- Donepezil-loaded Poly(D,L-lactic acid) Microspheres for Potent and Sustained Drug Release in the Treatment of Alzheimer’s Disease
Eunyoung Seol*, **, Kwonhyeok Yoon*, Heeyong Lee*, Seong-Jun Jo*****, Jong II Park****,†
 , Sung-Joo Hwang***,†
, Sung-Joo Hwang***,†  , and Cheong-Weon Cho**,†
, and Cheong-Weon Cho**,† 
*G2GBIO, Inc., 1646 Yuseong-daero, Science Park #302, Yuseong-gu, Daejeon 34054, Korea
**College of Pharmacy, Chungnam National University, 99 Daehak-ro, Yuseong-Gu, Daejeon 34134, Korea
***Yonsei Institute of Pharmaceutical Sciences & College of Pharmacy, Yonsei University, 85 Songdogwahak-ro, Yeonsu-Gu, Incheon 21983, Korea
****Department of Bio Nanomaterials, Bio Campus of Korea Polytechnics, #48, Dongan-ro 112, Ganggeong-up, Nonsan, Chungnam 32943, Korea
*****College of Pharmacy and Integrated Research Institute of Pharmaceutical Sciences, The Catholic University of Korea, 43 Jibong-ro, Wonmi-gu, Bucheon 14662, Korea- 알츠하이머 치료를 위한 PLA 서방형 미립구 제형 개발
설은영*, ** ·윤권혁* ·이희용* ·조성준***** · 박종일****,†
 · 황성주***,†
· 황성주***,†  · 조정원**,†
· 조정원**,† 
*㈜지투지바이오, **충남대학교 약학과, ***연세대학교 약학과 ****한국폴리텍대학 바이오나노소재과, *****가톨릭대학교 약학과
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
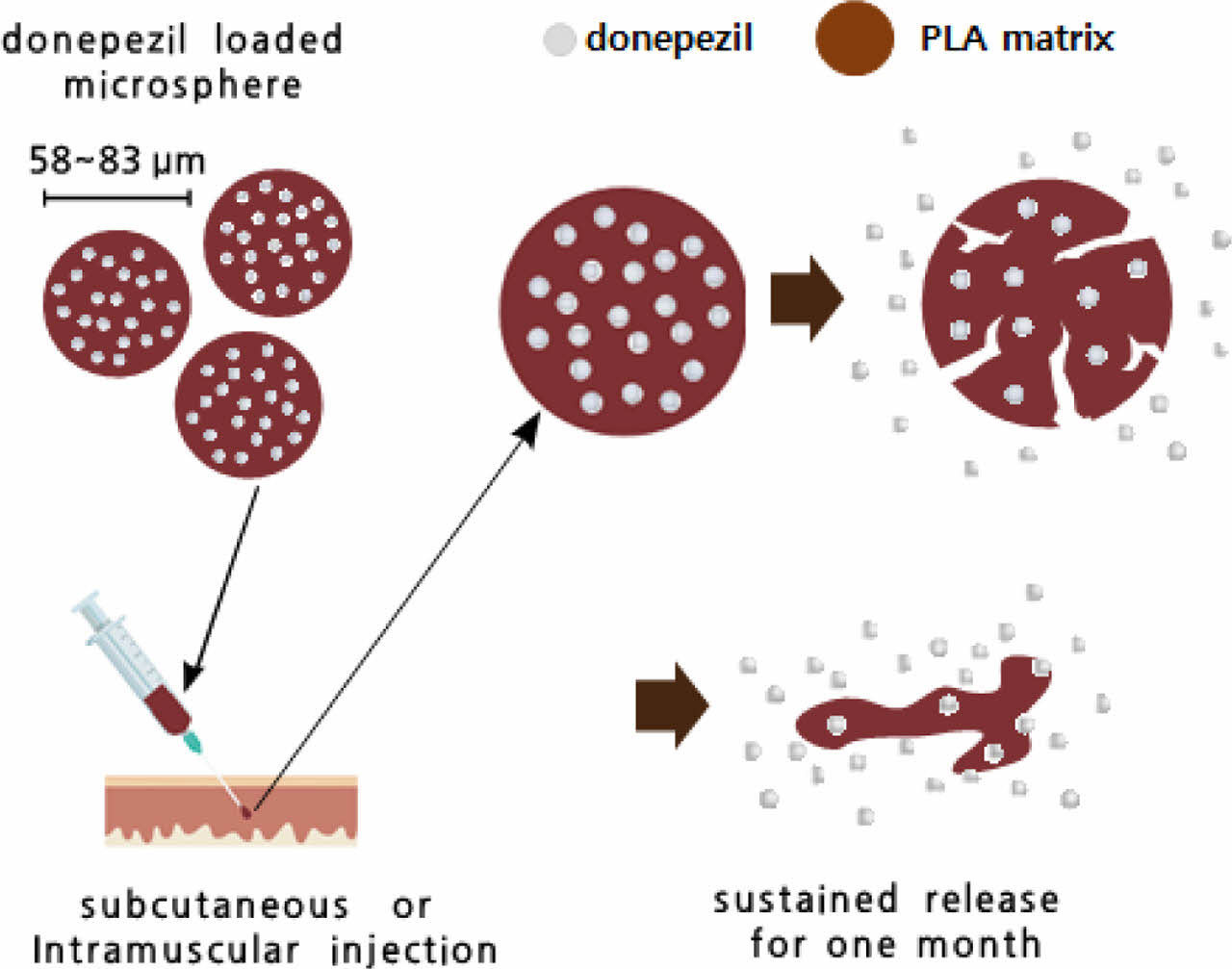
Alzheimer’s disease is a progressive neurodegenerative disease associated with memory deficit and cognitive decline. Herein, a new formulation of donepezil-loaded microspheres of uniform particle size was investigated. Donepezil-loaded microspheres were successfully prepared using a cross-flow membrane emulsification technology. The size analysis showed that the average diameter of the microspheres was 58-83 μm, indicating a narrow size distribution with a span value of less than 0.71. Scanning electron microscopy showed that the microspheres had a spherical shape. The loading efficiency of donepezil in the poly(D,L-lactic acid) (PLA) microspheres was 61.8±3.7% (w/w). Donepezil-loaded microspheres prepared using PLA (DM-L3) presented a more sustained release efficacy than poly(lactic-co-glycolic acid) (PLGA) microspheres in an in vitro accelerated release test and single-dose subcutaneous pharmacokinetic test in rats. The concentration-time profile analyzed using the non-parametric superposition method showed that the DM-L3 microspheres could achieve a sustained release of the drug for 1 month.
알츠하이머병은 기억력 결핍 및 인지 저하가 수반되는 진행성 신경 퇴행성 질환이다. 본 연구에서는 도네페질(donepezil)이 함유된 균일한 입자크기를 갖는 신규한 미립구 제형에 대하여 연구하였다. 도네페질이 함유된 미립구는 막유화 기술을 이용하여 제조하였으며, 평균 직경은 58-83 μm, 스팬값은 0.71 미만으로 입도가 균일하였고, 전자현미경 관찰결과 입자가 구형으로 제조되었음을 확인하였다. Poly(D,L-lactic acid)(PLA) 미립구에서 도네페질의 봉입 효율이 61.8±3.7%(w/w)이었고, 가속 방출 시험 및 쥐의 단일 용량 피하 약동학 시험에서 poly(lactic-co-glycolic acid)(PLGA)으로 제조된 미립구보다 더 오랫동안 방출이 지속되는 것을 확인할 수 있었다. 비모수 중첩법을 사용하여 분석한 농도-시간 프로파일은 PLA를 이용하여 제조된 미립구인 DM-L3가 1개월 동안 체내에서 약물이 지속적으로 방출될 수 있음을 확인하였다.
A new formulation of donepezil-loaded microspheres of uniform particle size was successfully prepared using a cross-flow membrane emulsification technology. The average diameter of the microspheres was 58-83 ¥ìm, indicating a narrow size distribution. Donepezil-loaded microspheres (DM-L3) prepared using poly(D,L-lactic acid) (PLA) showed more sustained release efficacy than poly(lactic-co-glycolic acid) (PLGA) microspheres and can be a suitable drug formulation for the release of donepezil for 1 month.
Keywords: donepezil, Alzheimer's disease, microsphere, poly(D,L-lactic acid).
This work was supported by the Industrial Strategic Technology Development Program [grant number 20001269, Enhancement Project of Global Long- acting Platform Technology InnoLAMP] funded by the Ministry of Trade, Industry & Energy (MOTIE, Korea) and the Bio & Medical Technology Development Program (NRF-2019M3E5D1A02068573).
The authors declare that there is no conflict of interest.
Alzheimer's disease (AD) is a neurodegenerative disorder characterized by progressive cognitive deterioration, a decline in the ability to perform daily activities, and behavioral changes. The disease initially damages the brain, thereby affecting learning, memorizing, thinking, and planning. This mental decline negatively affects the quality of life of patients because they have difficulties expressing themselves and organizing their thoughts.1 As the disease progresses, more changes occur in the patients’ personalities and behaviors, with patients requiring extensive help with daily activities. Over time, shrinkage of the brain results in more severe signs of AD, such as a decline in thinking and reasoning and an inability to respond to the environment or control movement.2
AD is the most common cause of dementia in individuals aged 65 years and older. Recently, the number of patients with dementia has rapidly increased, and the management of patients with dementia has become a serious social problem.3,4 Dementia is a central nervous system-related degenerative disease characterized by a loss or decline in memory and other cognitive abilities, resulting in irreversible dysfunction of the neural network due to the slow death of nerve cells, leading to a permanent loss of function.5 The cause of dementia has not yet been clarified, but includes various pathological and pathophysiological factors. Currently, there is no cure for dementia, and the treatments for AD are indirect methods, including the administration of acetylcholinesterase inhibitors, such as donepezil (trade name: Aricept), tacrine (trade name: Cognex), rivastigmine (trade name: Exelon), and galantamine (trade name: Reminyl). Donepezil is an acetylcholinesterase (AChE) inhibitor widely used to treat mild to severe AD.6,7
The available donepezil formulation is in the form of tablets, which are administered orally to patients. However, orally administered acetylcholinesterase inhibitors are generally associated with poor compliance; moreover, they can exert adverse effects, such as anxiety; nightmares; insomnia; and gastrointestinal adverse effects, including nausea, vomiting, and diarrhea. It is also difficult to administer these drugs orally to patients with advanced dementia. Therefore, studies have been actively conducted to develop new formulations for the long-term release of dementia drugs through injections and rectal or transdermal administration.8-10
Poly(lactic-co-glycolic acid) (PLGA) is a copolymer of poly lactic acid (PLA) and poly glycolic acid (PGA). The biodegradation rate of PLGA copolymers is dependent on the molar ratio of the lactic and glycolic acids in the polymer chain and the molecular weight of the polymer.11-14
In this study, we aimed to develop a method for AD treatment that maintains the efficacy of single administration of donepezil for a month compared to the once daily oral administration. To this end, PLGA was used to develop a one-month long-acting formulation of donepezil-loaded monodisperse microspheres using the membrane emulsification method.15 We then analyzed the potential of the donepezil-loaded microspheres, which had a uniform particle size, for the sustained and stable release of donepezil.
Materials. Dichloromethane and acetonitrile were purchased from Avantor (Phillipsburg, NJ, USA). Poly(vinyl alcohol) (PVA) was obtained from the Mitsubishi Chemical Corporation (Tokyo, Japan). Poly(lactide-co-glycolic acid) (lactide:glycolide=85:15) (MW=215000 g/mol) and Poly(D,L-lactic acid) (MW=25000 and 15000 g/mol) were purchased from Evonik Operations GmbH (Essen, Germany). The donepezil base was obtained from Neuland Laboratories Limited (Hyderabad, India). Dimethyl sulfoxide, monobasic potassium phosphate, triethylamine, and polysorbate 20 were purchased from Sigma-Aldrich (St. Louis, MO, USA). Sodium carboxymethylcellulose (AqualonTM 7LF PH BET) was obtained from Ashland (DE, USA). D-Mannitol (Pearlitol PF) was purchased from Roquette Freres (Lestrem, France). Sodium hydroxide and citric acid were obtained from Samchun Chemicals Corporation (Seoul, South Korea), and deionized (DI) water was prepared using an Arium® Advance Pure Water System (Sartorius AG, Gottingen, Germany).
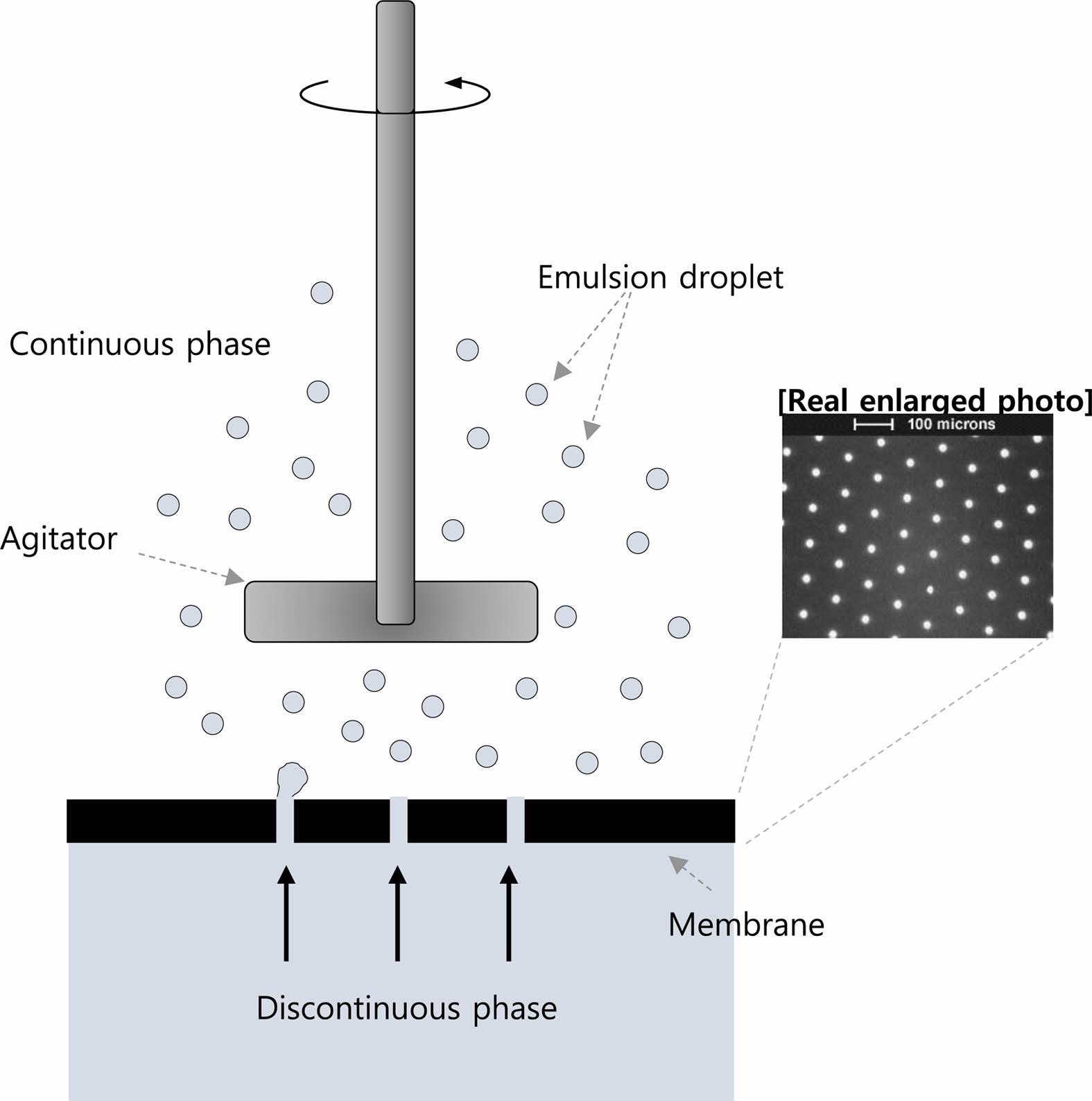
Preparation of Donepezil Microspheres. Donepezil-loaded microspheres were prepared using a cross-flow membrane emulsification technique (Figure 1). An emulsification device, a dispersion cell with a flat disc membrane under a paddle blade stirrer, was supplied by Micropore Technologies (Cleveland, UK).16 Pre-determined amounts of the donepezil base and polymers were dissolved in dichloromethane for 2 h to prepare the dispersed phase. The solution was transferred into a polypropylene syringe, and the syringe was placed on a syringe pump. The syringe and dispersed phase inlet of the emulsification device were connected to the PTFE (polytetrafluoroethylene) tubing. PVA solution (0.5% w/w) as the continuous phase was supplied to the reservoir. The reservoir and continuous phase inlet of the emulsification device were connected with platinum-cured silicon tubing fitted to a peristaltic pump. The prepared dispersed phase was supplied into the emulsification device using a syringe pump at a flow rate of 2.5 mL/min for a 30-μm pore membrane or 5.0 mL/min for a 40-μm pore membrane. Simultaneously, the continuous phase was supplied from the continuous phase reservoir into the emulsification device. The droplets produced in the emulsification device were transferred to an evaporation vessel equipped with an overhead stirrer to prevent the precipitation of the droplets. As the first hardening process, the droplets in the vessel were stirred for 30 min. Thereafter, the droplets were separated from the continuous phase by filtration using a sintered stainless-steel filter of pore size 25 μm. The droplets were re-dispersed in 0.5% w/w polyvinyl alcohol solution in the vessel. The solution was heated to 45 °C for 3 h. Subsequently, the temperature was maintained at 25 °C. The hardened microspheres were washed with DI water and collected using a 25-μm pore wire mesh. The microspheres were then freeze-dried.
Characterization of the Donepezil-loaded Microspheres.
Tapped Density: The tapped density was measured as described by Arvind et al.17 A 1.5-mL EP-tube was filled with dried microspheres, and the microspheres were weighed. The tube was tapped on the table 100 times to pack the microspheres. The volume of the packed microspheres was recorded and the tapped density was calculated using the following eq.:

Loading Content and Loading Efficiency: As a standard solution, 10 mg of donepezil was dissolved in 10 mL of dimethyl sulfoxide, and 30 mg of the sample was dissolved in 10 mL of dimethyl sulfoxide. As a mobile phase, 50 mM potassium phosphate buffer (pH 5.0) in 40% acetonitril was used. The donepezil loading content and loading efficiency of the microspheres were determined by measuring the absorbance of donepezil at 271 nm using a high-performance liquid chromatography (HPLC) system (Waters Alliance 2690; Waters, MA, USA).
A quantitative research technique was applied for the quantification of donepezil. The donepezil loading content and efficiency were calculated using the following eq.:

Particle Size Analysis: The particle size and distribution were analyzed using laser diffraction technology with a particle size analyzer (PSA990; Anton Paar, Graz, Austria). The sample (200 mg) was dispersed in 1 mL of 9% Tween20 solution, and the solution was added into the particle size analyzer. The results are expressed as volume fraction and size. The span value shows the distance between the 10% and 90% values, normalized with 50%. The span value was calculated using the following eq.:

Morphology (Scanning Electron Microscopy [SEM] and Optical Microscopy): The morphologies of the microspheres were observed by SEM (EM-30; COXEM Co., Ltd., Daejeon, South Korea) and optical microscopy (Olympus, BH-2). The microspheres were placed on a sample holder attached to the carbon tape and vacuum sputter-coated with a 0.1-mm-thick Pt layer. The SEM instrument was operated at 15 kV.
In-vitro Accelerated Dissolution Study. The in vitro accelerated dissolution study was performed using USP apparatus 2 (paddle). In brief, 900 mL of 0.05 M acetic acid solution with 0.05 M sodium acetate was added into the vessel of USP apparatus 2 (DS14000; Labindia Analytical Instruments Pvt. Ltd, Maharashtra, India). The temperature of the vessel was maintained at 37 °C under gentle stirring (paddle speed of 150 rpm). The microspheres (200 mg) were placed in the vessel. At pre-determined time intervals, 1 mL of the medium was collected. The amount of dissolved donepezil was determined by HPLC at 271 nm.
In-vivo Pharmacokinetics Study in Rats. Pharmacokinetic (PK) Parameters: The PK analysis in rats was performed by QuBest BIO Co., Ltd. (Yongin, South Korea). Pathogen-free male Sprague-Dawley rats (approximately 8 weeks old) were obtained from Samtako Ltd. (Osan, South Korea). The rats were acclimatized to the laboratory conditions, and the room temperature and humidity controls were set to 22±3 °C and 50±20%, respectively, for 5 days. A certified and irradiated rodent diet (Purina Rodent Chow 38057; Cargill Inc., South Korea) and tap water via polycarbonate bottles were provided to the rats ad libitum. The dose strength of donepezil was 86.8 mg/kg. The formulation was prepared by suspending a pre-determined amount of the microspheres in 0.35 mL of the diluent with 1% carboxymethyl cellulose sodium, 4.5% D-mannitol, and 0.1% Polysorbate20. The individual dose volume was slowly injected using a 1-mL disposable syringe with a 19G needle into the femoral muscles. Blood samples were collected from the jugular vein. The blood samples (0.5 mL) were collected using a heparin-coated disposable syringe at pre-determined time intervals. Plasma samples were analyzed according to the International Scientific Standards (Chuncheon, South Korea). The collected samples were centrifuged at 15000×g for 2 min. The concentration of donepezil in the plasma samples was determined by liquid chromatography–mass spectrometry (LC-MS/MS). The assay range of donepezil in rat plasma was from 0.1 to 100 ng/mL. To calculate the PK parameters, a non-compartmental PK analysis was performed using Phoenix WinNonlin® (8.0 version, Certara, St. Louis, MO, USA).
Non-parametric Superposition Analysis: Steady-state plasma profiles of various donepezil formulations were simulated using the non-parametric superposition module in PheonixTM WinNonlin from the mean plasma concentrations determined after administering each single formulation at 86.8 mg/kg, assuming linear kinetics upon repeated administration. They were simulated by non-parametric superposition for each formulation dose of 86.8 mg/kg administered every 4 weeks over 0-140 days. The lowest post-dose and steady-state plasma concentrations were obtained directly from each simulation.
|
Figure 1 Schematic diagram showing membrane emulsification device. |
Preparation of Donepezil-loaded Microspheres and Analysis of Loading Efficiency. First, we investigated the potential of PLA or PLGA for the development of microspheres of uniform particle size and for a stable and sustained release of donepezil. PLA and PLGA are polymers that are commonly used in the medical field for drug delivery.18,19 They are also easily soluble in several solvents, including chloroform and dichloromethane.20 In addition, donepezil shows good solubility in chloroform and dichloromethane. However, PLA and PLGA are insoluble in water. They absorb water and degrade owing to the hydrolysis of their ester linkages. Because of the hydrophobic side chain (methyl groups), PLA exhibits a lower water absorption property and slower degradation than PLGA. Therefore, drug release from PLGA is faster than that from PLA.21 Here, the donepezil-loaded microsphere were prepared using PLGA and PLA of different molecular weights as the dispersed phase.
Table 1 shows the preparation conditions of the dispersion phase (DP), continuous phase (CP), and donepezil-loading content in the microspheres. As shown in Table 1, the amount of donepezil in the microspheres ranged from 25.4% to 27.1% (w/w) and its loading efficiency ranged from 52.5±0.3% to 61.8±3.7% (w/w).
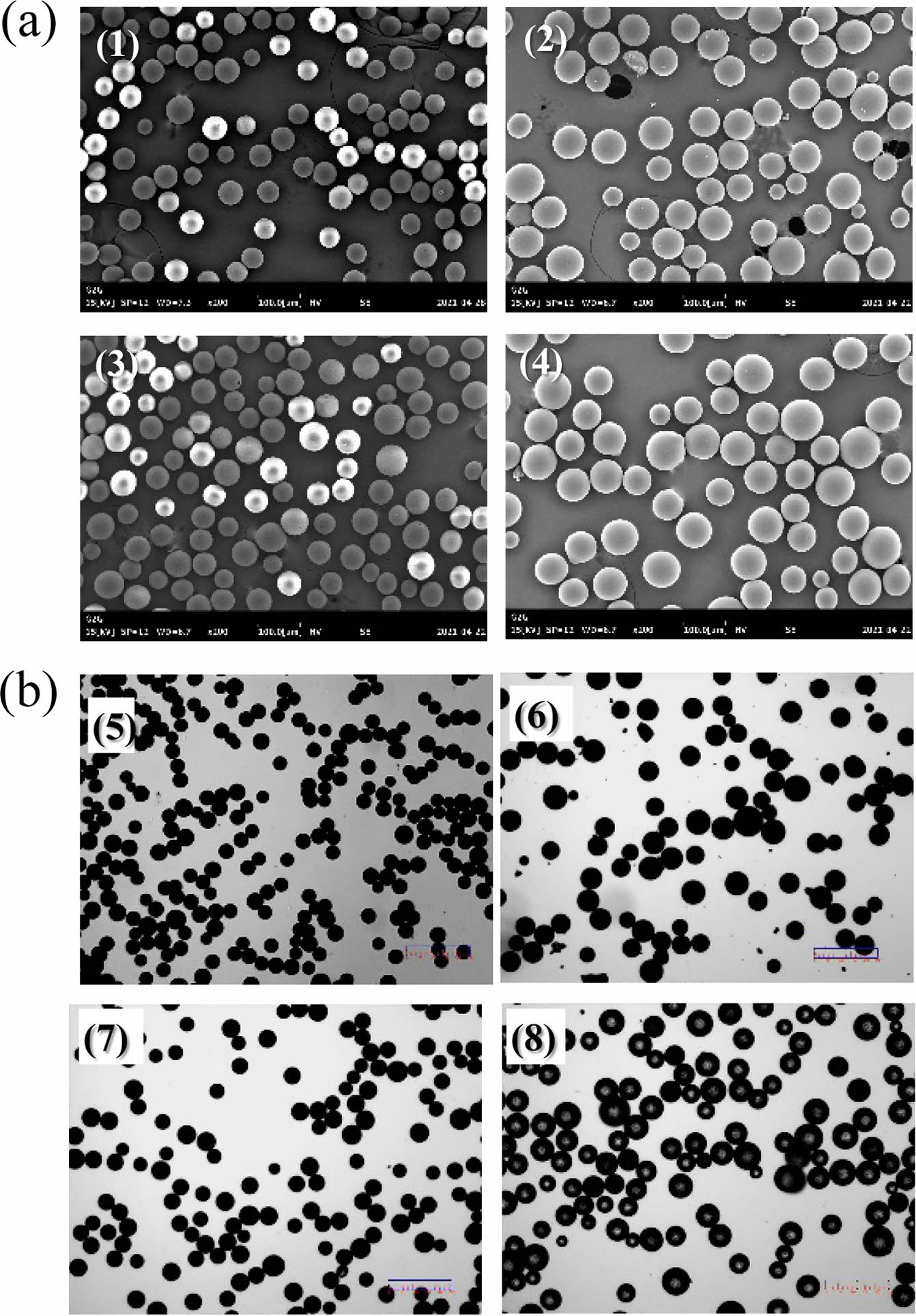
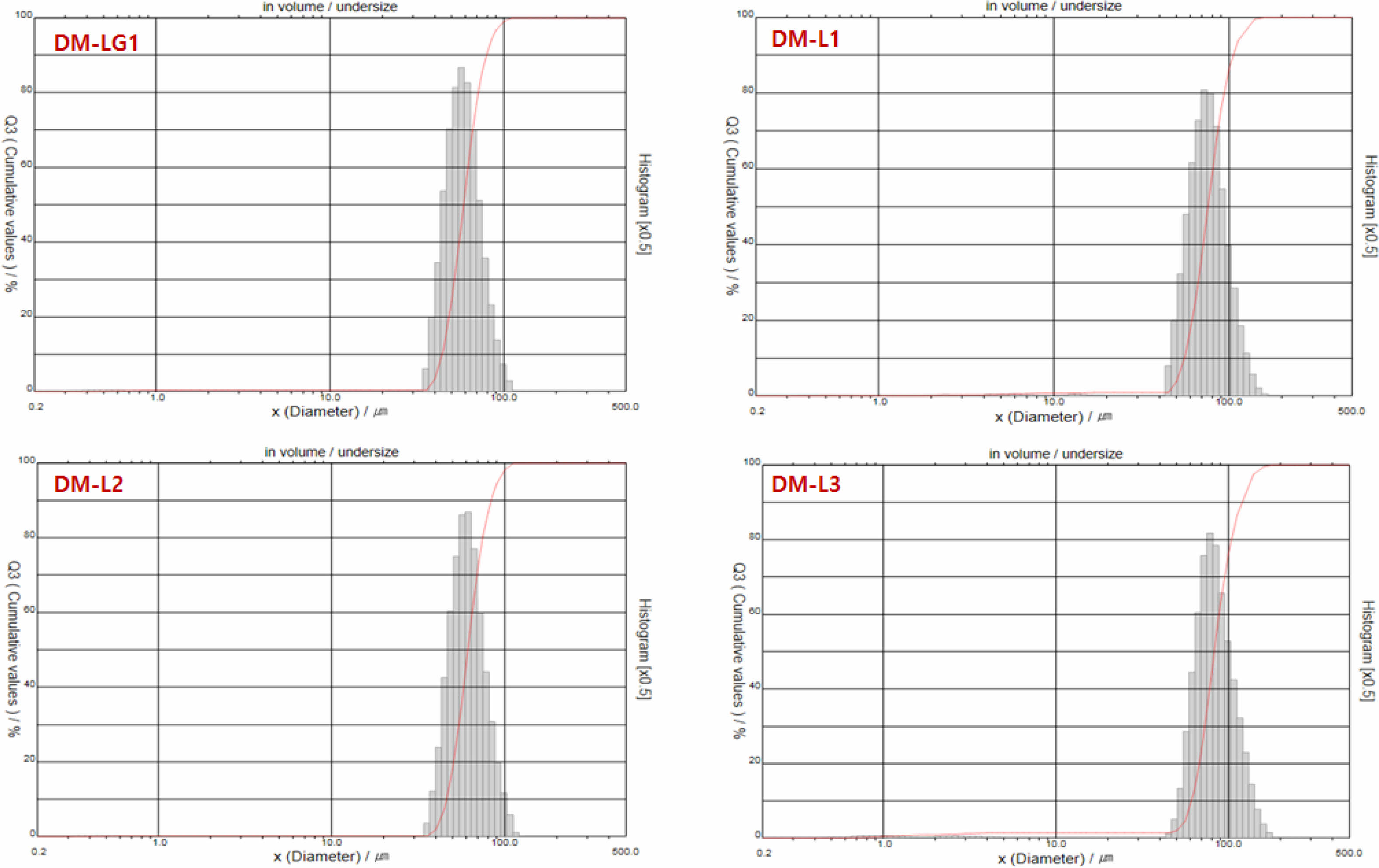
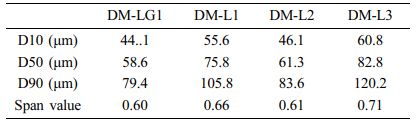
Morphological Characteristics of the Donepezil Micro- spheres. We aimed to develop microspheres with a uniform particle size required for a stable and sustained release of donepezil. As described in section 2.2, donepezil microspheres of uniform particle size were successfully prepared. SEM and optical microscopy were used to analyze the morphological characteristics of the prepared microspheres. Most of the microspheres were spherical, and there were no pores and drug crystals on their surface; furthermore, their surfaces were smooth (Figure 2). The particle size determined by SEM was consistent with that determined by optical microscopy and using a particle size analyzer. Table 2 and Figure 3 show that the average particle size of the donepezil microspheres was 58-83 μm; they showed a narrow size distribution with a span value of less than 0.71. The term “average particle size” refers to the median diameter, corresponding to 50% of the volume percentage of the particle size (w/w). The size distribution or span value is an index indicating particle size uniformity of the microspheres. The span value obtained in this study indicates that the donepezil microspheres had a uniform particle size distribution. Donepezil microspheres with a uniform particle distribution can be administered with a smaller deviation than non-uniform microspheres at the time of injection. It is well known that the particle size and distribution significantly influence the in vitro and in vivo release of drugs from microsphere systems. Donepezil microspheres with an average particle size of less than 30 μm can help achieve a rapid release of active donepezil from microspheres.21,22 The larger the average particle size, the better the sustained release of donepezil. However, a large particle size may require a thick syringe needle for injection, and this may cause pain during injection. Thus, the particle size obtained in this study is beneficial for a suitable drug release.
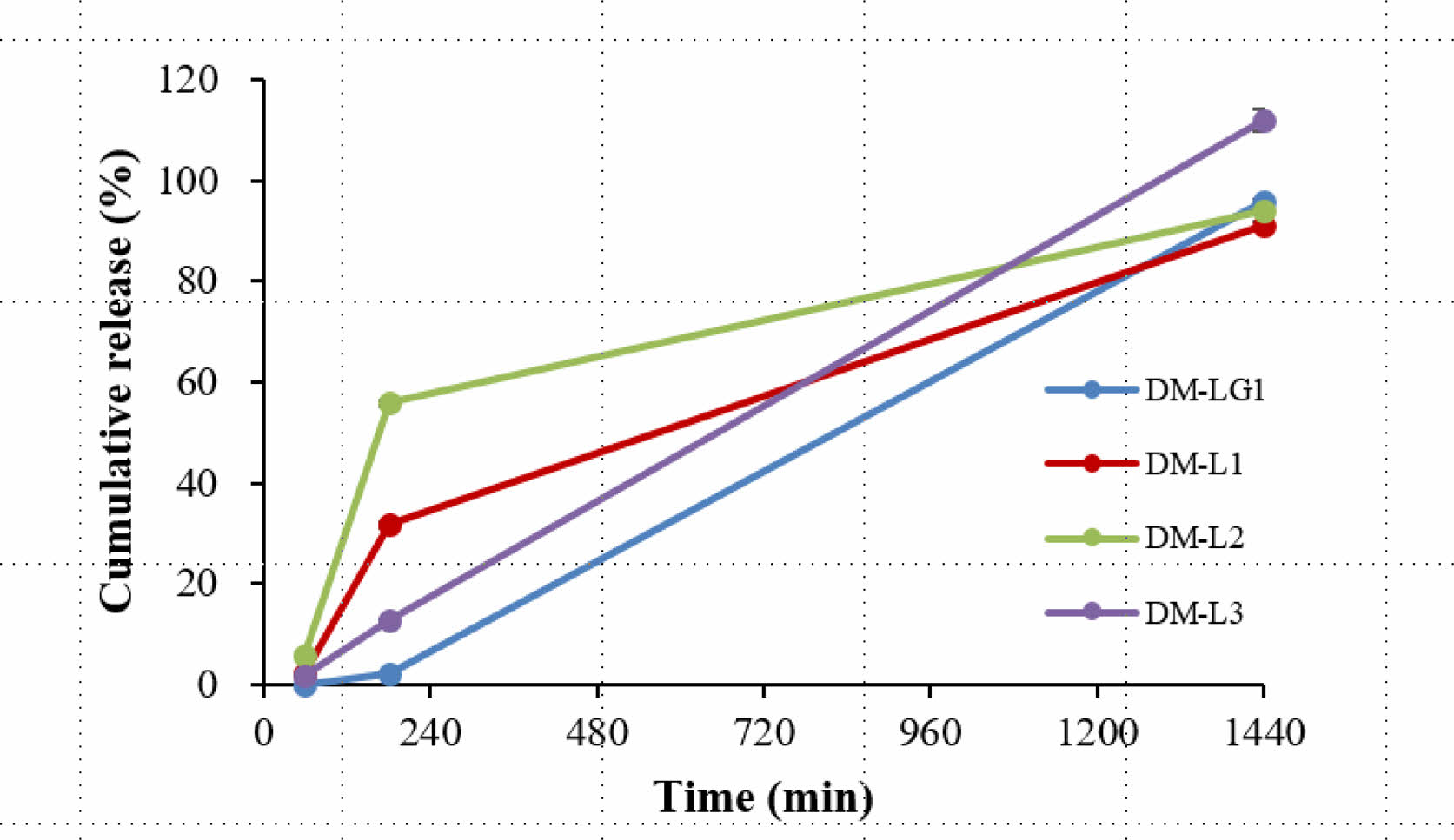
In-vitro Accelerated Release Test. The drug delivery capacity of the donepezil microspheres was evaluated using an in vitro accelerated release test. As shown in Figure 4, DM-LG1 and DM-L2, which were prepared with a membrane of the same pore size, 30 μm, had similar average particle sizes but different cumulative dissolution rates. The cumulative dissolution rate of DM-LG1 was low compared with that of DM-L2; thus, it was not suitable as a sustained-release microsphere. DM-L2 was also not suitable because of its relatively fast dissolution. This result indicates that the average particle size of the DM-L2 microspheres was very small to achieve an appropriate dissolution rate. Therefore, DM-L1 and DM-L3 were prepared with a membrane of pore size 40 μm, and their average particle size was larger than that of DM-L2; thus, their cumulative dissolution rate was slower than that of DM-L2. The cumulative dissolution rate of DM-L1 was higher than that of DM-L3. The difference between DM-L1 and DM-L3 is the weight of the polymer used; that is, DM-L1 was prepared with a low molecular weight polymer (Mw=15000 g/mol) compared with DM-L3 (Mw=25000 g/mol). Thus, DM-L3 may be suitable as a sustained-release microsphere of donepezil.
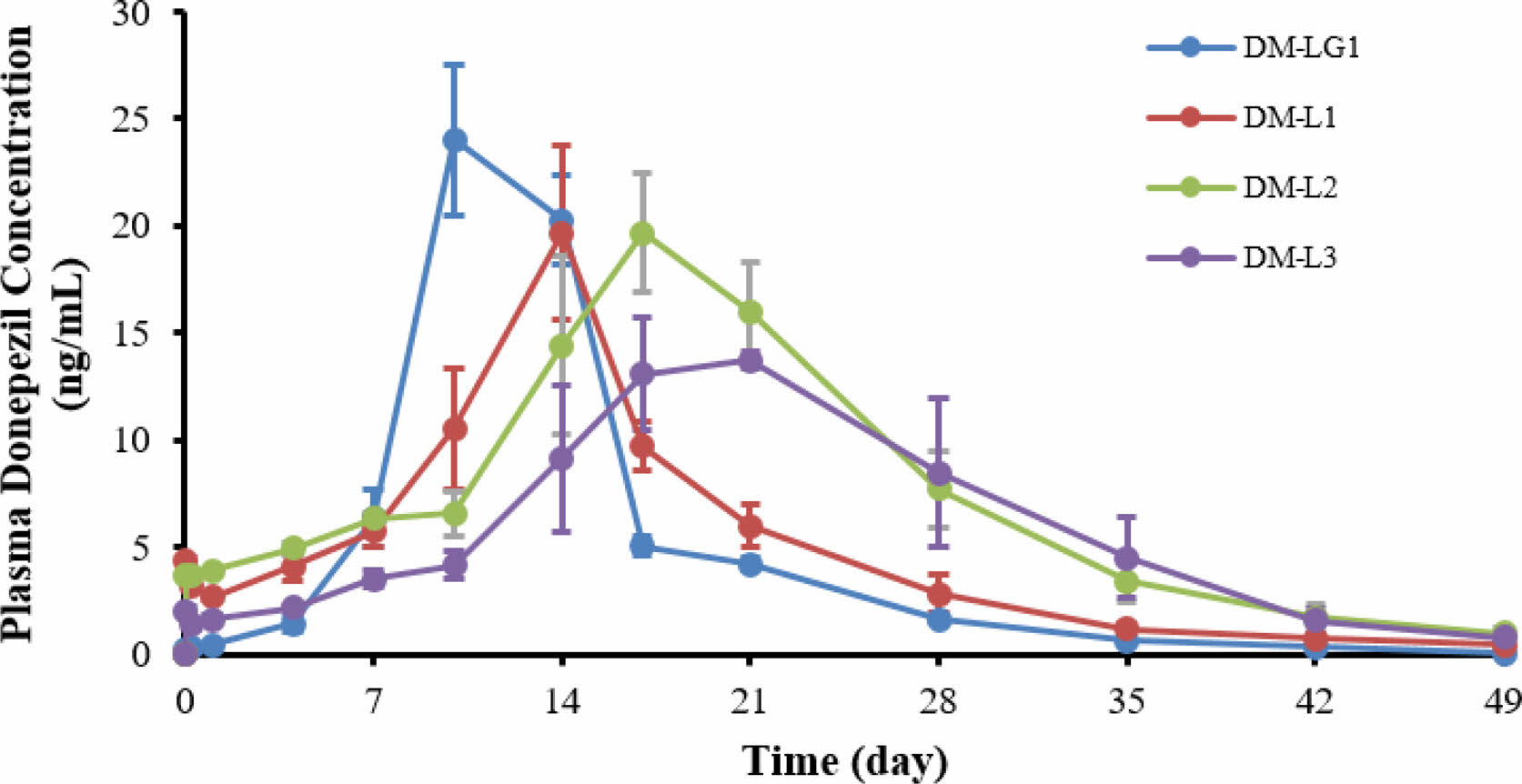
Single-dose Subcutaneous PK Test Using Sprague-Dawley Rats. To evaluate the potential of the donepezil microspheres for sustained-release treatment, the donepezil concentration in the rat plasma was measured. Figure 5 shows the mean plasma concentration-time profiles of the donepezil-loaded microspheres, DM-LG1, DM-L1, DM-L2, and DM-L3, after the intramuscular injection of microspheres into the rats.
As mentioned above, in the in vitro experiment, the dissolution rate of DM-LG1 was considerably slower than that of DM-L2. Unexpectedly, the initial plasma concentration of donepezil achieved with DM-LG1 was higher than that with DM-L2 in the in vivo experiment. This might be because DM-LG1 was prepared using PLGA of a high molecular weight (Mw=215000 g/mol) and high intrinsic viscosity (I.V.=1.5). This may lead to a rapid increase in the serum concentration of donepezil, which may be toxic; therefore, it is not suitable as a sustained-release microsphere. Hence, the polymer was changed, and DM-L1, DM-L2, and DM-L3 were prepared using 100% PLA with different molecular weights as the dispersed phase. The PK parameters of the microspheres are presented in Table 3. As shown in Table 3, compared with DM-LG1, donepezil-loaded PLA microspheres altered the distribution of donepezil in vivo, and the T1/2 values of DM-L1 (T1/2=91.4±23.5 h), DM-L2 (T1/2=268.8±40.8 h), and DM-L3 (T1/2=264.8±132.2 h) were 1.5-, 2.05-, and 2.02-fold higher than that of DM-LG1, respectively. These results indicate that the PLA microspheres had a more sustained release efficacy than the PLGA microspheres for donepezil in this system. In addition, the T1/2 values of DM-L2 and DM-L3 were higher than that of DM-L1, indicating that the average particle size and molecular weight of the polymer used together affected the sustained release efficacy. In other words, the average particle size of DM-L1 (D50=75.8 μm) is larger than that of DM-L2 (D50=61.3 μm), but the molecular weight of the polymer used is smaller than that of DM-L2 and DM-L3. These results show that drug release from the degradation matrix does not necessarily correlate with the rate of polymer degradation.23
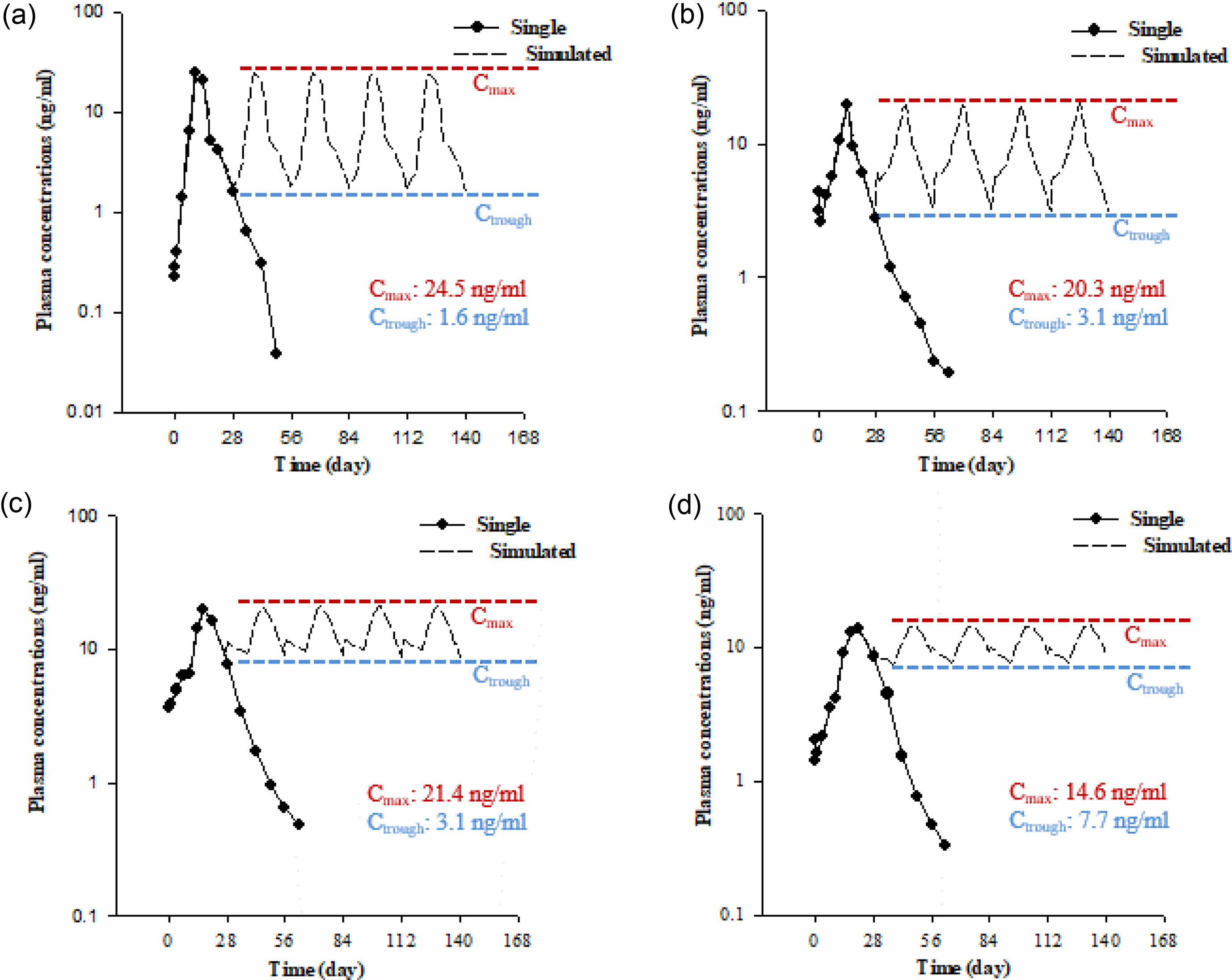
Steady-state Simulation Using a Non-parametric Super- position Method. To predict the concentration-time profile of plasma donepezil by repeated dose simulation with a single injection, a non-parametric superposition method was used, and suitable sustained-release donepezil microspheres were also selected. The data are shown in Figure 6. The difference between the Cmax and Ctrough of DM-LG1 was larger than that of any other formulation. On the contrary, the plasma concentration of DM-L3 fluctuated around approximately 20.3± 3.3. The difference in the Cmax and Ctrough was the smallest for DM-L3 among all formulations. Therefore, the plasma concentration of donepezil in DM-L3 can be stabilized to be used as a new formulation for dementia drugs.
|
Figure 2 (a) SEM micrographs of the (1) DM-LG1; (2) DM-L1; (3) DM-L2; (4) DM-L3 microspheres; (b) Optical micrographs of the (5) DM-LG1; (6) DM-L1; (7) DM-L2; (8) DM-L3 microspheres. |
|
Figure 3 Particle size distribution of the microspheres. |
|
Figure 4 In-vitro cumulative donepezil release profiles: (blue) DMLG1; (red) DM-L1; (green) DM-L2; (purple) DM-L3 using USP apparatus 2 at 37 ℃. |
|
Figure 5 Plasma concentration of donepezil after an intramuscular injection of the microspheres in SD rats: (blue) DM-LG1; (red) DM-L1; (green) DM-L2; (purple) DM-L3. |
|
Figure 6 Predicted concentration-time profiles of plasma donepezil following repeated doses simulated from a single injection using the nonparametric superposition method: (a) DM-LG1; (b) DM-L1; (c) DM-L2; (d) DM-L3. |
|
Table 1 Preparation Condition of DP and CP and Physicochemical Properties of Donepezil-loaded Microspheres |
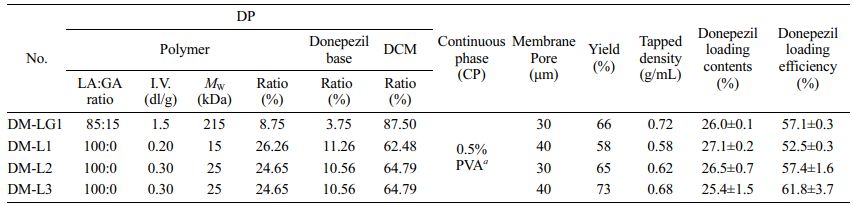
a Weight of the polymers in the continuous phase=50 times the weight of dichloromethane (DCM). |
|
Table 3 Pharmacokinetic Parameters of the Microspheres After Intramuscular Injection in SD Rats |
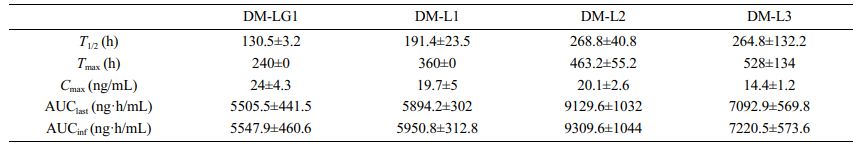
AUClast : AUC to the last measurable concentration; AUCinf : AUC extrapolated to infinity. |
Donepezil-loaded microspheres were successfully prepared using the cross-flow membrane emulsification technology with membranes of pore diameter 30 or 40 μm. The average particle size of the microspheres was 58-83 μm; the microspheres presented a narrow size distribution with a span value of less than 0.71. Donepezil-loaded microspheres prepared using PLA showed more sustained release efficacy than PLGA microspheres in the in vitro accelerated release test and single-dose subcutaneous pharmacokinetic test in rats. The concentration-time profile of plasma donepezil by repeated dose simulation from a single injection using a non-parametric superposition method showed that DM-L3 is a suitable drug formulation for the release of donepezil for 1 month. These results suggest the potential of donepezil-loaded PLA microspheres for the sustained release of donepezil in AD treatment.
- 1. Schultz, C.; Del Tredici, K. H. B. Neuropathology of Alzheimer's Disease. In Alzheimer's Disease. Current Clinical Neurology; Richter, R.W., Richter, B.Z., Eds.; Humana Press: Totowa, 2004; pp 21-31.
-

- 2. Citron, M. Alzheimer’s Disease: Strategies for Disease Modification. Nat. Rev. Drug Discov. 2010, 9, 387-398.
-

- 3. Khan, M. M.; Ahsan, F.; Ahmad, U.; Akhtar, J.; Badruddeen; Mujahid, M. Alzheimer disease: A Review, World J. Pharm. & Pharm. Sci. 2016, 5, 649-666.
- 4. Folch, J.; Petrov, D.; Ettcheto, M.; Abad, S.; Sánchez-López, E.; García, M. L.; Olloquequi, J.; Beas-Zarate, C.; Auladell, C.; Camins, A. Current Research Therapeutic Strategies for Alzheimer’s Disease Treatment. Neural Plast. 2016, 2016, 8501693.
-

- 5. Scott, K. R.; Barrett, A. M. Dementia Syndromes: Evaluation and Treatment. Expert Rev. Neurother. 2007, 7, 407-422.
-

- 6. Cummings, J.; Lee, G.; Ritter, A.; Sabbagh, M.; Zhong, K. Alzheimer’s Disease Drug Development Pipeline: 2019, Alzheimers Dement. 2019, 5, 272-293.
-

- 7. Yiannopoulou, K. G.; Papageorgiou, S. G. Current and Future Treatments for Alzheimer’s Disease. Ther. Adv. Neurol. Disord. 2013, 6, 19-33.
-

- 8. Priano, L.; Gasco, M. R.; Mauro, A. Transdermal Treatment Options for Neurological Disorders: Impact on the Elderly. Drugs Aging 2006, 23, 357-375.
-

- 9. Möller, H.-J.; Hampel, H.; Hegerl, U.; Schmitt, W.; Walter, K. Double-blind, Randomized, Placebo-controlled Clinical Trial on the Efficacy and Tolerability of a Physostigmine Patch in Patients with Senile Dementia of the Alzheimer type. Pharmacopsychiatry 1999, 32, 99-106.
-

- 10. Levy, A.; Brandeis, R.; Treves, T. A.; Meshulam, Y.; Mawassi, F.; Feiler, D.; Wengier, A.; Glikfeld, P.; Grunwald, J.; Dachir, S. Transdermal Physostigmine in the Treatment of Alzheimer’s Disease. Alzheimer Dis. Assoc. Disord. 1994, 8, 15-21.
- 11. Lu, L.; Peter, S. J.; Lyman, M. D.; Lai, H.; Leite, S. M.; Tamada, J. A.; Uyama, S.; Vacanti, J. P.; Langer, R.; Mikos, A. G. In vitro and in vivo Degradation of Porous Poly(-lactic-co-glycolic acid) foams. Biomaterials 2000, 21, 1837-1845.
-

- 12. Lu, L.; Garcia, C. A.; Mikos, A. G. In vitro Degradation of Thin Poly(D,L-lactic-co-glycolic acid) Films. J. Biomed. Mater. Res. 1999, 46, 236-244.
-

- 13. Park, T. G. Degradation of Poly(lactic-co-glycolic acid) Microspheres: Effect of Copolymer Composition. Biomaterials 1995, 16, 1123-1130.
-

- 14. Blasi, P. Poly(lactic acid)/poly(lactic-co-glycolic acid)-based Microparticles: An Overview. J. Pharma. Investig. 2019, 49, 337-346.
-

- 15. Piacentini, E.; Drioli, E.; Giorno, L. Membrane Emulsification Technology: Twenty-five Years of Inventions and Research Through Patent Survey. J. Membr. Sci. 2014, 468, 410-422.
-

- 16. Stillwell, M. T.; Holdich, R. G.; Kosvintsev, S. R.; Gasparini, G.; Cumming, I. W. Stirred Cell Membrane Emulsification and Factors Influencing Dispersion Drop Size and Uniformity. Ind. Eng. Chem. Res. 2007, 46, 965-972.
-

- 17. Parmar, A.; Mishra, A.; Pathak, A. Preparation and Evaluation of Mucoadhesive Microspheres of Repaglinide for Treatment of Diabetes Mellitus Type II. Int. J. Adv. Pharm. 2015, 4, 72-82.
-

- 18. Brown, A.; Zaky, S.; Ray Jr, H.; Sfeir, C. Porous Magnesium/PLGA Composite Scaffolds for Enhanced Bone Regeneration Following Tooth Extraction. Acta Biomater. 2015, 11, 543-553.
-

- 19. Ramot, Y.; Haim-Zada, M.; Domb, A. J.; Nyska, A. Biocompatibility and Safety of PLA and Its Copolymers. Adv. Drug Deliv. Rev. 2016, 107, 153-162.
-

- 20. Mohammadi-Samani, S.; Taghipour, B. PLGA Micro and Nanoparticles in Delivery of Peptides and Proteins; Problems and Approaches. Pharm. Dev. Technol. 2015, 20, 385-393.
-

- 21. Makadia, H. K.; Siegel, S. J. Poly Lactic-co-glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers 2011, 3, 1377-1397.
-

- 22. Guo, W.; Quan, P.; Fang, L.; Cun, D.; Yang, M. Sustained Release Donepezil Loaded PLGA Microspheres for Injection: Preparation, in vitro and in vivo Study. Asian J. Pharm. Sci. 2015, 10, 405-414.
-

- 23. Siggle, S. J.; Kahn, J. B.; Metzger K.; Winey, K. I.; Werner, K.; Dan, N. Effect of Drug Type on the Degradation Rate of PLGA Matrices. Eur. J. Pharm. Biopharm. 2006, 64, 287-293.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2022; 46(2): 238-245
Published online Mar 25, 2022
- 10.7317/pk.2022.46.2.238
- Received on Nov 21, 2021
- Revised on Jan 6, 2022
- Accepted on Feb 8, 2022
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Conflict of Interest
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Jong II Park**** , Sung-Joo Hwang*** , and Cheong-Weon Cho**
-
**College of Pharmacy, Chungnam National University, 99 Daehak-ro, Yuseong-Gu, Daejeon 34134, Korea
***Yonsei Institute of Pharmaceutical Sciences & College of Pharmacy, Yonsei University, 85 Songdogwahak-ro, Yeonsu-Gu, Incheon 21983, Korea
****Department of Bio Nanomaterials, Bio Campus of Korea Polytechnics, #48, Dongan-ro 112, Ganggeong-up, Nonsan, Chungnam 32943, Korea - E-mail: jipark@kopo.ac.kr, sjh11@yonsei.ac.kr, chocw@cnu.ac.kr
- ORCID:
0000-0002-4012-8821, 0000-0001-6521-3822, 0000-000









 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.