amine-type curing agent to obtain cross-linked elastomer samples of three series (FR-12, FR-22, FR-32). The following curing agents were used: low molecular weight amine 3-aminomethyl-3,5,5-trimethylcyclohexylamine (IPDA) (99.4% purity, Huntsman, USA) and high molecular weight polyoxypropylenediamine (D-2000) (99.8% purity, Huntsman, USA). The choice of molar ratios of the curing agent was substantiated experimentally. The molar ratios of the curing system’s components used are presented in Table 2. The mode of the third stage of synthesis included stirring the initial components for 5 minutes under vacuum (1-2 kPa) at 50±1 °C, pouring into slot-type metal molds and thermostating the latter for 2 days at 90 °С.22
The glass transition temperature Tg of the samples was determined by differential scanning calorimetry (DSC) on a DSC 822e device (Mettler Toledo, Switzerland) at a scanning speed of 0.08 deg s-1, according to ISO 11357-2:1999. The glass transition temperature measurement error is 0.2 K. The samples were mechanically tested on an Instron 3365 universal testing machine (UK) at a temperature of 25±1 °C according to ISO 37-2013. There were determined the following parameters: conditional strength (σp), relative critical strain (εp), and conditional modulus at 100% elongation (E100).
According to the procedure described in 20, the effective network density (Ndx), the network density due to chemical bonds (Nx) and hard block domains (Nd) were determined through the tensile modulus of samples swollen in solvents or plasticizers, according to the formula (1):

where h0 is the height (length) of a non-swollen, non-deformed sample;
S is the slope of the linear part of the graph of force vs. strain;
R is gas constant;
T is absolute temperature;
A0 - cross-sectional area of the sample.
To determine the S parameter, ring-shaped samples 2 mm thick were cut out and underwent 30% stretching. There were carried out three parallel tests, with the average value of the slope of the curve taken for calculation.29
The equilibrium degree of swelling in solvents and plasticizers was calculated using eq. (2)

where m1 is the mass of the initial polymer, g; m2 is the mass of the polymer swollen to the equilibrium state, g;
From formula (3), the volumetric degree of swelling Qv was determined from the Qp value:

where ρ1 is the polymer density, g/cm3; ρ2 - solvent density, g/cm3.
The findings obtained were used to calculate the thermodynamic interaction parameter between the polymer and the solvent (plasticizer) χ1 using the Flory-Rehner eq. (4)29:
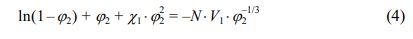
where N is the effective density of the elastic network limiting the swelling of the elastomer,
χ1 – Huggins parameter; V1 is the molar volume of the solvent (plasticizer), cm3/mol; φ2 is the volume fraction of the polymer in the equilibrium swollen sample, was determined by the formula (5):


|
Figure 1 Principal reaction scheme for epoxy-urethane oligomers. R1- diisocyanate fragment, R2- polyether fragment: (a) synthesis urethane oligomer with terminal isocyanate groups; (b) synthesis urethane oligomer with terminal epoxy groups. |
|
Table 1 Initial Components Used in the Synthesis of Oligomers and Intermediate Products |

|
Table 2 Physical and Mechanical Properties of Elastomers of the FR-12, FR-22 and FR-32 Series |

The curing of polyurethanes based on epoxy urethane oligomers gives rise to the appearance of some distinctive features of the final structure of their chains. The opening of the epoxy ring upon interaction with diamines by the polyaddition mechanism leads to forming hard segments with hydroxyl groups included in their composition.30 The examples of the chemical structure of hard segments in elastomers that can be formed during synthesis are shown in Figure 2(a) (the one based on the FR-11 oligomer and IPDA) and Figure 2(b) (the one based on the FR-11 oligomer and D-2000). The structures of possible hard segments based on FR-21 and FR-31 oligomers are similar.
FTIR spectroscopy and DSC methods were used to study the phase structure of polymers based on oligoetherurethane diepoxides.5 The results of spectral studies of polyurethane elastomers and model compounds were used to correlate the corresponding absorption bands and to qualitatively evaluate the phase separation in polyurethanes.7,13,24,25,27
FTIR Spectra Analysis. The presence of groups capable of forming hydrogen bonds in the polymer system affects the internal organization of domains, the orientation behavior of segments, and the mechanical properties of the polymer sample. As a rule, there are present several main types of hydrogen bonds in polyurethane elastomers that enable the structure of the resulting polymer to be characterized. These are hydrogen bonds between the amide group and the carbonyl group of the urethane fragments in Figure 3(a); these are the ones between the amide group of the urethane fragment and the oxygen of the ether group in Figure 3(b). Hydrogen bonds between the amide group and the oxygen of urethane group can be formed as well in Figure 3(c). The first and third types of bonds are characteristic of self-associates of urethane groups that are part of the domains of hard blocks. The second type of bond will be formed to a greater extent, with the soft and hard phases mixed.21
The FTIR spectra of the synthesized elastomers were analyzed in two characteristic absorption regions 3600-3000 cm-1 (absorption region of amide groups) and 1760-1600 cm-1 (absorption region of carbonyl groups). It should be noted that the FTIR spectra of the vibration region of amide groups are of a complicated character and described by the overlapping of several peaks. In fact, in the said regions there can be observable the absorption peaks of free amide (3430 cm-1), of amide bound to oxygen of urethane’s carbonyl groups (3295 cm-1), as well as the absorption peaks of free and bound hydroxyl groups (3550-3400 cm-1).7,21,26
The main difference between the FTIR spectra of the samples based on FR-11 oligomers and the ones based on FR-21 and FR-31 oligomers is a shift of the absorption band of the bound amide from 3330 to 3295 cm-1 (Figure 4(a)). At this point, when considering the vibration region of urethane groups’ carbonyl (the amide I band) at 1760-1600 cm-1, there can be noticeable that the samples produced with FR-11 oligomer (Figure 4(b)) are characterized by the peaks at 1730 cm-1 (in accord with vibrations of free carbonyl), and at 1700 cm-1 (vibrations of urethane groups’ carbonyl hydrogen-bound to amide groups). The markedly less intensity of the peaks at 1700 cm-1, as compared with the band at 1730 cm-1, permits the conclusion that, when using the FR-11 oligomer, the elastomers are characterized by the phase separation of a lower degree.
However, a different pattern of FTIR spectra is observed in the region of vibrations of amide and carbonyl groups for the other two series of elastomers FR-21 and FR-31 (Figures 5(a) and 5(b)). In this case, the bound amide band shifts to the region characteristic of the hydrogen bonded carbonyl of the urethane group (3330 cm-1), confirming the presence of a higher degree of the phase separation. At the same time, a fragment of the FTIR spectrum of the carbonyl region has a somewhat different character for the samples synthesized on the basis of FR-21 and FR-31. In this case, the intensity of the absorption band of the hydrogen-bonded carbonyl is much higher than that when using the FR-11 oligomer, but, as the intensity of the free amide is insignificant and there is free carbonyl present in the system, the system possibly contains hydrogen bonds between the amide group and the oxygen of the urethane alkoxy group.21 Thus, the use of oligoetherurethane diepoxides synthesized on the basis of an aliphatic and cycloaliphatic isocyanate leads to a high degree of phase separation, which may be due to the lower activity of aliphatic fragments of the polymer system, as a high intensity of the absorption band of the bound carbonyl of the urethane group is observed for the samples based on FR-31.
With the ratio between curing agents changing, the intensity of the band at 1700 cm-1 declines, that at 1730 cm-1 increases. However, the content of the bound amide practically either does not alter, or the alteration is insignificant. Thus, we can conclude that a decrease in the content of a high-molecular hardener in a mixture with IPDA leads to the formation of a structure with a higher degree of phase separation.
DSC Thermograms Analysis. An increase in the content of D-2000 in a mixture with IPDA evidently leads to the higher values of the glass transition temperature (Table 2). An increase in the glass transition temperature indicates an increase in the concentration of polar groups in the elastic matrix. Therein, there occurs a partial transition of hard segments into a relatively low-polarity oligoether matrix, indicating a decrease in the degree of phase separation. A similar dependence of the glass transition temperature on the molar ratio of hardeners is preserved for FR-22 and FR-32 series (Table 2). Thus, despite the degree of phase separation decreased, the use of POTMG with a molecular mass of 1400 g/mol maintains the segmental mobility of the polymer chain. The low level of the glass transition temperature of the synthesized elastomers below minus 60 °C enables preserving their elastic properties at low temperatures.
Effective Network Density. As mentioned above, the structure of polyurethanes is characterized by the presence of a large number of polar groups during the formation of a three-dimensional spatial network in these materials. In addition to cross chemical bonds, physical bonds of various nature are also formed, which contributes to the topological structure. As is known,14 for two-phase polyurethane elastomers, the total effective network density N can be represented by a combination of three contributions: the concentration of elastically active chains due to chemical crosslinks Nx, hard Nd domains, and the interchain physical bonds outside the domains Nh: N=Nx+Nd+Nh.
Determining the contribution of each parameter to the effective network density enables the overall topological structure of the synthesized elastomers to be characterized.
Interchain hydrogen bonds in elastomers swollen by more than 200% in solvents or plasticizers are almost completely destroyed, and the Nh parameter can be neglected.14 If, at the same time, the domains of hard blocks playing the role of additional nodes of the spatial network, remain stable, true is the equation N=Nx+Nd. However, when there are used solvents destroying hard domains, the Nd parameter can also be equated to zero, and this will allow one to calculate the network density due to Nx chemical bonds only. Thus, the presence of solvents or plasticizers as swelling agents that selectively break only physical bonds (due mainly to hydrogen bonds), or all the physical bonds, is absolutely necessary for the application of the above method.
To substantiate the application of toluene and tributyl phosphate (TBP) as swelling agents in the mentioned method, FTIR spectroscopy was accordingly used.14,15,18 The analysis of fragments of the FTIR spectra of FR-12 series, cured by IPDA, in the region of vibrations of the carbonyl of urethane groups (Figure 4, curve 3), shows the swelling in TBP to lead to the disappearance of the absorption band at 1702 cm-1, which characterizes H-bonds between hard blocks. The intensity of the band at 1730 cm-1 enhances. This band characterizes vibrations of free carbonyl.26 This proves that the use of TBP leads to the destruction of cross-links in the system due to domains of hard blocks.14,15 In case of swelling in toluene (Figure 6(b), curve 2), the intensity of this band remains unchanged, i.e. the molecular structure of the elastomer does not change. Elastomers of the series FR-22 and FR-32 behave similarly. Thus, the obtained results have proved the validity of the choice of toluene and TBP as swelling agents for the polymers under study. Thus, swelling in TBP allows estimating the contribution of chemical bonds to the total network density. Swelling in toluene allows estimating the sum of the above contribution and that of hard blocks.
Interactions in the soft phase and phase separation with the formation of domains of hard blocks, which plays the role of a reinforcing filler, significantly affect the physical and mechanical properties of polyurethanes.10 As is evident from the data in Table 2, the contribution of hard blocks to the total effective network density in the obtained elastomers does not exceed 20% for elastomers of the FR-12 and FR-22 series with the IPDA/D-2000 molar ratio equaling 1/0. A further increase in the content of D-2000 in a mixture with IPDA reduces the contribution of hard domains to the concentration of elastically active chains. It very nearly equals zero, and at a molar ratio of IPDA/D-2000 equals 0.5/0.5. With an increase in the mole fraction of D-2000 in a mixture with IPDA, the density of the network due only to chemical bonds decreases. This value decreases by 5.2 times for elastomers based on FR-11, by 2 times for the FR-31 series, by 1.4 times for elastomers based on the FR-21 series.
Thermodynamic Affinity and Polarity of Polymers. A successive alteration of the ratio between the curing agents used in the work changes the overall polarity of the polymer chain, which can be characterized by the equilibrium swelling method. Using this method, there were obtained the values of the Huggins parameter characterizing the thermodynamic affinity of a polymer to a solvent.
According to the data obtained (Table 2), with increasing content of D-2000 in the curing mixture, a decrease in the Huggins parameter is observed for both TBP and toluene for all the series: it changes by 0.14-0.16 for FR-12; by 0.08 for FR-22, and by 0.05-0.12 for FR-32. This indicates an increase in the thermodynamic affinity of the final elastomer to solvents. This effect can be associated with a decrease in the polarity of the resulting polymer matrix with IPDA substituted by D-2000.
This conclusion can be confirmed by considering the problem of thermodynamic interaction between a polymer and a solvent using the concept of solubility parameters, according to which the larger the difference between the values of the solubility parameter of a polymer to a solvent, the lower the thermodynamic affinity between them.28,29,31,32
There was made a preliminary assessment of the possible influence of the structure of amine hardeners on the polarity of the resulting polymer chains. The solubility parameters of IPDA and D-2000 were calculated according to the Askadsky increments method28; these values were equal to 20.86 (MJ/m3)0.5 for IPDA, and 17.80 (MJ/m3)0.5 for D-2000. The obtained calculation data enabled expecting a higher polarity of urethane fragments in polymer chains in IPDA-cured elastomers as compared with D-2000-cured analogs.
The value of the solubility parameter is 18.2 (MJ/m3)0.5 for toluene,33 and that for TBP is 17.5 (MJ/m3)0.5.34 The solubility parameter of oligoethers based polyurethanes can be estimated about 19.4÷19.9 (MJ/m3)0.5. Therefore, a decrease in the polarity of the polyurethane polymer chain with an increase in the content of D-2000 should be accompanied by a decrease in the solubility parameter of a polymer, and accordingly, an increase in the thermodynamic affinity of a polymer to a solvent. This assertion is observed practically.
Physic-mechanical Properties of Elastomers. As is evident from the data obtained (Table 2), the structure of the used diisocyanates markedly affects the physical and mechanical properties of the final polyurethanes. The highest strength was obtained for the elastomers based on IPDI (FR-22 series). For this entire series, the contribution of Nd reaches 19%, which has a positive effect on both modulus and strength. Figure 7 shows the stress versus strain plots for all the obtained elastomers.
As for the IPDA-based elastomers (the fraction of network density due to hard blocks domains is about 20%), the stress versus strain plots are characterized by the presence of a hardening effect at the final stage of tension, while for FR-22 series elastomers this effect is more pronounced. The absence of this effect for the FR-32 series and the significantly worse strain characteristics are the reason for their lower strength. The results obtained correlate with the previously obtained effect of tensile strengthening of polyurethane ureas based on IPDI.16,17 As the contribution of hard block domains to the effective network density decreases (with an increase in the fraction of D-2000), the strengthening effect disappears.
The results of interest were obtained for the FR-12 series: with a successive increase in the molar fraction of IPDA in a mixture with D-2000, a sharp decrease in the relative critical strain occured, but an increase in the conditional modulus led to an increase in the conditional strength by 4.7 times (Table 2). A similar dependence of physical and mechanical properties on the molar ratio of curing agents was also observed for the FR-22 and FR-32 series. Table 2 shows that an increase in the molar fraction of D-2000 in a mixture with IPDA reduces the conditional strength by almost 5 times for the FR-22 series, and 2.3 times for the FR-32 one. The elastomers of the FR-12 and FR-22 series cured by IPDA have the most optimal physical and mechanical properties.
Thus, the method used in the work for regulating the chemical structure of polymer chains of cross-linked elastomers enabled the relationship between the chemical structure and phase separation in the studied materials and the complex of their most important physical and mechanical characteristics to be determined. Such materials can be used as a wide range of binders, especially promising for use at low temperatures

|
Figure 2 Structure of hard segments obtained using the FR-11 oligomer and curing agents: (a) IPDA; (b) D-2000. |

|
Figure 3 Characteristic hydrogen bonds in polyurethanes. |

|
Figure 4 Fragments of FTIR spectra in (a) the field of amide vibrations; (b) the field of carbonyl ones for samples based on the oligoetherurethane diepoxides: 1 - FR-11, 2 - FR-21 3 - FR-31 and IPDA. |

|
Figure 5 Fragments of FTIR spectra in (a) the region of amide vibrations; (b) in the region of carbonyl ones for samples based on the FR-11 oligomer and a mixture of amines IPDA and D-2000, taken in various molar ratios: 1 - 1/0; 2 - 0.5/0.5; 3 - 0 /1. |

|
Figure 6 Fragment of the FTIR spectra of the elastomer based on FR-12 with IPDA: 1 - before swelling; 2 - after swelling in toluene; 3 - after swelling in TBP. |

|
Figure 7 Stress versus strain plots for elastomers of three series for various molar ratios between IPDA and D-2000 (mole fractions) in the mix of curing agents: FR-12 (a); FR-22 (b); FR-32(c). Plots 1 corresponds to 1/0 ratio, 2 - 0.7 /0.3, 3 - 0.5 /0.5, 4 - 0.3/0.7, 5 - 0/1. |
The appearance of the phase separation in elastomers based on oligoetherurethane diepoxides, whose chemical structure was characterized by the presence of urethane hydroxyl hard segments, was determined for all investigated samples cured with isophorone diamine, with this separation being absent for the samples cured with D-2000 diamine.
There was made an analysis of the influence of the effective network density and its components on the physical and mechanical properties of the mentioned elastomers synthesized using polyoxytetramethylene glycol with a molecular weight of 1400 g/mol and 2,4-toluene diisocyanate, 3-isocyanato- methyl-3,5,5-trimethylcyclohexyl isocyanate, and 1,6-hexamethylene diisocyanate. The effective network densities and their components were calculated using experimental data for swelling in toluene and tributyl phosphate as swelling agents. The Nd component (contribution of hard blocks in the network density) was found to attain a value of 0.014-0.016 kmol/m3. With increasing content of the D-2000 diamine in the mixture of hardeners, this component drastically decreases, which reflects a decrease in the degree of phase separation, a decrease in the contribution of hard blocks to the properties of polymers, and leads to a corresponding decrease in the strength.
An increase in the content of the D-2000 diamine in the hardeners mix leads to the glass transition temperature increase due to the transition of a part of the polar hard segments formed by urethane hydroxyl fragments of polymer chains from hard blocks to a soft polymer matrix.
The use of oligomers based on 3-isocyanatomethyl-3,5,5-trimethylcyclohexyl isocyanate enabled the best physical and mechanical properties of the synthesized polyurethane elastomers to be attained.
- 1. Hepburn, C. Polyurethane Elastomers; 2nd ed. Elsevier Science Publishers Ltd.: London, 2012.
- 2. Prisacariu, C. Polyurethane Elastomers from Morphology to Mechanical Aspects. Springer: New York, 2011.
- 3. Randall, D.; Lee, S. The Polyurethanes Book; Wiley: New York, 2003.
- 4. Saunders, J. H.; Frish, K. G. Polyurethanes. John Wiley and Sons: New York - London, 1968.
- 5. Omel'chenko, S. I.; Kadurina, T. I. Modified Polyurethanes. Naukova Dumka: Kyiv, 1983.
- 6. Harkal, U. D.; Muehlberg, A. J.; Edwards, P. A.; Webster, D. C. Novel Water-dispersible Glycidyl Carbamate (GC) Resins and Waterborne Amine-cured Coatings. J. Coat. Technol. Res. 2011, 8, 735-747.
-

- 7. Hsia, H. C.; Ma, C. C. M.; Chen, D. S. Adhesion Properties and Phase Separation Behavior of Glycidyl‐terminated Polyurethanes. Macromol. Mater. Eng. 1994, 220, 133-149.
- 8. Harkal, U. D.; Muehlberg, A. J.; Li, J.; Garrett, J. T.; Webster, D. C. The Influence of Structural Modification and Composition of Glycidyl Carbamate Resins on Their Viscosity and Coating Performance. J. Coat. Technol. Res. 2010,7, 531-546.
-

- 9. Makarova, M. A.; Slobodinyuk, A. I.; Tereshatov, V. V.; Volkova, E. R.; Kisel'kov, D. M. Elastic and Moisture-resistant Compounds Based on Mixtures Off Oligotetraurethane Diepoxide, Epoxydiane Resin, and Aromatic Diamine. Polym. Sci. Series D 2014, 7, 166-169.
-

- 10. Sidorov, O. I.; Kapustin, S. A.; Shragin, D. I.; Muzafarov, A. M.; Sidorova, N. I.; Pleshakov, D. V. The Influence of an Organosilicon Modifier on the Properties of a Compound Based on Epoxy-urethane Oligomers. Polym. Sci. Series D 2017, 10, 322-325.
-

- 11. Senichev, V. Yu.; Slobodinyuk, A. I.; Slobodinyuk, D. G.; Savchuk, A. V.; Kulakova, M. V.; Oshchepkova, T. E.; Borisova, I. A.; Dolinskaya, R. M. Frost-Resistant Elastomers with Controllable Microphase Segregation, Based on Epoxy-Ether-Urethane Oligomers. Russian J. Appl. Chem. 2020, 9, 1172-1178.
-

- 12. Tereshatov, V. V.; Senichev, V. Y. Stress-strain Dependence of Segmented Polyurethanes and Polyurethane Ureas. J. Macromol. Sci. Part B 2015, 54, 365-380.
-

- 13. Tereshatov, V. V. Influence of Strong Bonding with Impenetrable Substrate on Swelling of Elastomers in Low-Molecular-Weight Liquids. Russian J. Appl. Chem. 2010, 83, 1341-1344.
-

- 14. Tereshatov, V. V.; Tereshatova, E. N.; Volkova, E. R. Two Types of Physical Networks in Cross-linked Segmented Polyurethanes. Polym. Sci. 1995, 37, 1881-1887.
- 15. Tereshatov, V. V.; Tereshatova, E. N.; Makarova, M. A.; Tereshatov, S. V. Influence of Chemical Structure and Composition of Mixed Soft Segments on the Properties of Elastomers with Urethane-urea Hard Blocks. Polym. Sci. A 2002, 44, 275-281.
-

- 16. Tereshatov, V. V.; Vnutskikh, Z. A.; Slobodinyuk, A. I.; Makarova, M. A.; Senichev, V. Y. New Multi-block Isophorone Diisocyanate-based Copolymers with Urethane Urea Hard Segments. J. Elastomers Plast. 2016, 4, 289-304.
-

- 17. Tereshatov, V. V.; Makarova, M. A.; Senichev, V. Y.; Volkova, E. R.; Vnutskikh, Z. A.; Slobodinyuk, A. I. The Role of the Soft Phase in the Hardening Effect and the Rate Dependence of the Ultimate Physico-mechanical Properties of Urethane-containing Segmented Elastomers. Colloid Polym. Sci. 2015, 1, 153-164.
-

- 18. Tereshatov, V. V.; Makarova, M. A.; Senichev, V. Y.; Slobodinyuk, A. I. Interrelationship Between Ultimate Mechanical Properties of Variously Structured Polyurethanes and Poly(urethane urea) s and stretching rate thereof. Colloid Polym. Sci. 2012, 7, 641-651.
-

- 19. Strel'nikov, V. N.; Senichev, V. Yu.; Slobodinyuk, A. I.; Savchuk, A. V.; Volkova, E. R.; Makarova, M. A.; Nechaev, A. I.; Krasnosel’skikh, S. F.; Ukhin, K. O. Preparation and Properties of Frost-resistant Room-temperaturecurable Compounds Based on Oligoethertetraurethane Diepoxides of Various Chemical Structures. Russian J. Appl. Chem. 2018, 91, 463-468.
-

- 20. Strel'nikov, V. N.; Senichev, V. Y.; Slobodinyuk, A. I.; Savchuk, A. V.; Volkova, E. R. Frost-resistant Epoxy-urethane Binders Containing Diglycidyl Urethane. Int. J. Polym. Sci. 2019, 2019.
-

- 21. Vatulev, V. N.; Laptii, S. V.; Kercha, U. U. Infrared Spectra and Structure of Polyurethanes. Naukova Dumka: Kyiv, 1987.
- 22. Sun, W.; Yan, X.; Zhu, X. The Synthetic Kinetics and Underwater Acoustic Absorption Properties of Novel Epoxyurethanes and Their Blends with Epoxy Resin. Polym. Bull. 2012, 69, 621-633.
-

- 23. Fedoseev, M. S.; Devyaterikov, D. M.; Rybina, G. V.; Meshechkina, A. E. Curing of Oligo (diene-isoprene-urethane-epoxy) Oligomer in the Presence of an Active Plasticizer, 1,2-epoxycyclopentane, and of Its Adducts with Imidazoles. Russian J. Appl. Chem. 2013, 86, 1441-1446.
-

- 24. Strel'nikov, V. N.; Senichev, V. Yu.; Slobodinyuk, A. I.; Savchuk, A. V.; Volkova, E. R.; Makarova, M. A.; Belov, Yu. L.; Derzhavinskaya, L. F.; Selivanova, D. G. Preparation and Properties of Frost-Resistant Materials Based on Compounds of Oligoether Urethane Epoxides and Diglycidyl Urethane. Russian J. Appl. Chem. 2018, 91, 1937-1944.
-

- 25. Bakirova, I. N.; Kirillova, A. S. Effect of Organometallic Catalysts on the Synthesis Process and Properties of Molded Polyurethane. Russian J. Appl. Chem. 2013, 86, 1399-1403.
-

- 26. Socrates, G. Infrared and Raman Characteristic Group Frequencies:Tables and Charts; John Wiley & Sons: Chichester, New York, 2004.
-

- 27. Senichev, V. Y.; Slobodinyuk, A. I.; Makarova, M. A.; Savchuk, A. V.; Pogoreltsev, E. V. The Effect of Hard Segments Structure on the Functional Properties of Polyurethane Elastomers Based on Oligoesterurethane Epoxides. J. Phys.: Conf. Ser. 2019, 1399, 044060.
-

- 28. Bagdi, K.; Molnar, K.; Wacha, A.; Bota, A.; Pukánszky, B. Hierarchical Structure of Phase‐separated Segmented Polyurethane Elastomers and Its Effect on Properties. Polym. Int. 2011, 60, 529-536.
-

- 29. Van Krevelen, D. W. Properties of Polymers: Their Correlation with Chemical Structure; Their Numerical Estimation and Prediction from Additive Group Contributions. 4th ed., Elsevier: Amsterdam, 2009.
- 30. Malkin, A. Ya.; Begishev, V. P. Chemical Molding of Polymers; Moscow, Khimia, 1991.
- 31. Redman, R. P. Developments in Polyurethane-1. Applied Science Publishers Ltd.: London, 1978.
- 32. Askadskii, A. A. Computational Materials Science of Polymers. Cambridge Int Science Publishing: Cambridge, 2003.
- 33. George, W. Handbook of Solvents, Volume 1: Properties, 3rd ed.; Elsevier: Amsterdam, 2019.
- 34. Mills, A.; Monaf, L. Thin Plastic Film Colorimetric Sensors for Carbon Dioxide: Effect of Plasticizer on Response. Analyst 1996, 121, 535-540.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2022; 46(6): 766-774
Published online Nov 25, 2022
- 10.7317/pk.2022.46.6.766
- Received on Jun 24, 2022
- Revised on Aug 22, 2022
- Accepted on Aug 24, 2022
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Conflict of Interest
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Anna V. Savchuk
-
Institute of Technical Chemistry of Ural Branch of the RAS as Affiliated Branch of Perm Federal Research Center of Ural Branch of the RAS Acad., Korolev St. 3, 614013 Perm, Russian Federation
- E-mail: ataraksa@mail.ru
- ORCID:
0000-0002-0474-9735









 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.