- Fabrication of Tubular Scaffolds with Aligned Fibrous Structures Containing Cells within Pockets Using Electrohydrodynamic Methods
Inseong Choi*, **, Guk Young Ahn*, **, Dong-Hyun Paik*, **, and Sung-Wook Choi*, **,†

*Biomedical and Chemical Engineering, The Catholic University of Korea, 43 Jibong-ro, Wonmi-gu,
Bucheon-si, Gyeonggi-do 14662, Korea
**Department of Biotechnology, The Catholic University of Korea, 43 Jibong-ro, Wonmi-gu,
Bucheon-si, Gyeonggi-do 14662, Korea- 전기유체역학적 기법을 이용한 정렬된 섬유구조체를 갖는 세포 함유 스캐폴드 제조
*가톨릭대학교 바이오메디컬화학공학과, **가톨릭대학교 생명공학과
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
Fibrous tubular scaffolds with cells in pockets were fabricated by a combination of electrospinning and electrospraying methods. Cell dispersions were electrosprayed onto a rotating drum while a polymer solution was electrospun at the same time for the production of the fibrous structure. The electrospun fibers were aligned onto the rotating drum using conductive wires, creating pockets with cells present within the scaffold. The thickness of the scaffold and the number of the cells were controlled by varying the processing time. These scaffolds could be potnetially utilized for the tissue engineering of nerve, bone, and blood vessels.
전기방사 및 전기분무의 두 가지 전기유체역학적 기법을 이용하여 포켓 내에 세포가 함입되어 있고 정렬된 섬유구조체를 갖는 관형 스캐폴드를 제조하였다. 스캐폴드 제조를 위해 분산액을 회전 드럼에 전기 분무하는 동시에 섬유구조를 형성하기 위해 고분자 용액을 동시에 전기방사하였으며, 전도성 와이어를 통해 정렬된 구조의 스캐폴드가 형성되고, 전기분무를 통해 섬유구조체 내부에 존재하는 포켓에 세포를 함입시켰다. 제조된 스캐폴드의 두께 및 세포 농도는 분무와 방사시간을 통해 조절되었으며 해당 스캐폴드 제조기술은 신경, 뼈 및 혈관의 조직재생에 활용할 수 있다.
Fibrous tubular scaffolds with cells in pockets were fabricated by a combination of electrospinning and electrospraying methods. A cell dispersion was electrosprayed onto a rotating drum while a polymer solution was electrospun simultaneously for the production of the fibrous structure. The electrospun fibers were aligned onto the rotating drum using conductive wires and results showed the cells were present within the pockets in the scaffold. The thickness and the number of the cells were controlled by varying the processing time. These scaffolds could potentially be used for tissue engineering of nerve, bone, and blood vessels.
Keywords: electrospray, electrospinning, fibrous structure, cell entrapment, scaffold.
This work was supported by the Korea Medical Device Development Fund grant funded by the Korea government (the Ministry of Science and ICT, the Ministry of Trade, Industry and Energy, the Ministry of Health & Welfare, the Ministry of Food and Drug Safety) (Project Number: 202012D21-02), the Research Fund, 2020 of The Catholic University of Korea, and the National Research Foundation of Korea (NRF) grant funded by the Korea government (MIST) (No. 2021R1A2C1003865).
Electrospinning processes have been widely employed to fabricate fibrous-structured scaffolds for tissue engineering because of their large surface area, high aspect ratio, porosity, and the presence of small pore structures on the fibers. Compared to other scaffolds, the electrospun scaffold displays the characteristic features of extra cellular matrix (ECM)-like structures.1-3 Also, electrospun fibrous scaffold architectures may have various effects on cell behaviors.4 Yang et al. confirmed that cells stretched and grew parallel to the fiber, when neuronal stem cells were cultured in an aligned fibrous scaffold.5 Li et al. reported that the chondrogenesis of mesenchymal stem cells on nanofibrous scaffolds was better than that of high-density cell pellet culture.6 Xu et al. reported that the culture of human smooth muscle cells in an aligned fibrous scaffold resulted in directional growth in a form ideal for blood vessel engineering.7
Similar to electrospinning, electrospraying is an electrohydrodynamic method consisting of a single-stage technique of liquid atomization by means of electrical forces that enables the generation of micro/nanoparticles within a narrow size distribution.8 While the electrospraying process follows the same principles as electrospinning, the solution below a critical concentration is spun into a sphere rather than a fiber. Therefore, electrospraying methods can produce an aerosol of charged droplets, precisely controlled in size and shape. This feature can be used to produce various forms of polymer coatings on medical implants, which can help cellular adhesion to the implant surface.9 By electrospraying, living cells in the medium can form micro-droplets without harmful effects on the cells at the molecular level, and micro-sized cell-containing droplets are produced.10 By combining electrospinning and electrospraying, Bock et al. fabricated fibrous scaffolds containing microparticles for drug delivery of protein in a controlled manner.11
Despite the advantages of electrohydrodynamic methods, control of the pore size in the fibrous scaffold remains a major obstacle. The pore size of the electrospun scaffold is too small for cells to penetrate, making them unable to enter the scaffold.12,13 Many researchers have struggled to overcome this limitation. Several research groups attempted to fabricate a nano/micro fibrous structure to provide a high surface area and to enhance cell penetration. Lee et al. electrospun a poly(L-lactic acid) solution containing a leaching agent (ammonium bicarbonate), later removing the leaching agent to generate the micropores.14 Although the average porosity was increased to microscale, the pores were not well-interconnected, resulting in limited cell penetration. Burdick et al. fabricated a composite electrospun scaffold of poly(ɛ-caprolactone) (PCL) and poly(ethylene oxide) (PEO) and then selectively removed the water-soluble PEO to enhance pore size and interconnectivity, resulting in the presence of cells throughout the scaffold.15
Previously, we developed fiber aligned tubular scaffold using a conductive wire in electrospinning.16 In this study, we fabricated a tubular scaffold with an aligned fibrous structures, containing cells within the pockets of the matrix, using two electrohydrodynamic methods simultaneously. We believe that a fibrous scaffold, aligned in structure with sufficient pore size and interconnectivity, has great potential for use as an engineered tissue scaffold for artificial blood vessel, cartilage, and nerve conduit.
Materials. Poly(ε-caprolactone) (PCL, Mw 70000-90000, St. Louis, Sigma-Aldrich), chloroform, 2,2,2-trifluoroethanol, and 4,6-diamidino 2-phenyl-indole (DAPI) were purchased from Sigma-Aldrich (St. Louis, MO, USA). a-minimum essential medium (a-MEM, without L-ascorbic acid), penicillin, and streptomycin were supplied by Invitrogen (California, USA). Green fluorescent protein (GFP)-expressing NIH/3T3 were purchased from New England Biolabs (Beverly, MA, USA). The culture medium consisted of a-MEM supplemented with 10% fetal bovine serum (FBS) and 1% antibiotics (containing penicillin and streptomycin).
Preparation of Aligned-fibrous Tubular Scaffolds. The fabrication procedure for an aligned-fibrous scaffold was based on our previous study.16 PCL organic solution (10 wt%) in a mixture (8:2) of chloroform and 2,2,2-trifluoroethanol was used for electrospinning. The PCL solution was electrospun onto a rotating drum from a syringe needle (24 G) at flow rate of 0.01 mL/min using a syringe pump (NE-1000, New Era Pump Systems Inc.). The distance between the syringe needle and the rotating drum was kept at 10 cm. The relatively higher boiling point (74 ºC) of 2,2,2-trifluoroethanol than that of chloroform (61 ºC) reduced the evaporation rate, leading to the electrospinning without clogging at the needle tip. A voltage of 10 kV was applied between the syringe needle and the rotating drum/conductive wires (negative ground). The PCL solution was electrospun first using orthogonal conducting wires and then parallel conducting wires. The rotational speed of the drum was consistent at 150 rpm. During electrospinning, the dispersion of GFP-expressing NIH/3T3 cells in culture media (1×105 cells/mL) was simultaneously electrosprayed onto the rotating drum.
Characterization of Aligned-fibrous Tubular Scaffolds. Scanning electron microscopy (SEM, S-4800, Hitachi, Tokyo, Japan) was used to characterize the orientation of PCL fiber in tubular scaffolds. The presence of the electrosprayed living cells within the pockets were confirmed by fluorescence microscopy (FM, IX71, OLYMPUS Co. Ltd., Tokyo, Japan) with DAPI staining.
Cell Culture on Aligned-fibrous Tubular Scaffolds. The resultant tubular scaffolds containing the living cells were gently washed with PBS three times. The GFP-expressing NIH/3T3 cells within the pockets in the tubular scaffolds were cultured in an incubator at 37 °C in a humidified atmosphere containing 5% CO2 using an a-minimum essential medium supplemented with 10% FBS and 1% antibiotics. The media were changed every other day. Fluorescence microscopy was used to observe the proliferation of cells within the pockets at 1, 3, 5, and 7 days.
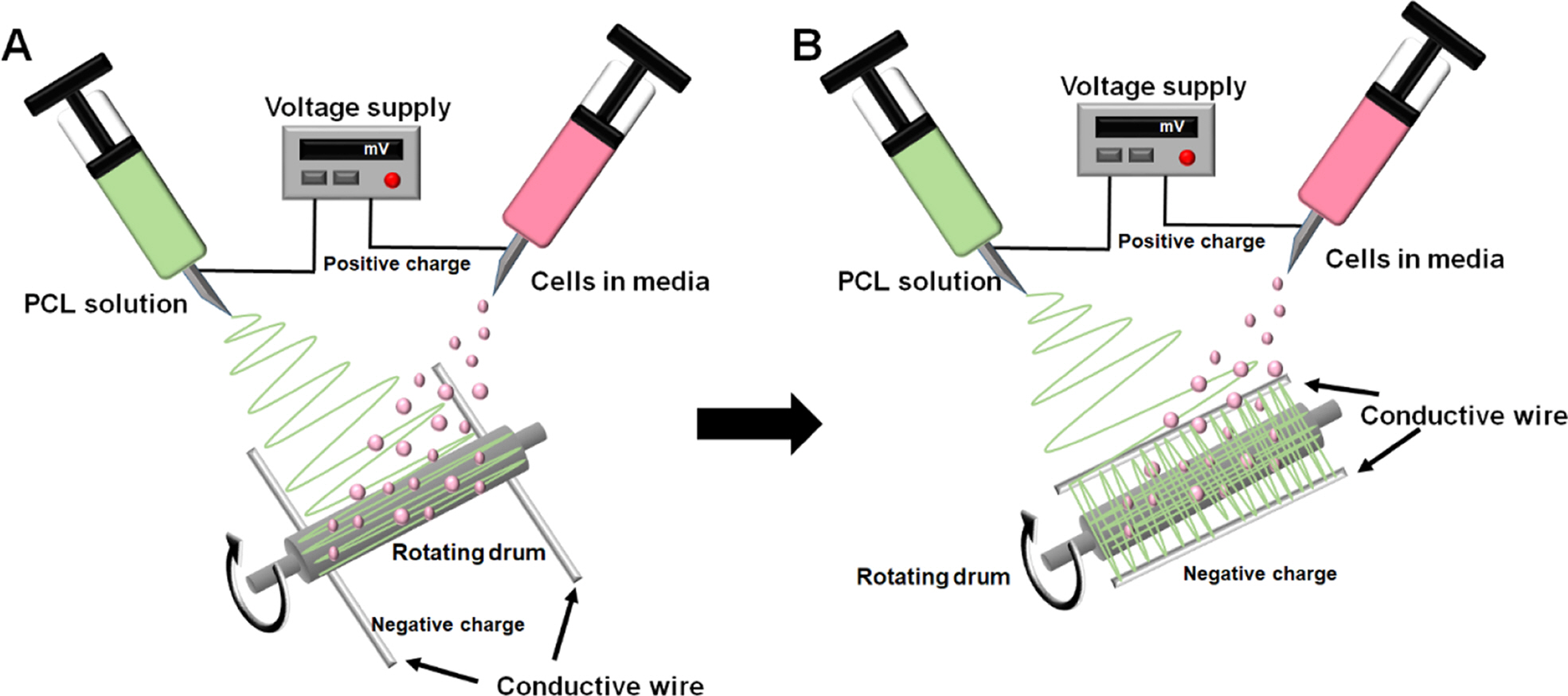
Preparation and Characterization of Tubular Scaffolds. The orientation of fibers can guide cell behaviors including adhesion, stretch, proliferation, and differentiation. Therefore, a rotating drum and two conductive wires were used to develop the aligned fibrous structure. Figure 1 shows the schematic diagram of the electrohydrodynamic setup for electrospinning of the PCL solution and electrospraying of the cells. Fiber direction was controlled by changing the placement of the conductive wires as previously reported.16 Both the electrospun fibers and electrosprayed cells were accumulated onto the rotating drum simultaneously. Figure 1(a) and 1(b) schematically show the fabrication of the parallel and orthogonal fibrous structures with cells, respectively. To mimic the aligned structure of blood vessels, the PCL solution was first electrospun in a parallel fibrous mode and then an orthogonal fibrous mode during the electrospraying of GFP-expressing NIH/3T3 cells.
Figure 2(a) and 2(b) show photographs of the fibrous tubular scaffold, which was prepared by electrospinning first in a parallel fibrous mode, then in an orthogonal fibrous mode. The fibrous tubular scaffold was 4 mm in diameter and 35 mm in length. Figure 2(c) and 2(d) show the SEM images of the inner surface with the parallel fibrous structure and the outer surface with the orthogonal fibrous structure, respectively. The orthogonal fibers exhibited a smaller diameter than the parallel fibers, which is due to the extension by the drum rotation during electrospinning for the orthogonal fibrous structure. It was known that the intimal layer of a natural blood vessel is composed of an endothelial cell layer lining the inner surface.17 Cartilage and nerves also have a structure in which tissues are oriented.18,19 Therefore, an inner surface with a parallel fibrous structure maybe be useful in mimicking various oriented tissues. The tubular scaffold with the opposite direction of fibers is shown in Figure 2(d). These tubular scaffolds with controlled fiber orientation can be used in a variety of tissue regeneration applications by mimicking the natural tissue structure. Tubular scaffolds with medial parallel and lateral orthogonal fiber orientations may serve as conduits for nerve regeneration by guiding nerve cell growth.20,21 The pore size of the fibrous scaffold is a critical factor for cell migration and the diffusion of nutrients and wastes. Therefore, the pore size was characterized from the SEM images. The pore sizes of the inner and outer electrospun layers were measured as 1.36±0.65 and 1.05±0.39 μm, respectively. These ranges of pore size can facilitate the nutrient supply and waste removal throughout the fibrous tubular scaffold. However, the cell migration can be limited due to the small pore size and thus the cells inside the pockets can be protected from other cells. Figures 2(e) and 2(f) show the SEM and fluorescence microscope images of the cross section of the tubular scaffold. The cells in the tubular scaffolds were imaged after with DAPI staining. The cells localized inside the pockets were observed between the electrospun layers, suggesting that the living electrosprayed cells were entrapped in the pockets in the tubular scaffolds during fabrication.
Figure 3(a) and 3(b) show SEM images and plots of thickness of tubular scaffolds with respect to electrospinning time while the flow rate of electrosprayed cell dispersion was kept at 0.06 mL/min. The average thickness of tubular scaffolds increased from 342.±24.8, 641.3±67.6, and 768.7±93.0 μm for 10, 20, and 30 min, respectively. As reported in our previous study, the fibers were more oriented into the guided directions when only electrospinning was employed.16 The fibers created in the combined process of electrospinning and electrospraying were relatively whipped and not well oriented to the conductive wires. The less oriented fibrous structures may be due to the interference by the electromagnetic field of the electrospraying because both the electrohydrodynamic methods are based on the electromagnetic field. It was confirmed that the thickness of tubular scaffolds could be controlled by varying the electrospinning time, suggesting that various organizations can be reproduced sufficiently. In addition, it is easy to control the inner diameter and overall thickness of the tubular scaffolds by controlling the size of the conductive wire.
Figure 4 shows fluorescence microscopy images of cell proliferation on the conventional tubular scaffold and the cell sprayed tubular scaffold with respect to time. In the typical tubular scaffold, the cells grew on the surface, but did not penetrate inside the scaffold. However, the cells of the cell sprayed tubular scaffold can be identified inside the scaffold. The cells also survived for up to 7 days, suggesting that the tubular scaffold had a structure that could supply oxygen and nutrients to the inner cells. Cell invasion and growth in the scaffold are the most important conditions for facilitating ideal tissue regeneration.22 However, there have been various limitations in relation to cell viability during long treatments in vitro and the reproducibility of porosity and pore size.23,24 The combination of our electrospinning and electrospraying methods allowed us to produce tubular scaffolds with aligned fibrous structures containing living cells, and allowed cells to penetrate and grow for a time likely long enough to facilitate efficient tissue regeneration.

|
Figure 1 Schematic diagrams of electrohydrodynamic setup: (a) parallel; (b) orthogonal fibrous structures with the pockets for cells |

|
Figure 2 (a, b) Photographs of the fibrous tubular scaffold; (c, d) SEM images of the inner and outer surface of the fibrous tubular scaffold; (e) SEM image; (f) fluorescence microscope image of the cross-section of the fibrous tubular scaffold with DAPI-stained cells |

|
Figure 3 (a) SEM image of a cross section of tubular scaffold; (b) average thickness size of the scaffold prepared at different processing time (10, 20, and 30 min). |

|
Figure 4 Fluorescence microscopy images of GFP-expressing NIH/3T3 cells seeded on the electrospun-fibrous and cell sprayed electrospun tubular scaffolds at different processing time (1, 3, 5, and 7 days). |
We have successfully fabricated a tubular scaffold with aligned fibers and large enough pockets for cell occupation using a combination of electrospinning and electrospraying. Our approach can be a solution for cell penetration into an electrospun scaffold which is typically pointed out as a major obstacle, because the cells were confined within the pockets during fabrication. The aligned structure of the scaffold may guide the stretch and differentiation of the cells in the pockets of the fibrous scaffolds. The cells within the pockets can be protected from the outer environment. The presence of cells (e.g., stem cells) also may release specific molecules for therapy. This combined strategy of electrospinning and electrospraying is not limited by cell types. Our next goal will be focused on the development of a cell-containing patch capable of releasing therapeutic molecules.
- 1. Agarwal, S; Wendorff, J. H.; Greiner, A. Progress in the Field of Electrospinning for Tissue Engineering Applications. Adv. Mater. 2009, 21, 3343-3351.
-

- 2. Teo, W. E.; Inai, R. Ramakrishna S. Technological Advances in Electrospinning of Nanofibers. Sci. Technol. Adv. Mater. 2011, 12, 013002.
-

- 3. Ahn, G. Y.; Ryu, T. K.; Choi, Y. R.; Lee, M. J.; Choi, S. W. Fabrication and Optimization of Nanodiamonds-composited Poly(ε-caprolactone) Fibrous Matrices for Potential Regeneration of Hard Tissue. Biomater. Res. 2018,22, 16.
-

- 4. Nisbet, D. R.; Forsythe, J. S. Review Paper: A Review of the Cellular Response on Electrospun Nanofibers for Tissue Engineering. J. Biomater. Appl. 2009, 24, 7.
-

- 5. Yang, F.; Murugan, R.; Wang, C.; Ramakrishna, S. Electrospinning of Nano/Micro Scale Poly(L-Lactic Acid) Aligned Fibers and Their Potential in Neural Tissue Engineering. Biomaterials 2005, 26, 2603-2610.
-

- 6. Li, W.; Tuli, R.; Okafor, C.; Derfoul, A.; Danielson, K. G.; Hall, D. J.; Tuan, R. S. A Three-Dimensional Nanofibrous Scaffold for Cartilage Tissue Engineering Using Human Mesenchymal Stem Cells. Biomaterials 2005, 26, 599-609.
-

- 7. Xu, C. Y.; Inai, R.; Kotaki, M.; Ramakrishna, S. Aligned Biodegradable Nanofibrous Structure: A Potential Scaffold for Blood Vessel Engineering. Biomaterials 2004, 25, 877-886.
-

- 8. Sosnik, A. Production of Drug-loaded Polymeric Nanoparticles by Electrospraying Technology. J. Biomed. Nanotechnol. 2014, 10, 2200-2217.
-

- 9. Wang, J.; Jansen, J. A.; Yang, F. Electrospraying: Possibilities and Challenges of Engineering Carriers for Biomedical Applications - A Mini Review. Front. Chem. 2019, 7, 258.
-

- 10. Yunmin, M.; Yuanyan, L.; Haiping, C.; Qingxi, H. Application and Analysis of Biological Electrospray in Tissue Engineering. Open Biomed. Eng. J. 2015, 9, 133-157.
-

- 11. Bock, N.; Woodruff, M. A.; Steck, R.; Hutmacher, D. W.; Farrugia, B. L.; Dargaville, T. R. Composites for Delivery of Therapeutics: Combining Melt Electrospun Scaffolds with Loaded Electrosprayed Microparticles. Macromol. Biosci. 2014, 14, 202-214.
-

- 12. Malakhov, S. N.; Khomenko, A. Y.; Belousov, S. I.; Prazdnichnyi, A. M.; Chvalun, S. N.; Shepelev, A. D.; Budyka, A. K. Method of Manufacturing Nonwovens by Electrospinning from Polymer Melts. Fibre Chem. 2009, 41, 355-359.
-

- 13. Shi, X.; Zhou, W.; Ma, D.; Ma, Q.; Bridges, D.; Ma, Y.; Hu, A. Electrospinning of Nanofibers and Their Applications for Energy Devices. J. Nanomater. 2015, 140716.
-

- 14. Lee, Y. H.; Lee, J. H.; An, I. G.; Kim, C.; Lee, D. S.; Lee, Y. K.; Nam, J. D. Electrospun Dual-porosity Structure and Bio- degradation Morphology of Montmorillonite Reinforced PLLA Nanocomposite Scaffolds. Biomaterials 2005, 26, 3165-3172.
-

- 15. Baker, B. M.; Gee, A. O.; Metter, R. B.; Nathan, A. S.; Marklein, R. A.; Burdick, J. A.; Mauck, R. L. The Potential to iMprove Cell Infiltration in Composite Fiber-aligned Electrospun Scaffolds by the Selective Removal of Sacrificial Fibers. Biomaterials 2008, 29, 2348-2358.
-

- 16. Jeong, K. Y.; Paik, D. H.; Choi, S. W. Fabrication of Tubular Scaffolds with Controllable Fiber Orientations Using Electro- spinning for Tissue Engineering. Macromol. Mater. Eng. 2014, 299, 1425-1429.
-

- 17. Vaz, C. M.; Tuijl, S. V.; Bouten, C. V. C.; Baaijens, F. P. T. Design of Scaffolds for Blood Vessel Tissue Engineering Using a Multi-layering Electrospinning Technique. Acta Biomater. 2005, 1, 575-582.
-

- 18. Bhattacharjee, M.; Miot, S.; Gorecka, A.; Singha, K.; Loparic, M.; Dickinson, S.; Das, A.; Bhavesh, N. S.; Ray, A. R.; Martin, I.; Ghosh, S. Oriented Lamellar Silk Fibrous Scaffolds to Drive Cartilage Matrix Orientation: Towards Annulus Fibrosus Tissue Engineering. Acta Biomater. 2012, 8, 3313-3325.
-

- 19. Huang, C.; Tang, Y.; Liu, X.; Sutti, A.; Ke, Q.; Mo, X.; Wang, X.; Morsic, Y.; Lin, T. Electrospinning of Nanofibres with Parallel Line Surface Texture for Improvement of Nerve Cell Growth. Soft Matter. 2011, 7, 10812.
-

- 20. Madduri, S.; Papaloïzos, M.; Gander, B. Trophically and Topographically Functionalized Silk Fibroin Nerve Conduits for Guided Peripheral Nerve Regeneration. Biomaterials 2010, 31, 2323-2334.
-

- 21. Wang, H. B.; Mullins, M. E.; Cregg, J. M.; McCarthy, C. W.; Gilbert, R. J. Varying the Diameter of Aligned Electrospun Fibers Alters Neurite Outgrowth and Schwann Cell Migration. Acta Biomater. 2010, 6, 2970-2978.
-

- 22. Wang, K.; Zhu, M.; Li, T.; Zheng, W.; Li, L.; Xu, M.; Zhao, Q.; Kong, D.; Wang, L. Improvement of Cell Infiltration in Electrospun Polycaprolactone Scaffolds for the Construction of Vascular Grafts. J. Biomed. Nanotechnol. 2014, 10, 1588-1598.
-

- 23. Stankus, J. J.; Guan, J.; Fujimoto, K.; Wagner, W. R. Microinteg- rating Smooth Muscle Cells into a Biodegradable, Elastomeric Fiber Matrix. Biomaterials 2006, 27, 735-744.
-

- 24. Baker, B. M.; Mauck, R. L. The Effect of Nanofiber Alignment on the Maturation of Engineered Meniscus Constructs. Biomaterials 2007, 28, 1967-1977.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2021; 45(6): 904-909
Published online Nov 25, 2021
- 10.7317/pk.2021.45.6.904
- Received on Jun 29, 2021
- Revised on Jul 30, 2021
- Accepted on Aug 13, 2021
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Sung-Wook Choi
-
*Biomedical and Chemical Engineering, The Catholic University of Korea, 43 Jibong-ro, Wonmi-gu,
Bucheon-si, Gyeonggi-do 14662, Korea
**Department of Biotechnology, The Catholic University of Korea, 43 Jibong-ro, Wonmi-gu,
Bucheon-si, Gyeonggi-do 14662, Korea - E-mail: choisw@catholic.ac.kr
- ORCID:
0000-0002-5075-8798









 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.