- Electrochemical Deposition of Polyaniline on Nanopillar Array Films for High-performance pH Sensors
Seo Jin Kim# , Sung Tae Jang# , Kyoung G. Lee*, and Bong Gill Choi†

Department of Chemical Engineering, Kangwon National University, Samcheok 25913, Korea
*National Nanofab Center, Center for Nano Bio Development, 291 Daehak-ro, Yuseong-gu, Daejeon 34141, Korea- 고성능 pH 센서를 위한 나노필라 필름의 폴리아닐린 전기증착
강원대학교(삼척) 화학공학과, *나노종합기술원 나노바이오센터
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
We developed a flexible polyaniline film with a one-dimensional nanopillar array to achieve high electrochemical performance of a pH sensor. Soft lithography was used to prepare a highly ordered nanopillar array substrate. Gold was deposited on the nanopillar surface using a sputtering process to provide high electrical conductivity. Electropolymerization of aniline monomers was next performed on the gold nanopillar arrays. The electrodeposition of polyaniline was systematically investigated by controlling the scan rate and cycle number using a cyclic voltammetry technique. Highly ordered nanopillar arrays of polyaniline films were obtained under optimized conditions. The as-obtained electrode exhibited excellent electrochemical sensing performance, including sensitivity, repeatability, selectivity, and long-term stability. In addition, the sensing performance of the electrode was maintained even under a mechanically bent state.
본 연구에서는 pH 센서의 우수한 전기화학적 성능을 위하여 1차원적 나노필라 어레이를 가진 유연한 폴리아닐린 필름 전극을 개발하였다. 소프트 리소그래피 방법을 사용하여 정밀하게 배열된 나노필라 어레이 기판을 제조하였다. 높은 전기적 전도성을 위해 스퍼터링 공정을 통한 나노필라 표면에 금을 증착시켰다. 이후, 금 나노필라 어레이 필름에 아닐린 단량체를 전기화학적으로 중합을 시켰다. 순환 전압전류법을 통한 폴리아닐린의 전기 증착은 주사 속도 및 순환 횟수를 조절함으로써 체계적으로 연구되었다. 상기 최적화 실험을 통하여 정밀하게 배열된 폴리아닐린 나노필라 어레이 전극을 제조하였다. 제작된 전극은 민감도, 반복성, 선택성, 내구성을 포함하여 우수한 전기화학적 센서 성능을 나타내었다. 또한, 기계적으로 구부러진 상태에서 전극의 센서 성능이 유지됨을 확인하였다.
A highly ordered nanopillar electrode of polyaniline was prepared by an electropolymerization of aniline monomers on gold nanopillar film. The as-obtained polyaniline electrode exhibited excellent electrochemical performances of sensitivity, response time, repeatability, and long-term stability.
Keywords: polyaniline, nanopillar, electrochemistry, pH sensor, electropolymerization.
This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Ministry of Science and ICT (No. 2021R1A2C1009926); the Technology Innovation Program (or Industrial Strategic Technology Development Program) (20015577) funded By the Ministry of Trade, Industry & Energy (MOTIE, Korea); and the Nanomedical Devices Development Project of NNFC in 2022.
The authors declare that there is no conflict of interest.
For good health, the pH level in the human body is tightly maintained by the primary mechanisms of a buffer system, respiratory system, and renal control.1 The pH of the blood is in a range of 7.34-7.45, but the pH of the human body is different. For example, natural human skin is mildly acidic (pH 4.7-5.75). The oral cavity has a pH of 6.8-7.5, whereas the pH of the stomach cavity is in the range of 1.5-2.0.2 Minor alterations in these ranges have severe implications. Thus, variations in pH are indicative of physiological problems such as ischemia, atherosclerotic plaque development, inflammation, and tumor growth. In particular, in the rapidly growing cosmetic industry, pH maintenance plays a major role in skin conditions. The acidic environment of the skin mantle provides a protective layer that inhibits bacterial growth and facilitates wound healing. The imbalance of pH in the skin (e.g., alkaline range) leads to dry and hypersensitive skin, making it susceptible to infections and diseases (e.g., atopic dermatitis).3
Electrochemical potentiometry has been widely used in the detection of hydronium ions in solutions because of its simple operation, low cost, efficient maintenance, and reliable sensing performance.4-6 The pH of solutions is measured by the difference in the electromotive force between the working and reference electrodes based on the Nernstian equation.5-10 A commercially available pH meter combined with a glass body can accurately measure the pH in various solution samples with a wide measuring range of pH 0-14 and a temperature range of 0-100 oC.11 However, this type of pH meter is too bulky and brittle, making it unsuitable for wearable human body sensors. Flexible polyaniline films have attracted considerable attention as potential pH sensing materials. Simple and efficient electropolymerization of aniline monomers enables the deposition of polyaniline on gold or carbon-based electrodes. The protonation and deprotonation of the polyaniline chains between emeraldine salt and emeraldine base forms induce pH-sensitive behavior in acidic or alkaline media.12,13 The resultant polyaniline electrodes are lightweight and flexible and are thus suitable for wearable pH sensors on the human body. Considering the small sample volumes and curved surfaces of the human body, pH sensors should be more flexible and miniaturized to achieve high sensing performance.14
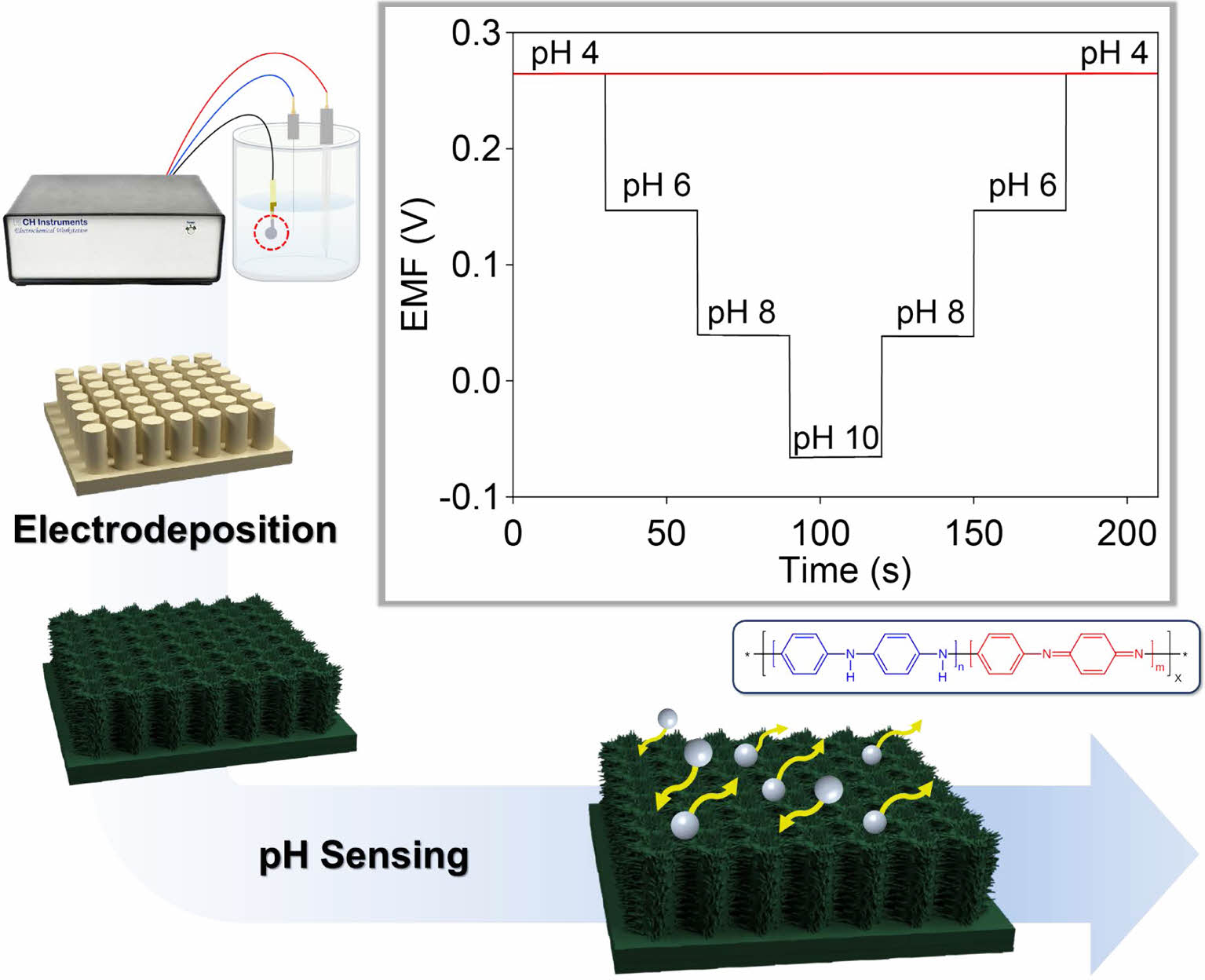
Herein, we developed a method for preparing polyaniline-deposited nanopillar array electrodes for high-performance pH sensors. Soft lithography was used to fabricate a highly flexible nanopillar array of a polymer film. After gold (Au) was deposited onto the nanopillar using the sputtering process, electropolymerization of aniline monomers was performed on the Au nanopillar arrays. Our previous report described that the electrodeposited polyaniline electrode based on one-dimensional array resulted in a high sensitivity for pH detection.15 However, this sensor suffered from reliability and repeatability, which are very important factors for the applications of pH sensors. In this work, the electropolymerization method and sensing performances were systematically optimized by controlled cyclic voltammetry technique. The as-obtained polyaniline nanopillar array (PNA) electrode exhibited excellent electrochemical sensing performance, including sensitivity, reliability, repeatability, selectivity, and long-term stability.
Reagents and Materials.Aniline, sodium chloride, potassium chloride, calcium chloride, sulfuric acid, and polyurethane were obtained from Sigma-Aldrich (USA). Norland Optical Adhesive 63 (NOA63) was obtained from Norland Products, Inc., USA.
Fabrication of PNA Electrode.Before the PNA electrodes were prepared, Au-coated nanopillar array (ANA) films were fabricated by photo- and soft lithography according to the previously reported methods.16-18 A polymeric nanopillar film was prepared by spin-coating a mixture of polyurethane and NOA 63 onto a silicone nanohole master mold. Following UV-assisted polymerization and curing, the polymer films were peeled off the mold. The as-obtained polymeric nanopillar film was coated with a gold layer using a sputtering process. The ANA films exhibited diameters of 500 nm and heights of 1.25 μm. The PNA electrodes were prepared through electrochemical polymerization of aniline monomers onto the surface of the ANA films. The polyaniline was electrochemically deposited on the ANA film using the cyclic voltammetry technique with a controlled number of scans (30 and 50 cycles) and scan rates (50 and 100 mV/s) at a potential of -0.2 to +0.8 V. A three-electrode system was set up with an Ag/AgCl reference and Pt wire counter electrodes. A 0.5 M aniline monomer dissolved in 1 M H2SO4 was used as an electrolyte.
Characterization.Scanning electron microscopy (SEM) images were obtained using a field-emission scanning electron microscope (JSM-6701F, JEOL, Ltd., Japan). Fourier-transform infrared (FTIR) spectra were collected using a JASCO FTIR 4600 spectrometer (Japan). Each spectrum was recorded from 3000 to 500 cm-1. Electrochemical characterization was performed using a CHI 760E instrument (CH Instruments, Inc., USA). The pH sensing performances of the PNA electrodes was investigated using a potentiometric technique. The electromotive force (EMF) signals between the PNA and Ag/AgCl electrodes were measured at pH values of 4-10. Note that the use of different electrodes (i.e., PNA and Ag/AgCl) in electrochemical characterization affects EMF responses.
One-dimensional polyaniline-structured arrays of PNA electrodes were prepared by electropolymerization of aniline monomers onto ANA films (Figure 1(a)). The Au substrate was fabricated using a combination of soft lithography and support processes. The polyaniline sensing materials were electrochemically synthesized on the ANA film by cyclic voltammetry with a controlled number of scans (30 and 50 cycles) and scan rates (50 and 100 mV/s) at a potential of -0.2 to +0.8 V under a 3-electrode cell (Figure 1(b)). As the CV scan increased, aniline monomers were gradually deposited on the ANA surface through oxidation and reduction reactions. These prominent redox peaks indicated the successful synthesis of polyaniline films. To investigate the effect of the scan rate and number of cycles on the electrochemical performance of the PNA electrodes, the electrode samples were prepared based on the scan cycles of 30 and 50 cycles (fixed at a scan rate of 50 mV/s) and a scan rate of 100 mV/s (fixed at a scan cycle of 50). The resulting three polyaniline electrodes were labeled as 30c-PNA, 50c-PNA, and 100m-PNA electrodes, respectively. Figure 1(c-e) shows typical SEM images of PNA films with a highly arranged one-dimensional morphology. Following electrodeposition of polyaniline, the 30c-PNA electrode exhibited a thin and uniform polyaniline film with ordered nanopillar structures (Figure 1(c)). As the number of scan cycles increased, a thick film of polyaniline was deposited onto the nanopillar array (SEM image of Figure 1(d) for 50c-PNA). The increase in scan rate and number of cycles resulted in the undesirable deposition of polyaniline covering the nanopillar structure in the 100m-PNA electrode (Figure 1(e)).
Figure 2 shows the FTIR spectra of ANA (based on poly- urethane) and PNA films. Compared to the bare polyurethane-based ANA film, the PNA film exhibited prominent peaks at 1563, 1450, 1281, 1125, 1025, and 718 cm-1, which were assigned to C=C stretching in the quinoid ring, C=C stretching in the benzenoid ring, C-N stretching in the benzenoid ring, C-N stretching in the quinoid ring, and C-H out-of-plane bending, respectively.19 Based on these bands, the synthesized poly- aniline had an emeraldine salt structure (inset of Figure 2). This result indicated that electropolymerization using cyclic voltammetry induced polymerization of aniline monomers onto the Au surface.
The pH sensing performances of the PNA electrodes were evaluated by measuring the potentiometric EMF responses of the electrodes immersed in a pH range of 4-10. A commercial Ag/AgCl electrode was used as a reference electrode. Figure 3(a) shows the cascade EMF responses of the PNA electrodes with increasing pH. The stable EMF values obtained for the 30c-PNA, 50c-PNA, and 100m-PNA electrodes were calibrated as a function of pH, as shown in Figure 3(b). Detailed EMF and sensitivity values for the different PNA electrodes are listed in Table 1. The 30c-PNA electrode exhibited a low sensitivity of 47.8 mV/pH with a poor coefficient of determination (R2=0.969). This is attributed to the inferior coating of polyaniline on nanopillar arrays. By contrast, the 50c-PNA electrode had an ideal Nernstian sensitivity of 56.0 mV/pH with high R2=0.999. With an increasing scan rate of 100 mV/s, 100m-PNA exhibited a sensitivity of 54.2 mV/pH and R2= 0.999. The decreased sensitivity value of 100m-PNA compared to the 50c-PNA is attributed to the thick polyaniline layer and reduced surface area of 100m-PNA. Based on these results, for the 50c-PNA electrode, the optimal electropolymerization condition between the scan cycle and rate was 50 cycles of cyclic voltammetry at a scan rate of 50 mV/s.
The 50c-PNA electrode was tested to investigate its reproducibility, response time, repeatability, selectivity, and long-term stability. Five samples of 50c-PNA were tested with results showing an average sensitivity of 54.8 mV/pH with a small standard deviation of 0.0004 (Figure 4(a)). The response time of 50c-PNA was tested by measuring the EMF signals at pH values in a range of 4 to 8 (Figure 4(b)). The response time of 10 s received a higher evaluation, indicating a fast sensing response for the pH sensors. Figure 4(c) shows the pH titration cycles of the 50c-PNA electrode at pH values of 4, 6, 8, and 10. Following the titration cycles, the EMF signals were successfully recovered to their initial values, indicating good repeatability of the 50c-PNA electrode. In general, the pH sensor should accurately and selectively measure the pH levels in the presence of interfering ions. The selective ability of the 50c-PNA electrode was evaluated by measuring the EMF responses of 50c-PNA by adding interfering ions of NaCl, KCl, and CaCl2 (0.1 M) in a pH 4 buffer solution (Figure 5(a)). After other ions were added, the 50c-PNA electrode did not affect the electrochemical sensing performance. The 50c-PNA electrode responded spontaneously with a pH buffer solution of decreasing pH from 4 to 2. Finally, the sensing stability of the 50c-PNA was tested by measuring the change in the EMF signals over a long period of 10 h. The 50c-PNA electrode exhibited a small change in the EMF signal (0.049 mV/h), indicating good long-term stability.
In general, the 50c-PNA electrode should be simultaneously sensitive and flexible for application as a pH sensor in wearable devices. The electromechanical properties of the 50c-PNA film were investigated by measuring the resistance change in a range of bending angles of 0-180o (Figure 6(a)). The 50c-PNA film exhibited small resistance variations of 1.04, 1.04, and 1.06 for 0, 90, and 180o, respectively. The change in the sensing performance of the 50c-PNA electrode was investigated by evaluating the sensitivity of the 50c-PNA electrode under different mechanical deformations of normal and bent states (Figure 6(b)). The 50c-PNA electrode exhibited nearly overlapping EMF responses under different mechanical states. The sensitivities of the 50c-PNA electrode were evaluated as 51.1 and 51.2 mV/pH under normal and bent states, respectively. The variation in sensitivity under both mechanical states of the 50c-PNA electrode was 0.11.

|
Figure 1 (a) Scheme of preparation of an PNA electrode using the electrochemical deposition method; (b) CV curves of aniline monomers dissolved in 1 M H2SO4. SEM images of (c) 30c-PNA; (d) 50c-PNA; (e) 100m-PNA. |

|
Figure 2 FTIR spectra of ANA and 50c-PNA electrodes. |

|
Figure 3 (a) EMF responses; (b) calibration curves of 30c-PNA, 50-cPNA, and 100m-PNA electrodes for pH values increasing from 4.0 to 10.0. |

|
Figure 4 (a) pH calibration curves of 50-cPNA electrodes (N=5) measured with pH values increasing from 4.0 to 10.0; (b) response time and repeatability of the 50-cPNA electrode. |

|
Figure 5 (a) Selectivity test of the 50-cPNA electrode when interfering ions of 0.1 M NaCl, 0.1 M KCl, and 0.1 M CaCl2 are added to a pH 4 buffer solution; (b) EMF responses of the 50-cPNA electrode measured in pH 8 for 10 h. |

|
Figure 6 (a) Resistance change of the 50-cPNA electrode with different bending angles of 0-180o ; (b) EMF responses of the 50-cPNA electrode under mechanically normal and bent states. |
|
Table 1 EMF, Eo , and Sensitivity Values of PNA Electrodes Measured at Different pH Solutions |

We developed a highly flexible polyaniline electrode based on a conductive nanopillar array film for use in high-performance pH sensors. The polyaniline sensing materials were electrochemically deposited onto the surfaces of the nanopillars with a uniform and conformal coating. The electrochemical sensing performance of the as-obtained PNA electrodes were investigated by measuring the EMF responses of the PNA and Ag/AgCl reference electrodes using a potentiometric technique. The 50c-PNA electrode exhibited excellent potentiometric performance in detecting pH levels in solution, including sensitivity, response time, selectivity, repeatability, reproducibility, and stability. In addition, the sensing performance of the 50c-PNA electrode was maintained even under a mechanically bent state. The electrochemical and mechanical properties of PNA electrodes thus show great potential for use in wearable and flexible pH sensors.
- 1. Aoi, W.; Zou, X.; Xiao, J. B.; Marunaka, Y. Body Fluid pH Balance in Metabolic Health and Possible Benefits of Dietary Alkaline Foods. eFood. 2020,1, 12-23.
-

- 2. Proksch, E. pH in Nature, Humans and Skin. J. Dermatol. 2018, 45, 1044-1052.
-

- 3. Ali, S. M.; Yosipovitch, G. Skin pH: from Basic Science to Basic Skin Care. Acta Derm Venereol. 2013, 93, 261-267.
-

- 4. Singh, E.; Kumar, U.; Srivastava, R.; Yadav, B. C. Catalytic Growth of MWCNT Using CVD and Its Application as Opto-electronic Humidity Sensor. Carbon Lett. 2020, 30, 215-224.
-

- 5. Nyein, H. Y. Y.; Gao, W.; Shahpar, Z.; Emaminejad, S.; Challa, S.; Chen, K.; Fahad, H. M.; Tai, L.; Ota, H.; Davis, R. W.; Javey, A. A Wearable Electrochemical Platform for Non-Invasive Simultaneous Monitoring of Ca2+ and pH. ACS Nano 2016, 10, 7216-7224.
-

- 6. Parrilla, M.; Cuartero, M.; Crespo, G. A. Wearable Potentiometric Ion Sensors. Trends Anal. Chem. 2019, 110, 303-320.
-

- 7. Hu, J.; Stein, A.; Bühlmann, P. Rational Design of All-solid-state Ion-selective Electrodes and Reference Electrodes. TrAC, Trends Anal. Chem. 2016, 76, 102-144.
-

- 8. Lindner, E.; Pendley, B. D. A Tutorial on The Application of Ion-selective Electrode Potentiometry: An Analytical Method with Unique Qualities, Unexplored Opportunities and Potential Pitfalls; Tutorial. Anal. Chim. Acta 2013, 762, 1-13.
-

- 9. Mazzaracchio, V.; Fiore, L.; Nappi, S.; Marrocco, G.; Arduini, F. Medium-distance Affordable, Flexible and Wireless Epidermal Sensor for pH Monitoring in Sweat. Talanta 2021, 222, 121502-121511.
-

- 10. Yoon, J. H.; Kim, S.; Park, H. J.; Kim, Y. K.; Oh, D. X.; Cho, H.; Lee, K. G.; Hwang, S. Y.; Park, J.; Choi, B. G. High Self-healable and Flexible Cable-type pH Sensors for Real-time Monitoringof Human Fluids. Biosens. Bioelectron. 2020, 150, 111946-111952.
-

- 11. Buck, R. P.; Rondinini, S.; Covington, A. K.; Baucke, F. G. K.; Brett, C. M. A.; Camões, M. F.; Milton, M. J. T.; Mussini, T.; Naumann, R.; Pratt, K. W.; Spitzer, P.; Wilson, G. S. Measurement of pH. Definition, Standards, and Procedures. Pure Appl. Chem.2002, 74, 2169-2200.
-

- 12. Ghoneim, M. T.; Nguyen, A.; Dereje, N.; Huang, J.; Moore, G. C.; Murzynowski, P. J.; Dagdeviren, C. Recent Progress in Electrochemical pH-sensing Materials and Configurations for Biomedical Applications. Chem. Rev. 2019, 119, 5248-5297.
-

- 13. Demuru, S.; Kunnel, B. P.; Briand, D. Thin Film Organic Electrochemical Transistors Based on Hybrid PANI/PEDOT:PSS Active Layers for Enhanced pH Sensing. Biosens. Bioelectron.: X 2021, 7, 100065-100073.
-

- 14. Manjakkal, L.; Dervin, S.; Dahiya, R. Flexible Potentiometric pH Sensors for Wearable Systems. RSC Adv. 2020, 10, 8594-8617.
-

- 15. Yoon, J. H.; Hong, S. B.; Yun, S.; Lee, S. J.; Lee, T. J.; Lee, K. G.; Choi, B. G. High Performance Flexible pH Sensor Based on Polyaniline Nanopillar Array Electrode. J. Colloid Interface Sci. 2017, 490, 53-58.
-

- 16. Park, Y. M.; Lim, S. Y.; Jeong, S. W.; Song, Y.; Bae, N. H.; Hong, S. B.; Choi, B. G.; Lee, S. J.; Lee, K. G. Flexible Nanopillar-based Electrochemical Sensors for Genetic Detection of Foodborne Pathogens. Nano Convergence 2018, 5, 15-23.
-

- 17. Park, H. J.; Yoon, J. H.; Lee, K. G.; Choi, B. G. Potentiometric Performance of Flexible pH Sensor Based on Polyaniline Nanofiber Arrays. Nano Convergence 2019, 6, 9-16.
-

- 18. Park, S. H.; Jeong, J.; Kim, S. J.; Kim, K. H.; Lee, S. H.; Bae, N. H.; Lee, K. G.; Choi, B. G. Large-area and 3D Polyaniline Nanoweb Film for Flexible Supercapacitors with High Rate Capability and Long Cycle Life. ACS Appl. Energy Mater. 2020, 3, 7746-7755.
-

- 19. Fei, J.; Cui, Y.; Yan, X.; Yang, Y.; Wang, K.; Li, J. Controlled Fabrication of Polyaniline Spherical and Cubic Shells with Hierarchical Nanostructures. ACS Nano 2009, 3, 3714-3718.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2022; 46(5): 695-700
Published online Sep 25, 2022
- 10.7317/pk.2022.46.5.695
- Received on Jun 16, 2022
- Revised on Jul 10, 2022
- Accepted on Jul 16, 2022
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Conflict of Interest
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Bong Gill Choi
-
Department of Chemical Engineering, Kangwon National University, Samcheok 25913, Korea
- E-mail: bgchoi@kangwon.ac.kr
- ORCID:
0000-0002-5452-4091









 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.