- Preparation of Porous Hydrogels Using Blowing Agents and Application to Contact Lenses
Vaovision Care, Mudong-Ro 226, Uichang-gu, Changwon, Gyeongnam 51106, Korea
*Department of Optometry & Vision Science, College of Bio and Medical Science, Daegu Catholic University, Hayang-Ro 13-13, Gyeongsan, Gyeongbuk 38430, Korea- 발포제를 이용한 다공성 하이드로젤의 제조 및 콘택트렌즈로의 응용성
바오비젼케어, *대구가톨릭대학교 안경광학과
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
This study aims to confirm the applicability of a contact lens by manufacturing a high-functional porous hydrogel containing a blowing agent and examining its properties. In addition, the physical properties according to the type and content of the blowing agent in the hydrogel were compared. Hydrogel contact lenses were prepared using 1% and 3% sodium carbonate, sodium bicarbonate, potassium carbonate and potassium bicarbonate as blowing agent, respectively. Light transmittance, water content, refractive index, oxygen permeability, contact angle, protein adsorption were measured for physical and chemical properties. As the amount of blowing agent increased, the water content, wettability, and oxygen permeability increased, but the refractive index and tensile strength decreased. The adsorption amount of protein also decreased as the amount of blowing agent increased. The hydrogel added with the foaming agent maintained the basic optical properties of the contact lens, and it was confirmed that it could be applied to the contact lens because it was very excellent in moisture content, oxygen permeability, and wettability.
본 연구에서는 발포제가 포함된 고기능의 다공성 하이드로젤을 제조하고 특성을 조사하여 콘택트렌즈로의 응용성을 확인하였다. 그리고 하이드로젤에 들어가는 발포제의 종류와 함량에 따른 물성도 비교하였다. 발포제로 각각 1% 및 3% 탄산나트륨, 탄산수소나트륨, 탄산칼륨 및 중탄산칼륨을 사용하여 하이드로젤 콘택트렌즈를 제조하였다. 물리화학적 특성은 광투과율, 수분함량, 굴절률, 산소투과도, 접촉각, 단백질 흡착을 측정하였다. 발포제의 함량이 증가할수록 수분함량, 습윤성, 산소투과성은 증가하고 굴절률과 인장강도는 감소하였다. 발포제의 양이 증가함에 따라 단백질의 흡착량도 감소하였다. 발포제를 첨가한 하이드로젤은 콘택트렌즈의 기본 광학적 물성을 유지하였으며, 함수율, 산소투과도, 습윤성 등이 매우 우수하여 콘택트렌즈에 적용 가능함을 확인하였다.
The porous hydrogel contact lens using a foaming agent has many pores inside the lens, so physical properties such as oxygen permeability and wettability are remarkably improved compared to a non-porous contact lens.
Keywords: contact lens, porous hydrogel, blowing agent, wettability, oxygen permeability.
This research was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2018R1A2B6008622)
The authors declare that there is no conflict of interest.
Hydrogels are stable 3D networks of hydrophilic polymers formed by hydrogen bonds, covalent bonds, Van der Waals bonds, or physical crosslinks. Hydrogels do not degrade in water and absorb large amounts of water while maintaining its original form.1-4 They are widely used in biomedical fields including biosensors, wet dressings, drug delivery systems, and soft contact lenses because of their high biocompatibility as they are physically and chemically similar to biological tissues.5-7
Superporous hydrogels absorb large amounts of water in a very short period of time. Superporous hydrogels have very fast hydration rates as pores connect to each other to form open channels and are produced by the gas bubble method.8 Therefore, the addition of blowing agents has a substantial effect on the water content of hydrogels, and the water content of hydrogels increases as the content of blowing agents increases. Due to these characteristics, superporous hydrogels are used in fields of medicine and biomedicine as drug delivery systems and dressings for wounds.9
Recently, many studies have been conducted on drug delivery using contact lenses for diagnosis and treatment.10-12 However, the space inside the contact lens is limited, so there is a need to improve the drug loading capacity.
Contact lenses are medical devices placed directly on the cornea that must satisfy conditions such as biocompatibility, physical properties, tensile strength, optical properties, and non-toxicity.13 Therefore, studies on high-functional contact lenses such as high oxygen permeability and high wettability, low protein and lipid adsorption, UV protection and antibacterial activity are active.14-16
Hydrogel contact lenses are preferred by many people because of their flexible and comfortable fit. However, they may cause problems such as corneal hypoxia and corneal edema due to low oxygen permeability. Therefore, hydrogel lenses need to have high wettability and high oxygen permeability while maintaining comfort. Silicone hydrogels containing silicon monomers exhibit improved oxygen permeability compared to hydrogel contact lenses. However, they also have limitations such as reduced wettability due to hydrophobic surfaces and are not comfortable because of the adsorption of proteins and lipids.17,18
Porous hydrogels have been widely used in other industries so far, but not in contact lenses. Contact lenses with pores can be applied to smart contact lenses because they can contain more drugs than conventional contact lenses. Therefore, this study aims to overcome problems such as low oxygen permeability of conventional hydrogel contact lenses and low wettability of silicone hydrogel contact lenses by manufacturing porous hydrogel contact lenses.
Reagent. In this study, we used hydrophilic monomers (2-hydroxyethyl methacrylate (HEMA) & methacrylic acid (MAA)), a polymerization initiator (2,2'-azobis (isobutyronitrile) (AIBN)), which were produced by JUNSEI (Japan), and a hydrophobic monomer (styrene) and crosslinking agent (ethylene glycol dimethacrylate (EGDMA)), which were produced by Sigma-Aldrich (Germany), in order to polymerization the hydrogels.
Figure 1 shows the structural formulas of the blowing agents including sodium carbonate (SC), sodium bicarbonate (SB), potassium carbonate (PC), and potassium bicarbonate (PB), which are all products of Sigma-Aldrich.
Preparation of Porous Hydrogel Film. Table 1 shows the monomers and ratios used to fabricate the hydrogel films with blowing agents in this study.
The basic sample (Ref) was made of HEMA (89.6%), MAA (5%), styrene (5%), EGDMA (0.3%), and AIBN (0.1%). We used 4 types of blowing agents SC, SB, PC, and PB to make the hydrogel films with blowing agents. In order to prepare the samples containing blowing agents, we first prepared the blowing agents at a concentration of 10%, and added the 10% aqueous blowing agents to the basic samples at a ratio of 1% and 3% to the whole sample.
The samples were fabricated by placing PP film on two glass plates and positioning a 0.5 mm silicone gasket in between the plates to make the mold. The hydrogels were polymerized according to the composition ratio in Table 1. In order to facilitate the evaluation of physical properties, we cut the samples that were polymerized in the form of films into circles with a diameter of 12 mm.
The reagents were mixed according to the ratios in Table 1, mixed with a vortex for 1 hour, and stabilized for 1 hour. The stabilized reagents were injected into the fabricated mold and polymerized at 100 °C for 2 hours using a drying oven.
In terms of the blowing agents, the samples were prepared by adding 1% and 3% of SC, SB, PC, and PB which included Na+ and K+, respectively. Table 1 shows the name of each sample used in the experiment. The basic sample was referred to as Ref, and the first letter and concentration of the blowing agent were used for the naming process. For example, the sample with SC 1% was named Sc1 and the sample with PB 3% was named Pb3.
Physical Properties. Physical properties of hydrogel were evaluated by moisture content, swelling behavior, refractive index, light transmittance, oxygen permeability, and contact angle, and measurements were averaged 5 times.
We used a UV-Visible Spectrophotometer (EVOLUTION 201) from Thermo SCIENTIFIC to measure the light transmittance. The sample was placed in saline in the jig and we measured the transmittance of visible light (380-780 nm) based on ISO standards.
The water content was measured by using the gravimetric method specified in ISO 18369-4:2006, Ophthalmic optics-Contact lenses-Part 4: Physicochemical properties of contact lens materials.
The refractive index was measured in accordance with ISO 18369-4: 2006. We used an Abbe Refractometer (ATAGO DR-A1) to measure the index after removing water on the surface of the samples, which were hydrated in 0.9% saline at room temperature for more than 24 hours.
In order to measure the swelling behavior and the equilibrium swelling ratio of the hydrogels, we measured the weight of the completely dried sample. We then immersed the sample in 0.9% saline and measured the weight of the swollen sample after 10 seconds, 30 seconds, 1 minute, 3 minutes, 5 minutes, 10 minutes, 30 minutes, 1 hour, 6 hours, 12 hours, and 24 hours. We removed the excess water on the surface when measuring the weight of the swollen sample. When the weight of the sample no longer changed, the swelling ratio was expressed as the equilibrium swelling ratio. The swelling ratio of the samples was calculated by using the following formula.
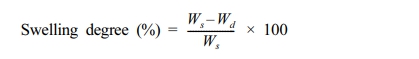
Ws is weight of the hydrated hydrogel according to time.
Wd is the initial weight of the dried hydrogel.
Oxygen permeability was measured by using the polarographic method specified in ISO 18369-4:2006. After hydrating the samples in 0.9% saline for more than 24 hours, we measured the center thickness with a VL-50 (thickness gauge) from Mitutoyo. After measuring the thickness, the sample was fixed to a flat cell inside a thermo-hygrostat with a temperature of 35°C and a humidity of 98% to measure the current with a O2 PERMEOMETER_201T from Rehder. The measured thickness and current were calculated and expressed as Dk values in units of 10-11(cm2/sec)(mL·O2/mL∙mmHg).
The contact angle was measured by the Sessile drop method with a DSA30 Analyzer from Kruss GMBH. We fabricated film samples and hydrated them in 0.9% saline for more than 24 hours and slowly dried them at room temperature. Next, we dropped 3 mL of ultrapure distilled water on the surface of the dried hydrogel film and measured the angle 10 times for 10 seconds.
Protein Quantification. This study examined the degree of protein adsorption of the fabricated hydrogel films by using spectrophotometry. We used bovine saline albumin (BSA, 66.4 kDa) from Sigma-Aldrich, which is similar in the shape and chemical properties of human albumin.
First, we dissolved bovine saline albumin (BSA) in 0.9% saline at a rate of 5 mg/mL. After injecting 2 mL of the BSA aqueous solution into a glass vial, we put the samples which were hydrated in 0.9% saline for more than 24 hours, in each vial and absorbed protein at 37 °C for 24 hours in a dry oven.
In order to desorb protein, we prepared a 3% aqueous solution by adding sodium dodecyl sulfate (SDS) in triple distilled water. After taking out the samples that absorbed protein and washing them with saline a total of 3 times, we put the samples in glass vials containing 2 mL of the 3% SDS aqueous solution, heated them at 95 °C for 15 minutes in a dry oven, and separated the protein from the samples using a shaking incubator for 3 minutes.
Each sample was measured 10 times, in which we measured the absorbance of the 3% SDS solution containing desorbed protein by using a 3% SDS aqueous solution as the baseline to measure the concentration of protein absorbed by the samples. We measured the absorbance at 280 nm, the absorption wave- length of protein, using a Cary 60 UV-Vis Spectrophotometer from Agilent.
The amount of protein adsorption (mg/g) was calculated using the following formula.

Q is the protein adsorption amount.
v is the volume of the solution.
c is the protein concentration in the solution.
m is the mass of the hydrated test specimens.
Surface Analysis. A scanning electron microscope (SEM) was used to view the surface and pores of the sample, and Tescan's Mira III was used as the measuring device. Each sample was pre-treated with alcohol for surface analysis, and the sample was dried by infiltrating liquid carbon dioxide using a critical point dryer (CPD). The surface of the dried sample was coated with platinum to a thickness of 3.25 nm, and the surface of the sample was observed at a magnification of 3000 to 10000.
Atomic force microscopy (AFM) was used to check the roughness of the surface of the sample, and Park Systems' NX10 was used as the measurement equipment. For surface analysis, each sample was pre-treated with alcohol, and the sample was dried by penetrating liquid carbon dioxide using a critical point dryer (CPD). By measuring the interaction force between atoms, the sample area of 5×5 mm was imaged.

|
Figure 1 Chemical structure of foaming agent: (a) sodium carbonate; (b) sodium bicarbonate; (c) potassium carbonate; (d) potassium bicarbonate |
Light Transmittance. The light transmittance of samples with 1% blowing agents ranged from 98.3 to 99.0%, and samples with 3% blowing agents showed a maximum transmittance of 99.4% (Figure 2). Although the difference according to the addition, type, and content of the blowing agent was not significant, the transmittance of the samples using blowing agents was slightly higher than that of the samples without blowing agents.
Specifically, the light transmittance was slightly higher when the amount of the blowing agent was high. In general, the visible light transmittance of the samples must be at least 88% to be used as contact lens materials.19 As all of the samples in this study have a visible light transmittance higher than 97%, they can be used as contact lens materials.
Water Content and Refractive Index. Water content is closely related to the comfort of contact lenses and is a very important factor because it affects the refractive index, oxygen permeability, protein adsorption, and polymer density.20,21
The results of measuring the water content and the refractive index of the hydrogel films fabricated in this study are in Figure 3.
The water content of Ref without any blowing agent was 32.61%. In terms of the water content according to the type and content of the blowing agent, the water contents of Sc1 and Sc3, with a sodium-based blowing agent, was 45.38% and 72.74%, respectively. The water content of Sb1 was 39.88% and that of Sb3 was 65.18%. The water contents of Pc1 and Pc3, with a potassium-based blowing agent, were 40.79% and 69.50%, and the water contents of Pb1 and Pb3 were 37.37% and 60.13%, respectively. The samples with large amounts of blowing agents showed higher water content.
The refractive index of the basic sample without any blowing agent was 1.452. The refractive index of Sc1 and Sc3, with a carbonate-based blowing agent, was 1.428 and 1.377, respectively. The refractive index of Pc1 was 1.436 and that of Pc3 was 1.383. The refractive indexes of Sb1 and Sb3, with a bicarbonate-based blowing agent, were 1.438 and 1.390, respectively, and the refractive indexes of Pb1 and Pb3 were 1.441 and 1.399, respectively. In the case of SC, the refractive indexes decreased by 1.7% and 5.2%, respectively, when the water contents of Sc1 and Sc3 increased by 39.2% and 123.1%, respectively, compared to Ref. Likewise, we can see that the higher the content of the blowing agent, the higher the water content and the lower the refractive index. This is because the blowing agents generate bubbles during polymerization which create numerous pores in the hydrogel. These pores increase the absorption of water by the hydrogels while reducing the density, and therefore reduce the refractive index.
As a result of examining the water content according to the blowing agent concentration, a difference in water content depending on the type of blowing agent was identified. However, the water content increased by at least 14.6% and up to 39.2% when adding 1% of the blowing agent compared with Ref which did not have any addition of the blowing agent. In terms of adding 3% of the blowing agent, the water content increased by at least 84.4% and up to 123.1% compared with Ref depending on the type of blowing agent. This shows that the water content according to the blowing agent content improves significantly in the case of 3% samples compared with 1% samples.
These results show that the amount of blowing agent has a substantial effect on the water content of hydrogels, and are consistent with a prior study8 which found that a large amount of blowing agent increased the water content of hydrogels.
In terms of comparing the water contents of samples with carbonate and bicarbonate with the same metal ion, carbonate samples Sc and Pc showed higher water contents than those of bicarbonate samples Sb and Pb. The water content of Sc was 12.1% higher than that of Sb1, and the water content of Sc3 was about 11.6% higher than that of Sb3. The reason is that samples with the same metal ion and carbonate absorb more water because they have more ions than the samples with bicarbonate. Sc is a strong alkali and diprotic while Sb is a weak alkali and monoprotic. Therefore, Sc has a higher water content because of the higher reactivity with the water protons than that of Sb.
In terms of comparing the water contents of samples with a different metal ion and the same carbonate and bicarbonate, the water content of Sc with sodium metal was higher than that of Pc with potassium, and the water content of Sb was higher than that of Pb. The water content of Sc1 was 11.3% higher than that of Pc1 and the water content of Sc3 was about 4.7% higher than that of Pc3. This is because the atoms of the sodium ions are smaller than the atoms of the potassium ions. As a result, many small pores are formed when forming networks in the hydrogel with high connectivity between the pores.
Among the four types of blowing agents, the samples using SC showed the highest water contents at 45.38% and 72.74%. Therefore, adding SC to hydrogels is considered most effective to increase the water content.
When the content of SC was 1% and 3%, the refractive index decreased by 1.7% and 5.2%, respectively, and the refractive index decreased as the blowing agent content increased even when using other blowing agents. We already confirmed that the water content increased as the blowing agent content increased. In general, the refractive index shows an inverse relationship. The reason is that as the water content increases, the spacing between the polymer networks constituting the hydrogel increases and the density decreases.22 This is consistent with the results of this study as the refractive index decreased as the water content increased, and the refractive index increased as the water content decreased.
A porous hydrogel using a blowing agent has a large space inside the hydrogel, which increases the moisture content and can contain a lot of drugs. Therefore, it can be applied as a smart contact lens for drug delivery.
Swelling Behavior. Figures 4 and 5 show the results of measuring the swelling behavior of the hydrogels using 1% and 3% blowing agents. In the case of Ref without any blowing agent, the swelling reached equilibrium after 6 hours.
All of the samples with 1% blowing agents reached a near-equilibrium state after 6 hours, but the samples with 3% blowing agents reached equilibrium within 1 hour regardless of the type of blowing agent. Therefore, the time required to reach equilibrium decreased as the content of the blowing agent increased. This is because the amount of bubbles increases as the blowing agent content increases, which in turn, form many pores in the hydrogel to absorb a large amount of water quickly.
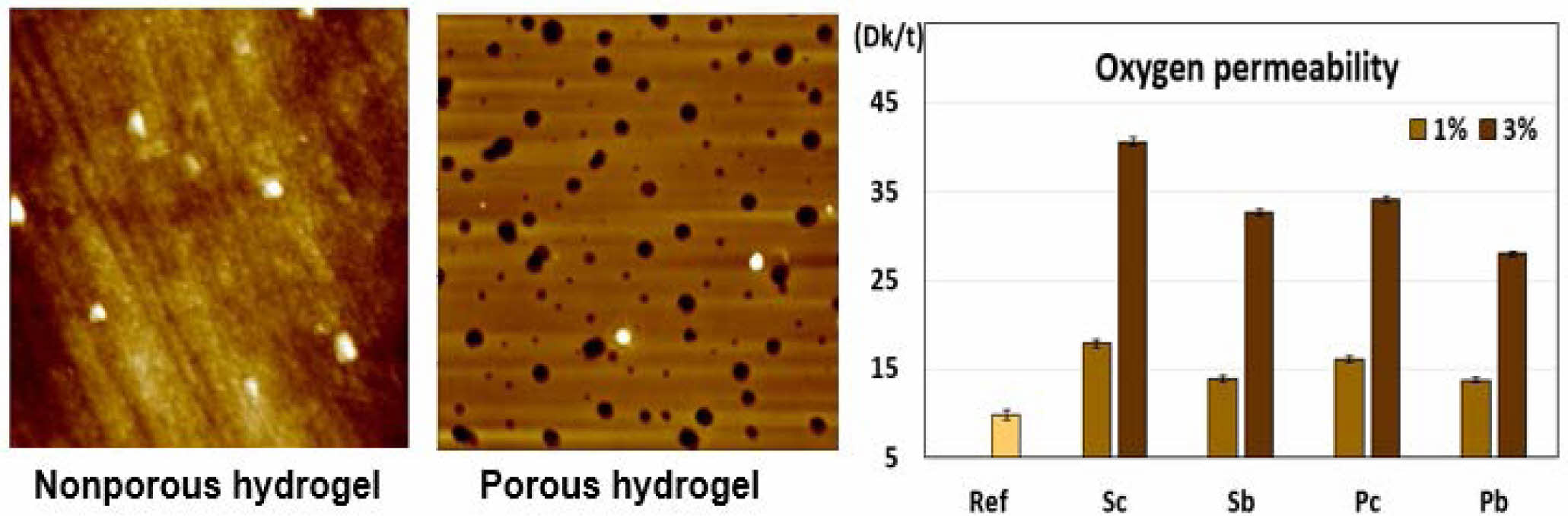
Oxygen Permeability. The oxygen permeability measurement results are shown in Figure 6. The oxygen permeability of Ref without any blowing agent was 9.84 Dk/t. In terms of the oxygen permeability of samples with a sodium-based blowing agent, the oxygen permeability of Sc1 and Sc3 with 1% and 3% SC were 17.86 Dk/t and 40.69 Dk/t, respectively. In addition, the oxygen permeability of Sb1 and Sb3 were 13.92 Dk/t and 32.66 Dk/t, respectively. The oxygen permeability of Sc1 and Sc3 were 28.3% and 24.6% higher than that of Sb1 and Sb3, respectively.
In terms of the samples with a potassium-based blowing agent, the oxygen permeability of Pc1 and Pc 3 were 16.07 Dk/t and 34.23 Dk, respectively. When adding Pb, the results were 13.79 Dk/t and 28.02 Dk/t for Pb1 and Pb3, respectively. Similarly, for potassium, the oxygen permeability of Pc1 and Pc3 were 14.2% and 18.1% higher than that of Pb1 and Pb3, respectively. Sc3 exhibited the highest oxygen permeability among the 9 samples used in this study.
Figure 6 shows the tendency of water content and oxygen permeability according to the content of SC, in which the water content and oxygen permeability increased as the content of the blowing agent increased. For example, in the case of SC, when the water contents of Sc1 and Sc3 increased by 12.77% and 40.13% compared to that of Ref, the oxygen permeability also increased by 8.02 Dk/t and 30.85 Dk/t, respectively. In the case of hydrogels, these results are consistent with the research results23-25 which show that oxygen permeability has a close relationship with water content and that oxygen permeability increases as the water content increases.
The oxygen permeability of Ref, the basic sample, was 9.84 Dk/t, which is similar to that of polymacon of hydrogel contact lenses made of pHEMA. Regardless of the type of blowing agent, the oxygen permeability of all of the samples increased by adding blowing agents. In terms of comparing the oxygen permeability according to the content of the blowing agent, oxygen permeability increased significantly when adding 3% compared to 1%. For example, compared to Ref in the case of SC, the oxygen permeability of Sc1 increased by 8.02 Dk/t, and that of Sc3 increased by 30.85 Dk/t. Oxygen permeability according to the blowing agent content correlates to the increased number of pores as the amount of gas increased due to the increased amount of blowing agent. In addition, when adding the same amount of blowing agent, the oxygen permeability of Sc and Pc with carbonate was higher than that of Sb and Pb with bicarbonate. In particular, the oxygen permeability most increased when using SC.
Wettability. Wettability is determined by the contact angle of the sample surface. The higher the contact angle, the lower the wettability due to the sample’s hydrophobic properties, and the lower the contact angle, the higher the wettability due to the sample’s hydrophilic properties.
Figure 7 show the results of measuring the contact angle to examine the wettability of the samples. The contact angle of Ref without any blowing agent was 78.46°.
When adding each type of blowing agent in order for the content to reach 1% and 3%, the contact angles of Sc1 and Sc 3, with a sodium-based blowing agent, were 73.53° and 47.28°, respectively. The contact angles of Sb1 and Sb3 were 74.06° and 55.81°, respectively. The contact angles of Pc1 and Pc3, with a potassium-based blowing agent, were 74.80° and 49.01°, respectively, and the contact angles of Pb1 and Pb 3 were 75.36° and 57.59°, respectively. Pb1 showed the largest contact angle, while Sc3 exhibited the smallest contact angle and the best wettability.
In this study, Ref without any blowing agent showed the lowest wettability with a contact angle of 78.46°. The contact angles of the samples with 1% and 3% of blowing agents decreased compared with Ref, and the angles decreased significantly when using 3% of blowing agents compared with 1%. In addition, even in the case in which the same amount of blowing agent was added, the contact angles of Sc and Pc samples with carbonate were lower than those of Sb and Pb samples with bicarbonate, showing better wettability. In terms of comparing the wettability through the contact angle of each sample, we can see that it is closely related to the water content. We can confirm that the tendency of water content according to the concentration and type of blowing agent was consistent with the tendency of wettability. This is because the wettability of the sample surface increases as the water content increases.
Surface Analysis of Porous Hydrogels. The surface and cross-section of the hydrogel depending on whether or not a foaming agent is added, and the roughness of the surface were imaged and examined. The surface and surface roughness of the porous hydrogel contact lens depending on whether a foaming agent was added were examined through scanning electron microscope (SEM) and atomic force microscope (AFM). SEM and AFM images of the surface of the non-porous and porous hydrogel are shown in Figure 8 and Figure 9, respectively.
As shown in Figure 8, the surface of (a) without addition of foaming agent appeared smooth without pores, but the number of (b) with addition of blowing agent showed many pores on the surface. As shown in the AFM image, it was confirmed that many pores were formed on the surface of the porous contact lens using 3% sodium carbonate.
Protein Adsorption. The adsorption of proteins can cause several side effects, such as decreased visual acuity, decreased comfort, and the denaturation of contact lens materials.26-28 If the hydrogel is anionic and hydrophilic and has high water content, the adsorption of albumin may be reduced. In addition, a higher surface wettability can reduce the adsorption of precipitates such as proteins.29
Figure 10 show the results of measuring the degree of protein adsorption of the samples according to the type and content of the blowing agent. The protein adsorption of Ref without any blowing agent was 7.5720 mg/g. The protein adsorption of Sc1 and Sc3, with a sodium-based blowing agent, were 4.5788 mg/g and 3.3981 mg/g, respectively. The protein adsorption of Sb1 and Sb3 were 5.0562 mg/g and 4.3490 mg/g, respectively. The protein adsorption of Pc1 and Pc3, with a potassium-based blowing agent, were 4.8246 mg/g and 3.9997 mg/g, respectively. When adding PB, the protein adsorption of Pb1 and Pb3 were 5.5835 mg/g and 4.2490 mg/g, respectively.
In terms of the tendency of protein adsorption and water content according to the content of SC, the water content increased as the blowing agent content increased while the protein adsorption decreased. For example, compared to Ref in the case of SC, when the water contents of Sc1 and Sc3 increased by 12.77% and 40.13%, respectively, the protein adsorption decreased by 2.9932 mg/g and 4.1739 mg/g, respectively.
In the case of Ref, the amount of protein absorbed was 7.5720 mg/g, which means that Ref absorbed the most albumin. By adding blowing agents, the protein adsorption decreased in all of the samples regardless of the type or content of the blowing agent. When adding the same amount of blowing agent, Sc and Pc with carbonate absorbed less protein compared to Sb and Pb with bicarbonate. For example, in the case of SC and SB, Sc3 adsorbed 21.9% less protein than Sb3. This is consistent with the results of prior studies that show that the addition of blowing agents increases the water content and improves the surface wettability, which in turn, reduces the precipitation of proteins and lipids.30,31 In terms of comparing the degree of protein precipitation according to the type of blowing agent, Sc1 and Sc3 show the greatest decrease in protein precipitation. Therefore, we believe that improved wettability due to the addition of blowing agents will also contribute to the reduced precipitation of proteins and lipids.

|
Figure 2 Transmittance of contact lens with blowing agent. |

|
Figure 3 Water contents and refractive index of samples: water contents and refractive index are represented by gray bars and solid black line, respectively. |

|
Figure 4 Swelling kinetics of sample with 1% blowing agent. |

|
Figure 5 Swelling kinetics of sample with 3% blowing agent. |

|
Figure 6 Comparison of oxygen permeability according to the amount of blowing agents. |

|
Figure 7 Comparison of contact angle according to the amount of blowing agents. |

|
Figure 8 SEM images of the surface of (a) the non-porous; (b) porous contact lens. |

|
Figure 9 AFM images of (a) the non-porous; (b) porous contact lens. |

|
Figure 10 Protein adsorption according to the amount of blowing agents. |
The purpose of this study was to examine whether hydrogels with various types of blowing agents could be used as contact lens materials. In addition, we compared and analyzed the physical properties of the hydrogels according to the type, and concentration of blowing agents.
In terms of visible light transmittance, the most basic optical property of contact lenses, all of the samples showed high visible light transmittance regardless of the type and content of the blowing agent. Compared with the sample without adding any blowing agent, the water content, oxygen permeability, and wettability of the samples were improved by adding and increasing the content of the blowing agents, while the refractive index, tensile strength, and protein adsorption decreased.
Among the 4 blowing agents, adding 3% sodium carbonate showed the best results in light transmittance, water content, wettability, protein adsorption, and oxygen permeability.
By adding blowing agents to hydrogel contact lens materials, we can maintain the basic optical properties of contact lenses, and improve the moisture content, oxygen permeability, and wettability, while reducing protein adsorption. A porous hydrogel using a blowing agent is sufficiently applicable as a contact lens.
- 1. Ahmed, Enas M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105-121.
-

- 2. Sun, Y.; Kaplan J. A.; Shieh. A.; Sun. H. L.; Croce, C. M.; Grinstaff, M. W. Parquette JR Self-assembly of a 5-fluorouracil-Dipeptide Hydrogel. Chem. Comm. 2016, 52, 5254-5257.
-

- 3. Kim, S. H.; Sun, Y.; Kaplan, J. A.; Grinstaff, M. W.; Parquette, J. R. Photo-crosslinking of a Self-assembled Coumarin-dipeptide Hydrogel. New J. Chem. 2015, 39, 3225-3228.
-

- 4. Verhulsel, M.; Vignes, M.; Descroix, S.; Malaquin, L.; Vignjevic, D. M.; Viovy, J. L. A Review of Microfabrication and Hydrogel Engineering for Micro-organs on Chips. Biomaterials 2014, 35, 1816-1832.
-

- 5. Ding, R.; Yu, X.; Wang, P.; Zhang, J.; Zhou, Y.; Cao, X.; Tang, H.; Ayres, N.; Zhang, P. Hybrid Photosensitizer Based on Amphiphilic Block Copolymer Stabilized Silver Nanoparticles for Highly Efficient Photodynamic Inactivation of Bacteria. RSC Advances. 2016, 6, 20392-20398.
-

- 6. Zhai, D.; Liu, B.; Shi, Y.; Pan, L.; Wang, Y.; Li, W.; Zhang, R.; Yu, G. Highly Sensitive Glucose Sensor Based on Pt nanoparticle/polyaniline Hydrogel Heterostructures. ACS Nano 2013, 7, 3540-3546.
-

- 7. Li, L.; Wang, Y.; Pan, L.; Shi, Y.; Cheng, W.; Shi, Y.; Yu, G. A Nanostructured Conductive Hydrogels-based Biosensor Platform for Human Metabolite Detection. Nano Lett. 2015, 15, 1146-1151.
-

- 8. Xiangming, H.; Weimin, C.; Zhenlu, S. Novel Authigenic Gas Foaming Hydrogels for Preventing Coal Spontaneous Combustion. e-Polymers 2015, 15, 361-368.
-

- 9. Jishan, A. A.; Arindam, C.; Bhupendra, S. C.; Manish, J.; Himanshu, M. V. A Conceptual Overview on Superporous Hydrogels. Int. J. Pharm. Sci. Rev. Res. 2014, 25, 166-173.
- 10. Mandal, D.; Bandyopadhyay, D. Comparison of Safety and Efficacy of Drug Delivery by Topical Application versus Drug-eluting Contact Lens in Cataract Surgery. Indian J. Opthalmol. 2021, 69, 466-467.
-

- 11. Liana, D. W.; Stephen, A. D.; Mark, E. B. In vivo Drug Delivery via Contact Lenses: The Current State of the Field from Origins to Present. J. Drug Del. Sci. Tech. 2021, 63, 102413.
-

- 12. Subir, C.; Prashant, U.; Manjul, M.; Srividya, M., Akshara, M. R.; Kamali, N.; Zahra, S. Z.; Sayeda, F. I.; Santosh, K. M. Advances in Chemistry and Composition of Soft Materials for Drug Releasing Contact Lenses. RSC Adv. 2020, 10, 36751-36777.
-

- 13. Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic Resistance of Bacterial Biofilms. Int. J. Antimicrob. Agents 2010, 35, 322-332.
-

- 14. Kusuma, V. A.; Gunawan, G.; Smith, Z. P.; Freeman, B. D. Gas Permeability of Cross-linked Poly(ethylene-oxide) Based on Poly(ethylene glycol) Dimethacrylate and a Miscible Siloxane Co-monomer. Polymers 2010, 51, 5734-5743.
-

- 15. Parsons, C.; McCoy, C. P.; Gorman, S. P.; Jones, D. S.; Bell, S.; Brady, C. McGlinchey, S. M. Anti-infective Photodynamic Biomaterials for the Prevention of Intraocular Lens-associated Infectious Endophthalmitis. Biomaterials 2009, 30, 597-602.
-

- 16. Murakami, K.; Aoki, H.; Nakamura, S.; Nakamura, S. I.; Takikawa, M.; Hanzawa, M.; Ishihara, M. Hydrogel Blends of Chitin/chitosan, Fucoidan and Alginate as Healing-impaired Wound Dressings. Biomaterials 2010, 31, 83-90.
-

- 17. Young, M. D.; Benjamin, W. J. Oxygen Permeability of the Hypertransmissible Contact Lenses. Eye Contact Lens 2003, 9, 17-21
-

- 18. Carney, F. P.; Nash, W. L.; Sentell, K. B. The Adsorption of Major Tear Film Lipids in vitro to Various Silicone Hydrogels over Time. Investig. Ophthalmol. Vis. Sci. 2008, 49, 120-124.
-

- 19. Ophthalmics-Contact Lenses-Standard Terminology, Tolerances, Measurements and Physicochemical Properties; ANSI Z80.20-2004, https://webstore.ansi.org/.
- 20. Elisseeff, J.; Puleo, C.; Yang, F.; Sharma, B. Advances in Skeletal Tissue Engineering with Hydrogels. Orthod. Craniofac. Res. 2005, 8, 150-161.
-

- 21. Lin, C. C.; Andrew, T. M. Hydrogels in Controlled Release Formulations: Network Design and Mathematical Modeling. Adv. Drug Deliv. Rev. 2006, 58, 1379-1408.
-

- 22. Gun'ko, V. M.; Savina, I. N.; Mikhalovsky, S. V. Properties of Water Bound in Hydrogels. Gels 2017, 3, 37, 1-30.
-

- 23. Efron, N. Contact Lens Practice; Elsevier Health Sciences: Brisbane, 2010; pp 67-83.
- 24. Musgrave, C. S. A.; Fang, F. Contact Lens Materials: A Materials Science Perspective. Materials 2019, 12, 261-296.
-

- 25. Pozuelo, J.; Compañ, V.; González-Méijome, J. M.; González, M.; Mollá, S. Oxygen and Ionic Transport in Hydrogel and Silicone-hydrogel Contact Lens Materials: An Experimental and Theoretical Study. J. Membr. Sci. 2014, 452, 62-72.
-

- 26. Luensmann, D.; Jones, L. Albumin Adsorption to Contact Lens Materials: A Review. Cont. Lens Anterior Eye. 2008, 31, 179-187.
-

- 27. Holden, B. A.; Sweeney, D. F.; Vannas, A.; Nilsson, K. T.; Efron, N. Effects of Long-term Extended Contact Lens Wear on the Human Cornea. Investig. Ophthalmol. Vis. Sci. 1985, 26, 1489-1501.
- 28. Alfonso, E.; Mandelbaum, S.; Fox, M. J.; Forster, R. K. Ulcerative Keratitis Associated with Contact Lens Wear. Am. J. Ophthalmol. 1986, 101, 429-433.
-

- 29. Yuan, Z. Physical and Cytological Characters of Carbon, Titanium Surface Modified Intraocular Lens in Rabbit Eyes. Graefes Arch. Clin. Exp. Ophthalmol. 2003, 241, 840-844.
-

- 30. Tighe, B.; Franklin, V. Lens Deposition and Spoliation. In The Eye in Contact Lens Wear; Butterworth Heinemann: Boston, 1985; pp 49-100.
- 31. Santos, L.; Rodrigues, D.; Lira, M.; Real Oliverira, M. E. C. D.; Oliveira, R.; Vilar E. Y.-P.; Azeredo, J. The Influence of Surface Treatment on Hydrophobicity, Protein Adsorption and Microbial Colonisation of Silicone Hydrogel Contact Lenses. Cont. Lens Anterior Eye 2007, 30, 183-188.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2022; 46(1): 47-55
Published online Jan 25, 2022
- 10.7317/pk.2022.46.1.47
- Received on Aug 28, 2021
- Revised on Oct 29, 2021
- Accepted on Oct 30, 2021
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Conflict of Interest
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Hyun Mee Lee
-
Department of Optometry & Vision Science, College of Bio and Medical Science, Daegu Catholic University, Hayang-Ro 13-13, Gyeongsan, Gyeongbuk 38430, Korea
- E-mail: hmlee@cu.ac.kr
- ORCID:
0000-0001-6668-5864











 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.