- Adjusting Water Absorption, Stimulus-Response and Adsorption Capacity for Toxic Heavy Metal Ions of Poly(acrylamide-co-potassium methacrylate) Hydrogel by Poly(ethylene glycol) Addition
Syed Wasim Ali†
 , Munib Ahmed Shafique*, Muhammad Arif Malik**, Muhammad Saad, Syeda Rida Amber***, and Aatiqa Jabeen****
, Munib Ahmed Shafique*, Muhammad Arif Malik**, Muhammad Saad, Syeda Rida Amber***, and Aatiqa Jabeen****Chemistry Division, Pakistan Institute of Nuclear Science and Technology (PINSTECH), PO 45650, Nilore, Islamabad, Pakistan
*Central Analytical Facility Division, Pakistan Institute of Nuclear Science and Technology (PINSTECH), PO 45650, Nilore, Islamabad, Pakistan
**Department of Chemistry and Biochemistry, Hampton University, Hampton, VA 23668, USA
***Chemistry Department, University of Wah, Quaid Avenue, PO 47040, Wah Cantt, Pakistan
****Department of Chemistry, Quaid-i-Azam University, Islamabad 45320, Pakistan- Poly(ethylene glycol) 첨가에 따른 Poly(acrylamide-co-potassium methacrylate) 하이드로젤의 독성 중금속 이온에 대한 수분흡수, 자극반응 및 흡착 능력 조절
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
Hydrogels, composed of poly(acrylamide-potassium methacrylate), cross-linked with N,N'-methylene bis-acrylamide, and having different proportions of poly(ethylene glycol) were synthesized by a free radical copolymerization technique. The effect of the cross-linkage and poly(ethylene glycol) contents on swelling in water, diffusion kinetics of water in the hydrogel matrix, stimuli response to pH, ionic strength of the external solution, and capacity for chromium(III) and uranyl ions from polluted water samples were evaluated. The results show that the swelling in water decreased. Still, the diffusion rate of water in the copolymer matrix increased with an increase in the cross-linkage and an increase in the poly(ethylene glycol) amount under the conditions of this study. Poly(ethylene glycol) addition provided another tool to adjust the swelling, diffusion kinetics, and adsorption characteristics of the hydrogel. For example, poly(ethylene glycol) addition made the hydrogel adsorb uranyl ions from polluted water.
Interpenetrating structure of poly(ethylene glycol) and poly(acrylamide-potassium methacrylate) hydrogel provides a new tool to adjust important hydrogel characteristics, like swelling in water, absorption kinetics and capacity for heavy metal pollutant removal from wastewater.
Keywords: free radical polymerization, diffusion coefficient, chromium(III), uranyl ion.
Hydrogels are three-dimensional polymer networks that can accommodate a massive quantity of water in their matrix. They carry ionic and polar functional groups like amides,1-3 amidoxime,4 carboxylic acid,3,5-8 hydroxamic acid,9 hydroxyl,5,10 phosphoric acid,11 sulphonic acid1 etc. on a flexible polymer backbone that make these polymers hydrophilic and swell-able. The hydrogels are usually employed for pollution remediation from water because they can absorb and retain the pollutants by ion-exchange, ion-dipole interactions, hydrogen bonding, dipole-dipole interaction and/or van der Walls forces. For example, they are employed to remove dyes from textile industry wastewater,1,2,9 bovine serum albumin,6 drug delivery system,7 insect pest management,12 entrapment of protein and peptides,11 removal of heavy metal ions,1,3 extraction of uranium compounds from seawater4 etc. One of the focus of this study is water absorption capacity and kinetics of water absorption-desorption of the hydrogel synthesized as these are essential characteristics of the hydrogels for multiple applications.6,9,13-17
A combination of two or more polymers or additives in polymers may enhance the characteristics of the hydrogels. For example, poly(arylamide) mixed with poly(acrylic acid) results in faster swelling kinetics, which is beneficial for removing unwanted water from the oil field.8 Other examples include: addition of maleic acid in acrylamide hydrogel improved swelling and adsorption of BSA,6 poly(hydroxyethyl methacrylate-hydroxyethyl acrylate) for fabrication of soft contact lenses,10 temperature-responsive properties by adding N-isopropyl acrylamide in acrylamide hydrogels,2 chitosan grafted with poly(acrylic acid-acrylamide) for high swelling behavior with salt and pH-response properties.18 Removal of uranium in the presence of other metal ions from an aqueous solution is a challenging job that often required special matrix or special preparation methods/techniques. To solve this problem, the combination of polymeric hydrogels was found very useful, e.g. poly(acrylamide-ethylenediaminetetraacetic acid) hydrogel,3 poly(2-hydroxyethyl methacrylate/maleic acid) hydrogels,5 natural occurring polymer chitosan with polyvinyl- alcohol,19 poly(vinylpyrrolidone/acrylic acid) hydrogels,20 chitosan entrapped in poly acrylamide,21 poly(N-iso-propyl acryl amide/maleic acid) hydrogels22 etc. This study explored improving uranyl ion removal from polluted water by addition of poly(ethylene glycol) in poly(acrylamide-potassium methacrylate).
Chemicals. All chemicals were of analytical grade and were used as received. Acrylamide, poly(ethylene glycol) [Mw ~6000 Da], uranyl nitrate, chromium nitrate, sodium acetate, boric acid, sodium chloride, sodium hydroxide, ammonium chloride, benzene, n-heptane, and potassium hydroxide were purchased from Merck. N,N'-methylene-bis-acrylamide, and methacrylic acid were obtained from Fluka. Potassium persulphate, potassium sulphate, calcium chloride, potassium and hydrochloric acid were purchased from Riedel-de-Haёn. Demineralized distilled water having conductivity ~1.5 μS/cm was used in all the experiments.
Synthesis of Hydrogels. The known amount of poly(ethylene glycol) was dissolved in 50 mL of water at 65 °C under constant stirring for one hour, and then the solution was cooled to room temperature. Acrylamide solution (10%) was prepared by dissolving 5.0 g acrylamide in 50 mL of distilled water. Then 6.02 g of methacrylic acid was neutralized in an ice bath by drop-wise addition of 4 N KOH solution to 75% neutralization. In the subsequent step, all three solutions were mixed together under constant stirring of 200 rpm for one hour at room temperature. The pH of the resulting mixture was 4.9 to 5.0 in all the experiments. N,N'-methylene-bis-acrylamide was added as a cross-linking agent.
Potassium persulphate was used as an initiator in this study and was kept at 1% of the total monomers used. The mixture was transferred to poly(vinyl chloride) straws of 7 mm diameter, having one end sealed. After sealing the other end of the straws, the mixture was polymerized in a water bath at 75 °C for 2 h. Both monomers i.e. potassium methacrylate & acrylamide undergone polymerization in the presence of N,N'-methylene-bis-acrylamide as a cross-linking agent as given in Scheme 1. Poly(ethylene glycol) formed an interpenetrating network with newly formed cross-linked poly(potassium methacrylate-acrylamide). The straws were then cut into 25 mm pieces and the hydrogel was removed from the poly(vinyl chloride) shell material of the straws. The pieces of hydrogels were made to swell in excess demineralized distilled water until equilibrium was established, followed by repeated washing with fresh demineralized distilled water in order to remove any unreacted monomers left. Then, these synthesized hydrogels were repeatedly subjected to five water absorption/desorption cycles with the subsequent inspection to ensure no sign of any damage. The hydrogel samples were then air-dried in a fume cupboard at room temperature in a dust-free environment until they reached a constant weight. A NICOLET 6700 FTIR spectrophotometer from Thermo Fisher Scientific, US was employed to record the FTIR spectra from 4000-400 cm-1 using Attenuated Total Reflectance mode (ATR). Moisture contents present in the samples were minimized prior to analysis.
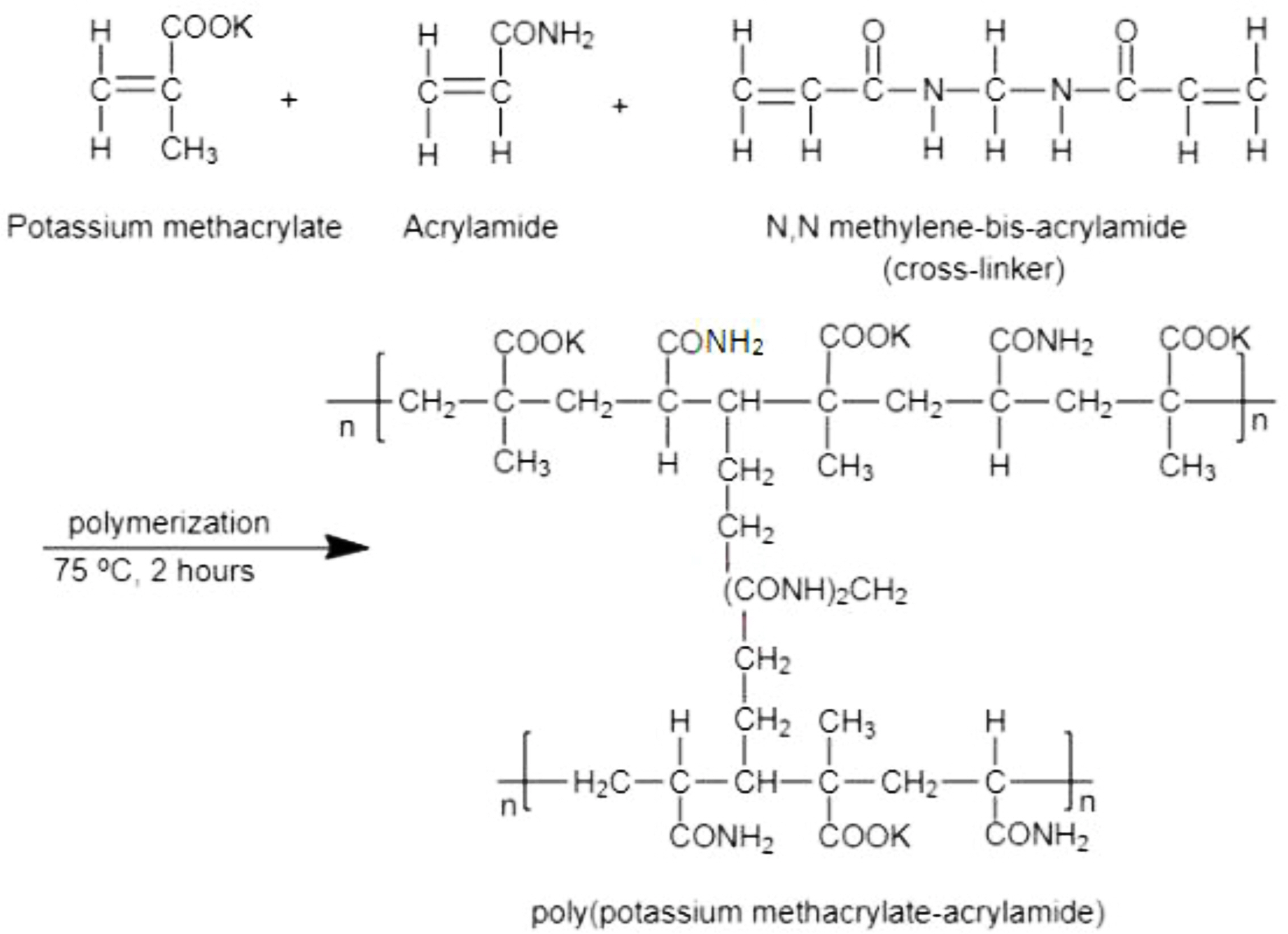
Scheme 1. Polymerization of potassium methacrylate and acrylamide in the presence of N,N'-methylene-bis-acryamide as a cross-linking agent.
Equilibrium Swelling Studies. A piece of the dried hydrogel was weighed and immersed in water at 25±0.1 °C. The hydrogel was then removed from the water after a known interval of time (t), and subsequently surface dried with the help of a laboratory tissue paper, and weighed. The hydrogel was immersed in the water again. The process was repeated until a constant swollen weight was achieved. The percentage mass swelling and the equilibrium water content of the hydrogel were calculated by using the following eq.
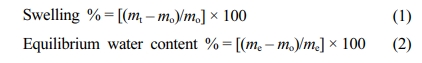
Where mt is the mass of the swollen hydrogel at time t, me is the mass of the swollen hydrogel at equilibrium, and mo is the mass of the initially dried hydrogel. The diffusion exponent n was calculated by using the following formula.13,15,20
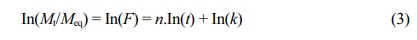
Where Mt and Meq are masses of solvent diffused into the hydrogel at time t and at equilibrium respectively, and k is the diffusion constant. A straight line was obtained by plotting In(F) versus In(t) where the slope of the line was n, and the intercept of the line was In(k). Out of the total swelling at equilibrium, slope of the line was obtained on about 60% hydrogel swelling.
The following equation was used to calculate water diffusion coefficient (D) into the hydrogels matrix.6,7,13

Where t1/2 is the time at which the swollen volume of the hydrogel is one half of the swollen volume at equilibrium and l is the radius of the cylindrical hydrogel samples at the time of completion of the polymerization i.e. 4 mm, which is the inner radius of the poly(vinyl chloride) straw in which the polymerization reaction was carried out.
Effect of pH and Ionic Strength. To investigate the swelling of a synthesized hydrogel in different pH media, buffer solutions were prepared by using the following mixture solutions:23 KCl-HCl 0.1 M in Cl- for pH 1-3, CH3COONa-CH3COOH 0.1 M in CH3COO- for pH 4-6, NH4Cl-NH4OH 0.1 M in NH4+ for pH 6-7.5, H3BO3-NaCl-NaOH 0.1 M in Borate for pH 8-10, and a KCl-NaOH mixture 0.2 M was used for pH 12-13. The salt solutions of KI, KCl, or CaCl2 were 0.9% w/v in water.
Adsorption Study. Standard solutions of different concentrations of chromium nitrate and uranyl nitrate were prepared. In each case, dried hydrogel samples weighing 0.15 g were added into 50 mL of various solutions of uranium or chromium in a conical flask with a stopper and left at room temperature without stirring for 24 h. The residual solution in the flask was analyzed for chromium or uranium content in ppm by an ICP 6500 inductivity coupled plasma-optical emission spectrometer (ICP-OES) from Thermo Fisher Scientific, UK with ITEVA (Ver. 8.0) operating software. The instrument was equipped with a high performance solid state charge injection device camera system. The high precision peristaltic pump was used for sample injection into plasma with 12 rollers and 4 channels with adjustable speed (0-12pm). Uranium and chromium standards were used for the calibration of ICP-OES. The adsorption capacity of the hydrogel was calculated from the difference between the initial and the final concentrations of the metal ions by using following relationship.

Where qe is the adsorption capacity in mg/g, C0 and Ce are the initial and final concentrations of the metal ions, respectively in mg/L, V is the total volume of solution in liters, and m is the weight of the dried hydrogels in grams.
The hydrogel exhausted after the adsorption was regenerated with 2 M HCl and repeatedly re-used for about 5 or 6 cycles with no damage noticed in the hydrogel.
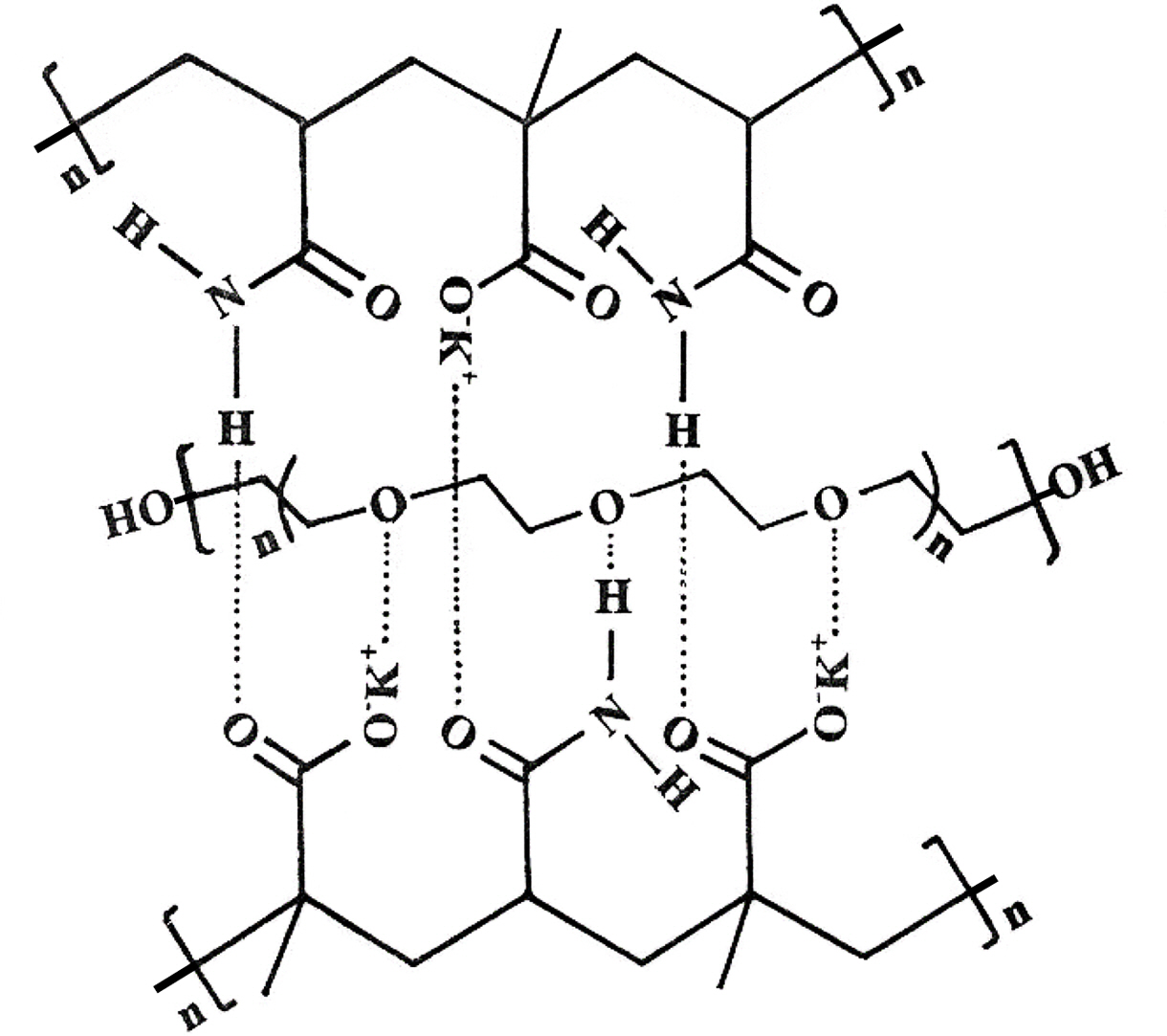
The hydrogels prepared without poly(ethylene glycol) were transparent, and those with poly(ethylene glycol) were opaque in appearance. Hydrogen bonding between oxygen atoms of poly(ethylene glycol) and protons of the amide groups hold the poly(ethylene glycol) in the matrix of the copolymer.24 The hydrogen bonding increases upon acidification of the medium because protons replace potassium ions on the carboxylate groups. The hydrogen bonding also exists between proton of -NH2 and oxygens of -COOK groups, as shown in Scheme 2. The dependence of hydrogen bonding on the acidity of the medium, i.e., pH, imparts a pH stimulus-responsive character to the hydrogel, as will be described later.
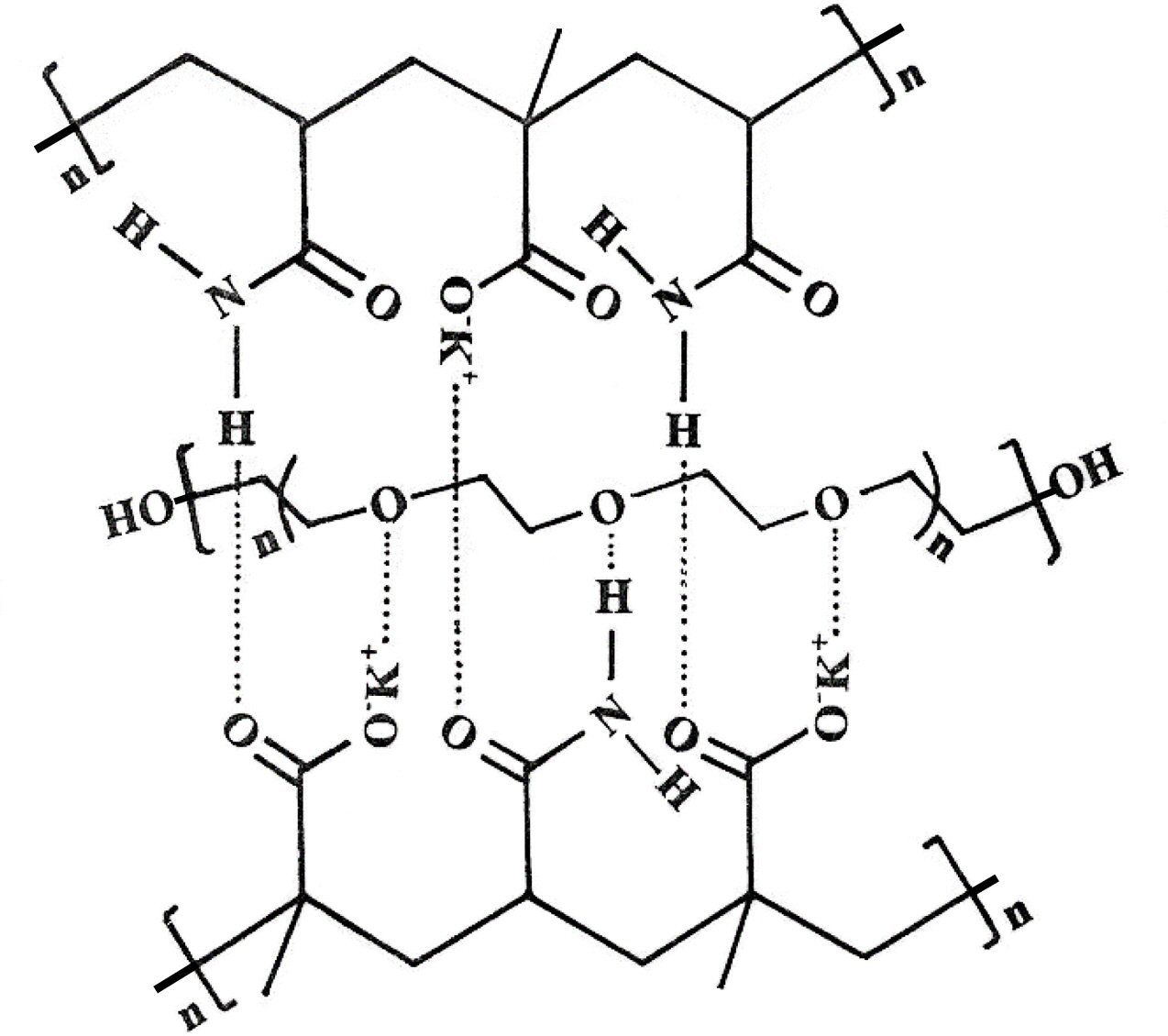
Scheme 2. Shows the intermolecular bonding between the -COOK and -NH2 groups of two polymeric chains and ether oxygen of entwined grafted chain of poly(ethylene glycol) in copolymeric potassium methacrylate and acrylamide hydrogels.
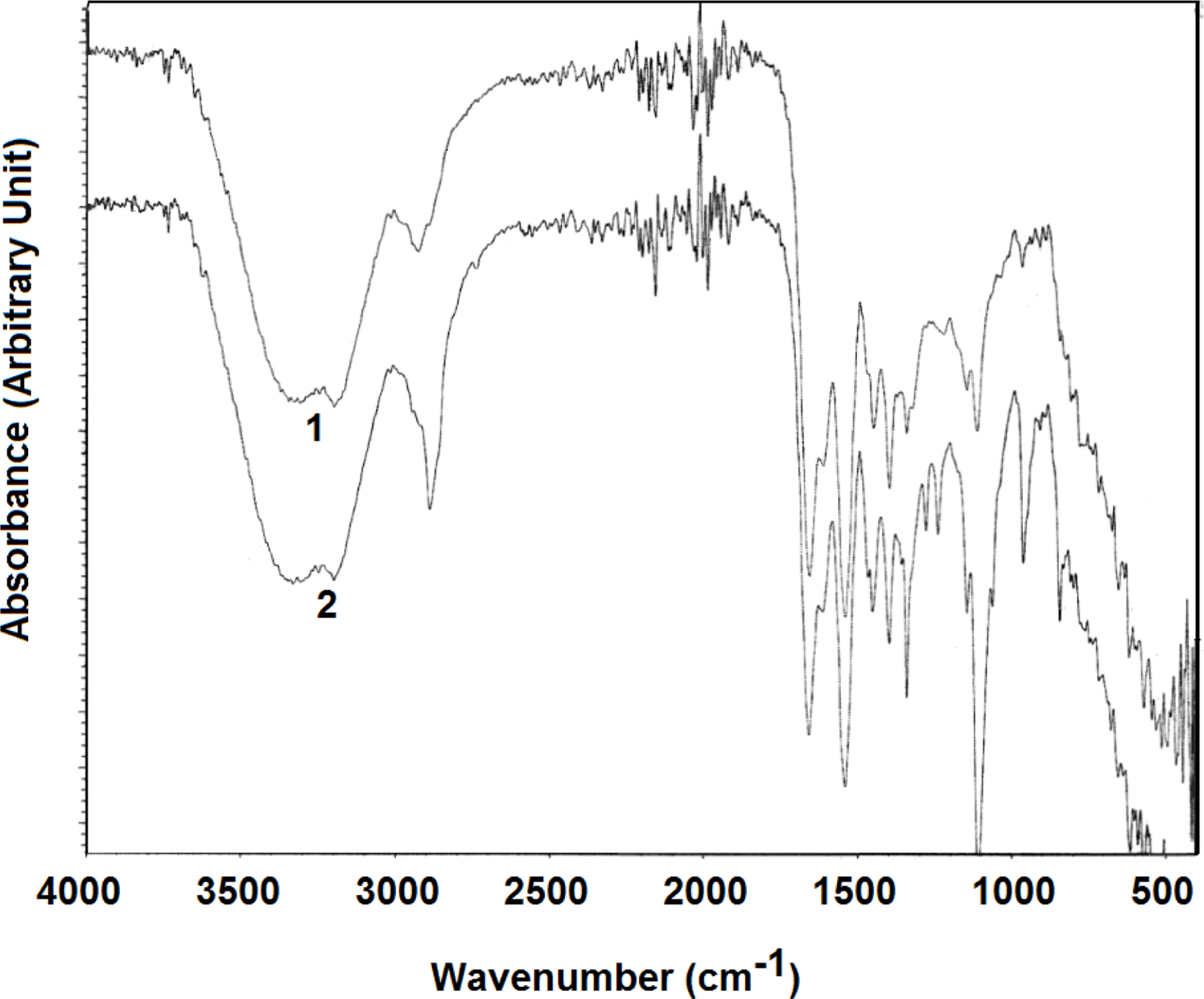
Figure 1 shows the FTIR spectra of the hydrogel with and without poly(ethylene glycol). Both spectra show a broad signal in the 3100-3600 cm-1, characteristic of the amide N-H. Residual moisture absorbs in the same region, but this interference can be considered negligible because copolymers were oven-dried till constant weight to remove the water. The C=O signal of amide and carboxylate groups are observed at around 1657 cm-1 and 1520 cm-1, respectively, in both spectra. Characteristic C-H single of hydrogen on sp3-hybridized carbon around 2930 cm-1 significantly increased upon addition of poly(ethylene glycol). Each repeating segment in poly(ethylene glycol) contains two sp3-hybridized carbons, i.e., -CH2CH2- part, which is responsible for the increased signal around 2930 cm-1. Similarly, a significantly increased absorption around 1108 cm-1 upon addition of poly(ethylene glycol) is attributed to ether groups (C-O bonds) in poly(ethylene glycol). These results confirm poly(ethylene glycol) additive in the hydrogel.
Effect of Cross-linkage on Swelling in Water. The percentage of cross-linking monomer, i.e. the percentage of N,N'-methylene-bis-acrylamide in the monomer mixture is expressed as %-cross-linkage in this manuscript. It should be mentioned here that the actual cross-linkage might be less than the percentage of cross-linking monomer added. Some of the cross-linking monomers might not have polymerized or might have one of the two vinyl groups left unreacted. Based on the fact that the monomer and the cross-linking monomer have similar functional groups and similar reactivity, it can be said that the actual cross-linkage is directly proportional to the proportion of the cross-linking monomer in the polymerization mixture.
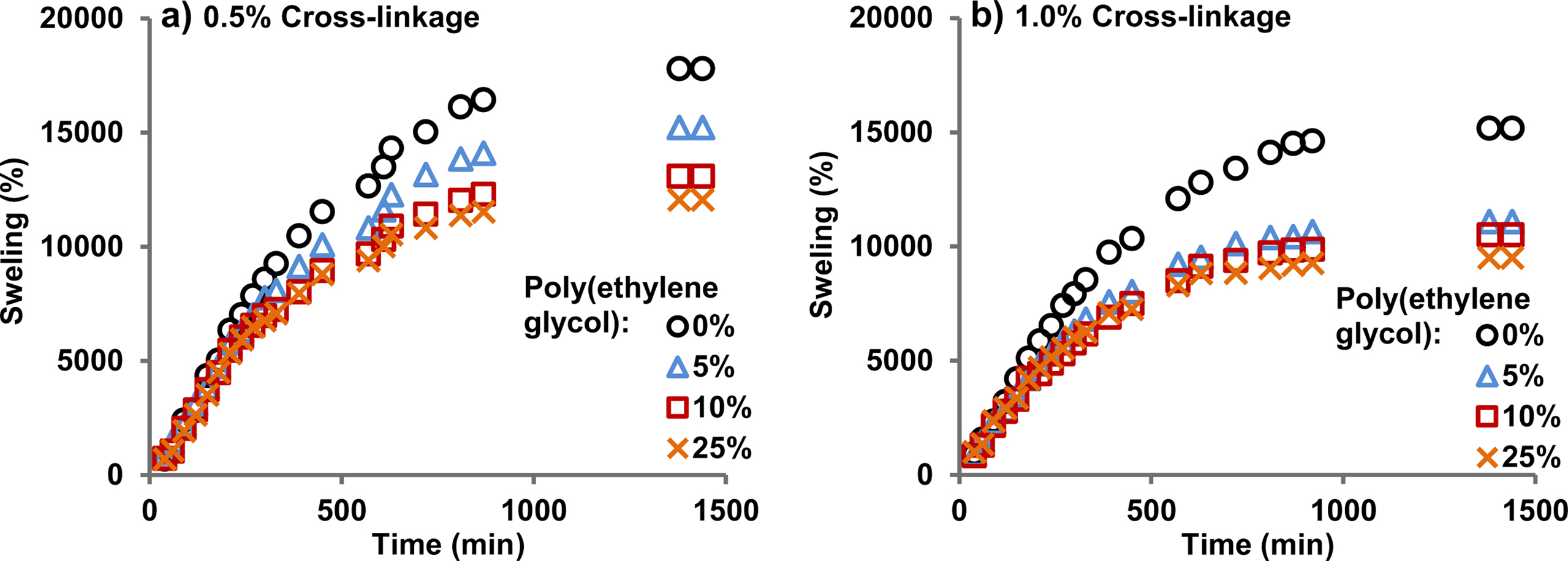
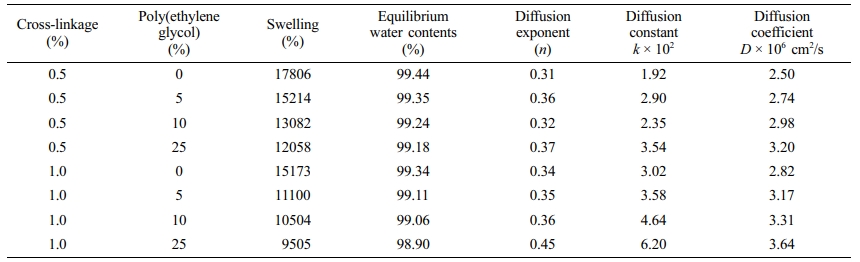
Table 1 shows the swelling percentage, equilibrium water contents percentage, diffusion exponent (n), diffusion constant (k), and diffusion coefficient (D) of the synthesized hydrogels for 0.5% and 1.0% cross-linkage in the monomers. The swelling percentage shows the total water uptake by the hydrogels, whereas the equilibrium water content percentage is the ratio of the water to the polymeric hydrogels in the matrix. The results show that the swelling percentage is significantly higher for the case of 0.5% cross-linkage compared to the 1.0% cross-linkage for the same poly(ethylene glycol) content in all the cases compared. The same trend is observed in the case of equilibrium water content percentage though the difference in the values was smaller. The increase in the cross-links makes the copolymer chains less flexible. It reduces the pores size i.e. the distances between cross-links and chains in the copolymer matrix resulting in lesser swelling in water.
Figure 2 shows the time-dependent swelling curves of hydrogels in water with various poly(ethylene glycol) and 0.5% and 1.0% cross-linkage concentrations. All the hydrogels swelled with time until an equilibrium was reached. According to equilibrium swelling theory, the absorption of solvent continues until the solvent chemical potentials in the polymer phase and the free solutions are equal.14 A comparison of Figure 2(a-b) shows that hydrogels with 0.5% cross-linking swell more than 1.0% cross-linking. This conclusion is supported by the swelling percentage and equilibrium water content percentage data, shown in Table 1. Filling of the pre-existing or dynamically formed spaces between the cross-links and the copolymer chains by the solvent causes the swelling. Cross-linking reduces the spaces between the cross-links and the chains, and it also hinders the mobility of the polymer chains resulting in lesser swelling.13
Effect of Poly(ethylene glycol) Content on Swelling in Water. Figure 2 shows that with an increase in the amount of poly(ethylene glycol) from 0% to 25% in the polymer network, the equilibrium swelling decreased for the same degree of cross-linkage. Although the ether groups in poly(ethylene glycol) are hydrophilic, they are less hydrophilic than the ionic groups such as -COO-K+. The reduced ratio of the ionic groups is one factor that explains the observed decrease in swelling upon poly(ethylene glycol) addition. This phenomenon may be explained in terms of network density because as the amount of poly(ethylene glycol) increases more chains entwined the polymeric chains of poly(acrylamide-co-potassium methacrylate. Eventually, the density of network structure increases and resulted less swelling and this observation is consistent with our previously reported results in which poly(vinyl alcohol) was incorporated in acrylamide/potassium acrylate hydrogel system.13 A second factor explaining it is that oxygen atoms in poly(ethylene glycol) chains form hydrogen bonds with protons of amide groups that has almost the same effect as an increase in cross-linkage.
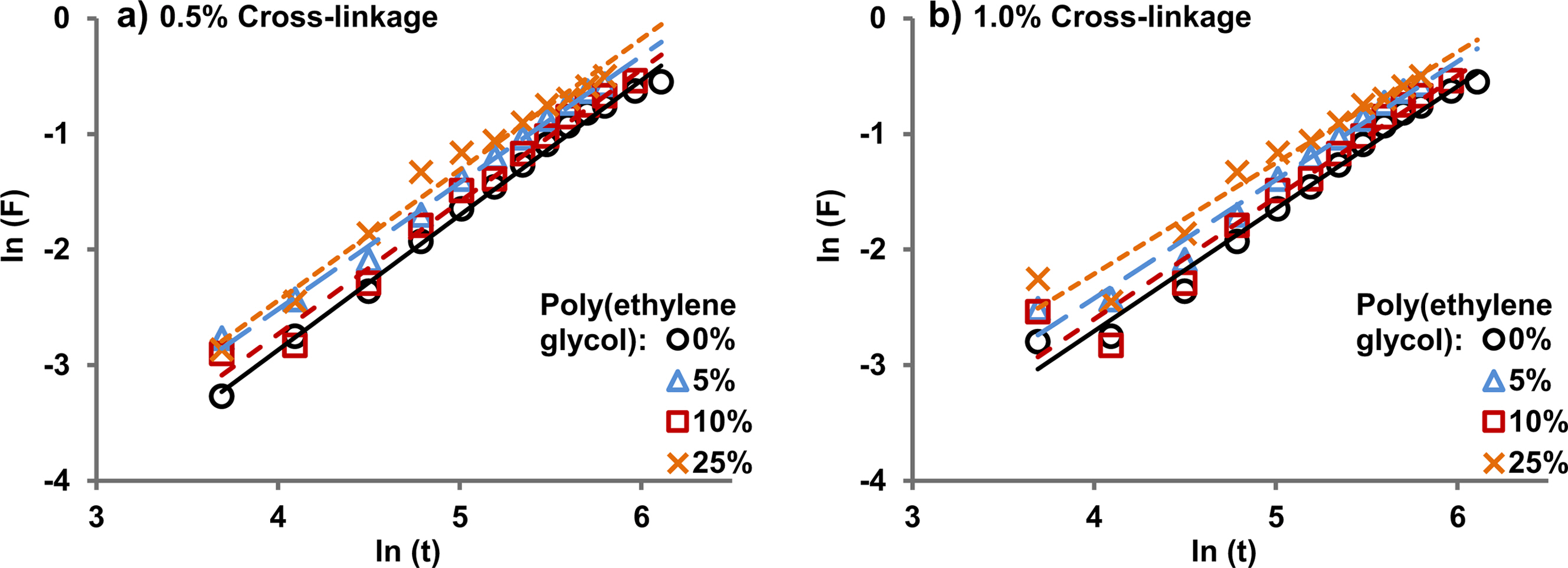
Figure 3 shows the kinetic curves for poly(acrylamide-potassium methacrylate)/poly(ethylene glycol) for 0.5% and 1.0% cross-linking. The diffusion exponent (n) and diffusion constant (k) were calculated from these curves. The value of n indicates the type of diffusion. If n=0.5 indicates Fickian diffusion.15,25 The n values obtained in our experiments were in the range of 0.31 to 0.45, which are beyond the value of classical Fickian theory, i.e. the value of n represents pseudo-Fickian in all the cases.
It can be observed from Table 1 that the diffusion constant and diffusion coefficient increase with an increase in poly(ethylene glycol) content in the hydrogel for the same cross-linkage. In other words, the diffusion of water takes place faster in the presence of more poly(ethylene glycol) in the hydrogel. Only one exception to this general trend was observed, i.e., for the case of 10% poly(ethylene glycol) at 0.5% cross-linking in which the kinetic of water diffusion was found to be slower compared to 5% poly(ethylene glycol) for the same cross-linkage. Currently, the authors do not have any plausible explanation for the exception.
It is clear from the results, described above that the addition of poly(ethylene glycol) decreases the swelling percentage due to hydrogen bonding that increases the effective cross-linkage, but it increases the kinetics of water diffusion in the matrix, which is desirable. Oxygen atoms in poly(ethylene glycol) are hydrogen bond accepter from water molecules which makes poly(ethylene glycol) water-soluble. The same effect explains the faster diffusion of water in the presence of poly(ethylene glycol) in the hydrogel. It can be concluded from these results that the addition of poly(ethylene glycol) provides a new tool to adjust the diffusion kinetics in the hydrogel. Since the hydrogel with 0.5% cross-linking had faster diffusion of water in the copolymer matrix and a higher swelling percentage than 1.0% cross-linking, it was selected for further studies described in the following sections.
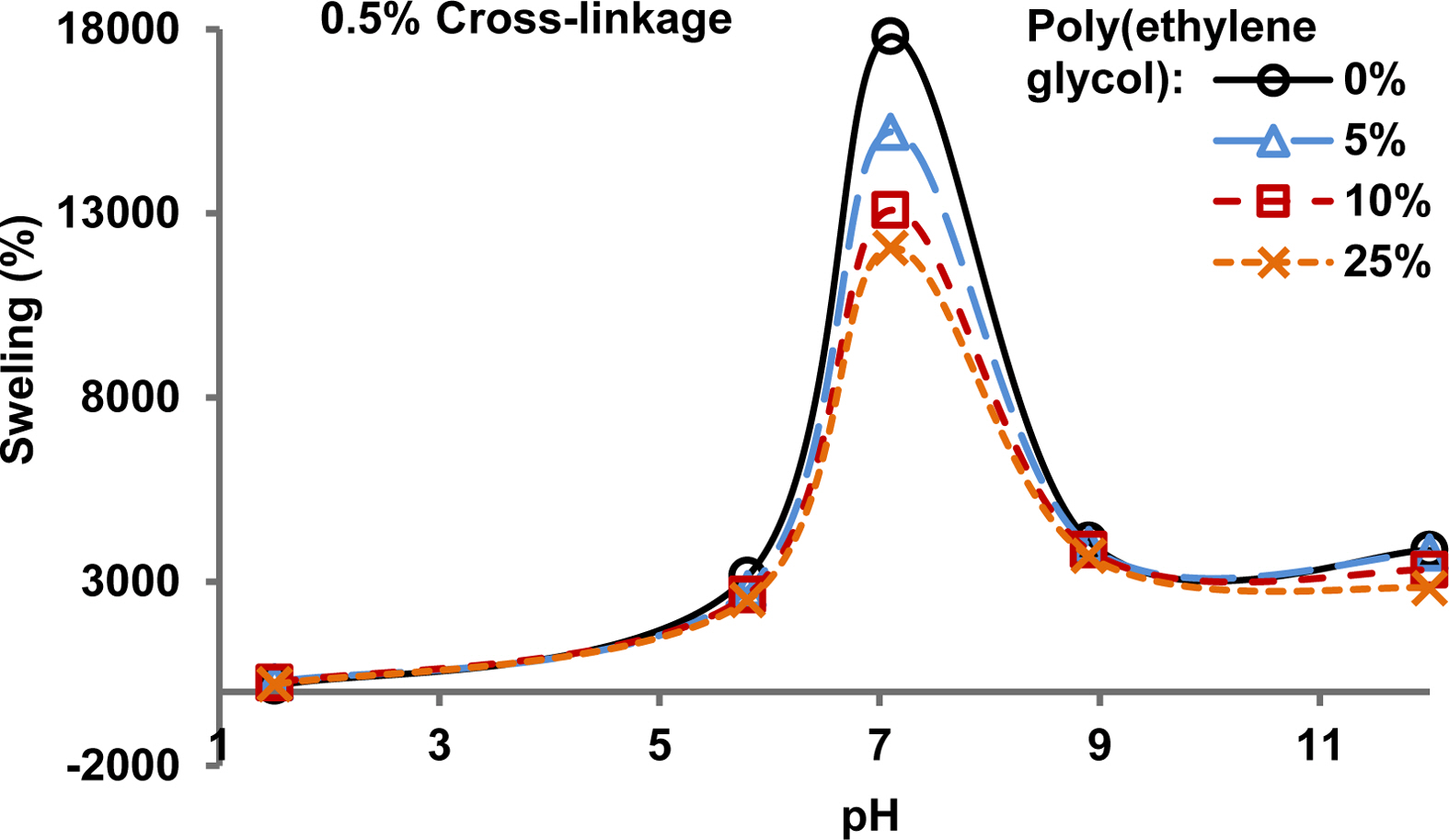
Effect of pH on Swelling in Water. Figure 4 shows the pH-dependent swelling of Poly(acrylamide-potassium methacrylate) hydrogels of 0.5% cross-linkage and poly(ethylene glycol) in the range of 0 to 25%. With an increase in pH from 1.5 to 5.8 there was some increase in the swelling percentage. A sudden increase in the swelling percentage was observed when the pH changed from 5.8 to 7.1. A sudden decrease in swelling percentage was observed when pH changed from 7.1 to 8.9. A further increase in pH from 8.9 to 12 showed almost no change in the swelling percentage. At low pH, the -COO- groups become protonated, i.e., -COOH, with a negligible degree of ionization due to a vast excess of protons in the surrounding solution. Under slightly alkaline conditions of pH 7.1, the -COOH groups become ionized. The hydrogel absorbs a tremendous amount of water due to an osmotic pressure imbalance because of the above-mentioned effect. When the pH increases further, the -COOH groups do not change much as few groups are left for the alkali to neutralize, and, consequently, the osmotic pressure imbalance reduces. It decreases swelling of the hydrogel, as observed in this study at pH ≥ 8.9. This trend follows the typical behavior of hydrogel carrying weak acid functional groups.26
The pH sensitivity of the hydrogels is a desirable characteristic for drug delivery applications.7,27 This is because pH varies in different parts of the body. For example, the pH in the stomach is < 3 which is much lower than the pH of about 7 in the intestines.27 So, the pH stimulus-response can be utilized to deliver the drug loaded on the hydrogel at a desired location in the body.7 The results described above show that the addition of poly(ethylene glycol) in this study decreased the swelling of the hydrogel to some extent. Still, it provided a tool to control the kinetic parameters and adjust the pH stimulus-response of the hydrogel.
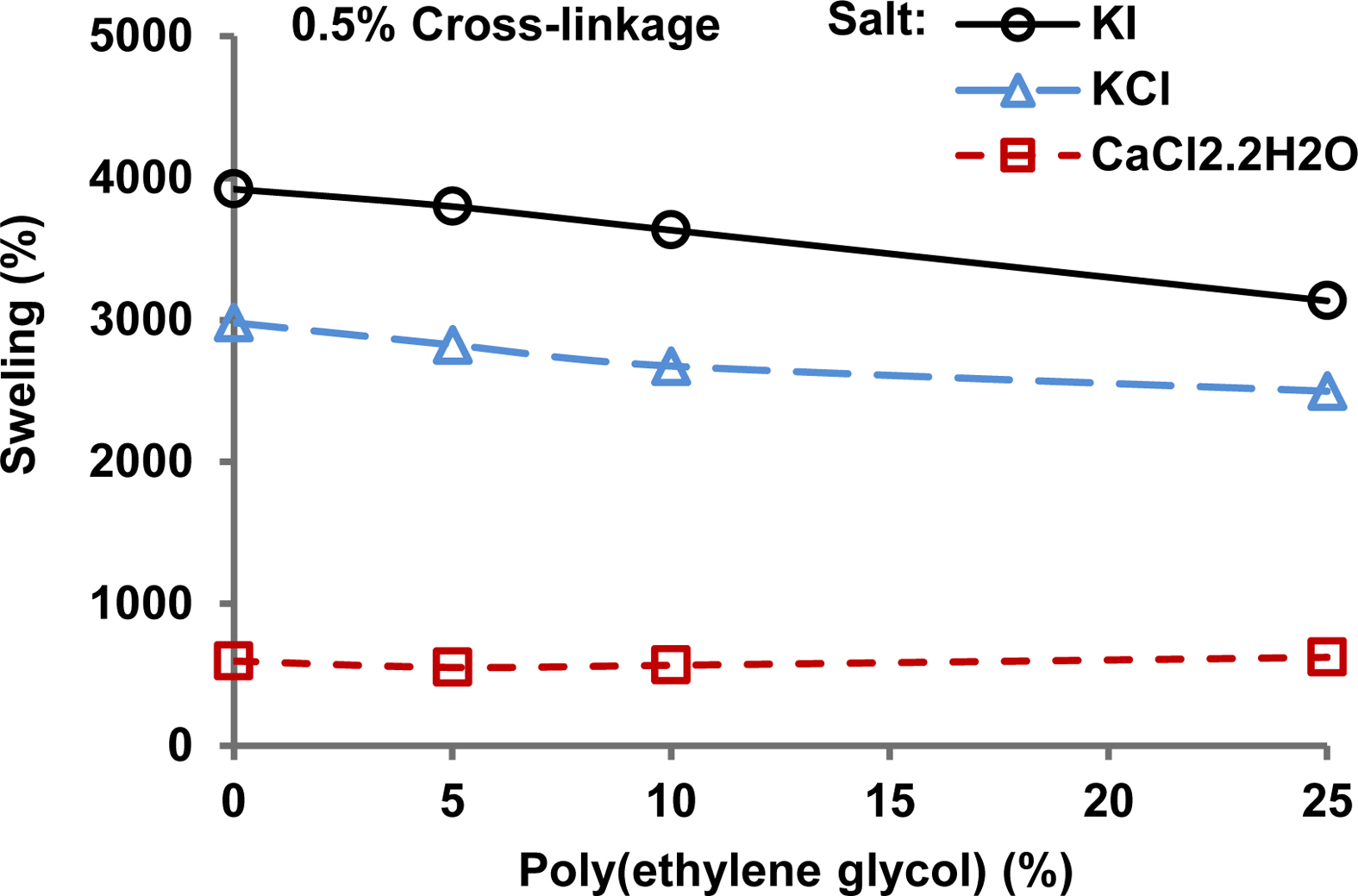
Effect of Ionic Strength on Swelling in Water. Figure 5 shows the equilibrium swelling of 0.5% cross-linked hydrogels in 0.9% w/v salt solution of KI, KCl, and CaCl2·2H2O. The swelling percentage decreased in the following order CaCl2·2H2O > KCl > KI. The ionic strength of the solution followed the same order. The ionic strength (I) of a solution is related to the ionic concentration (Ci) and charge on the individual ion (Zi) by the following eq.28

With increased ionic strength, the osmotic pressure difference between the polymer matrix and the external salt solution decreases. It results in less absorption of water and, consequently, less swelling of the hydrogel following the general trend reported in earlier literatuere.16,28
The hydrogel showed more swelling in KI than the KCl solution because the iodide ions have less ionic strength due to less charge-to-volume ratio than chloride ions. The ionic strength of divalent ions is more than monovalent ions, and hence hydrogel samples in a 0.9% w/v solution of KCl swell more than in a solution of CaCl2·2H2O.
Another factor explaining less swelling in the case of CaCl2·2H2O is that it forms intramolecular and intermolecular complexes with carboxyl, hydroxyl, and amide groups of hydrogels.14,25 It decreases the electrostatic repulsion among the polymeric network26 resulting in a decrease in the swelling following the Donnan theory for the case of weakly charged hydrogels.15
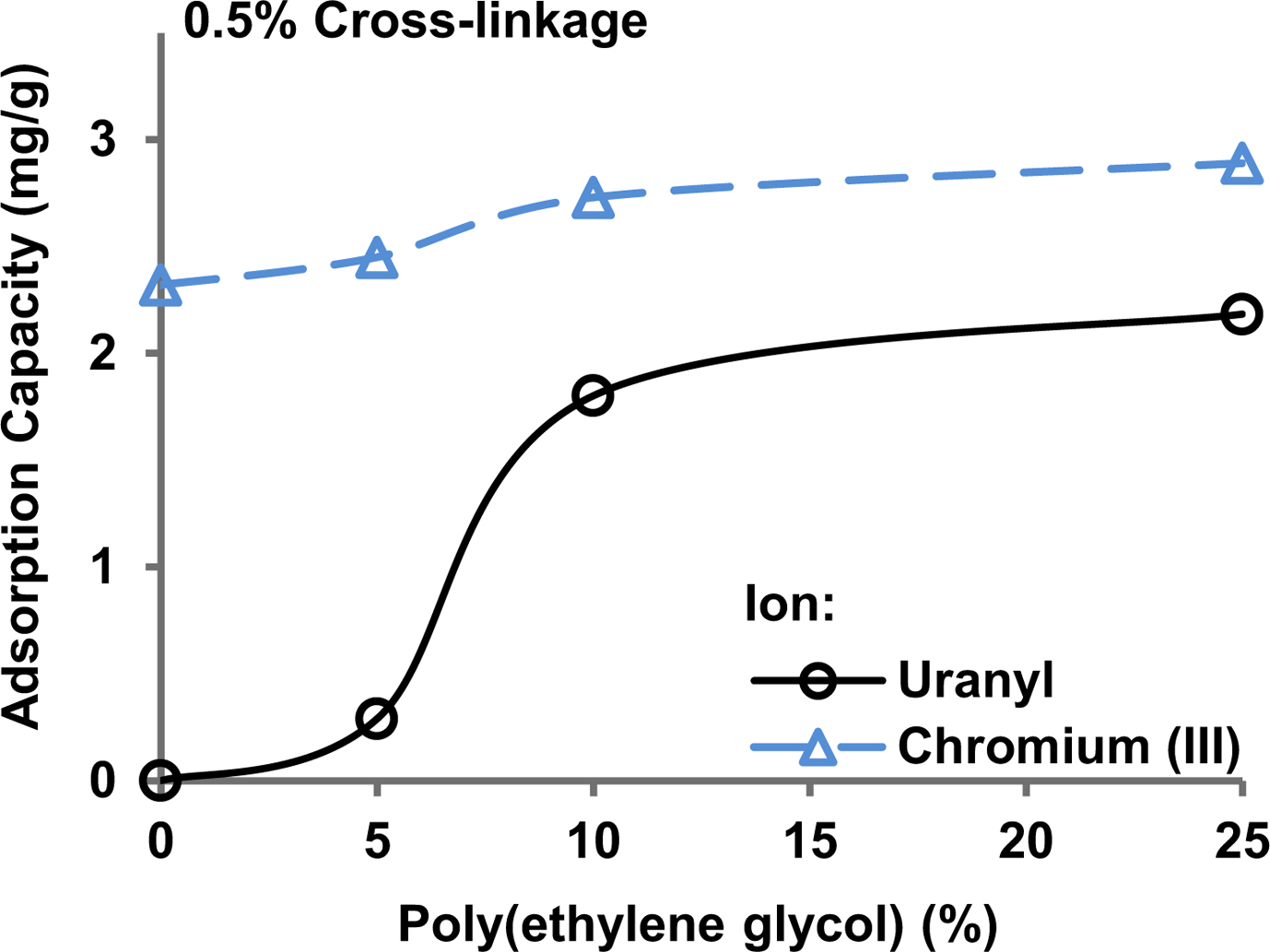
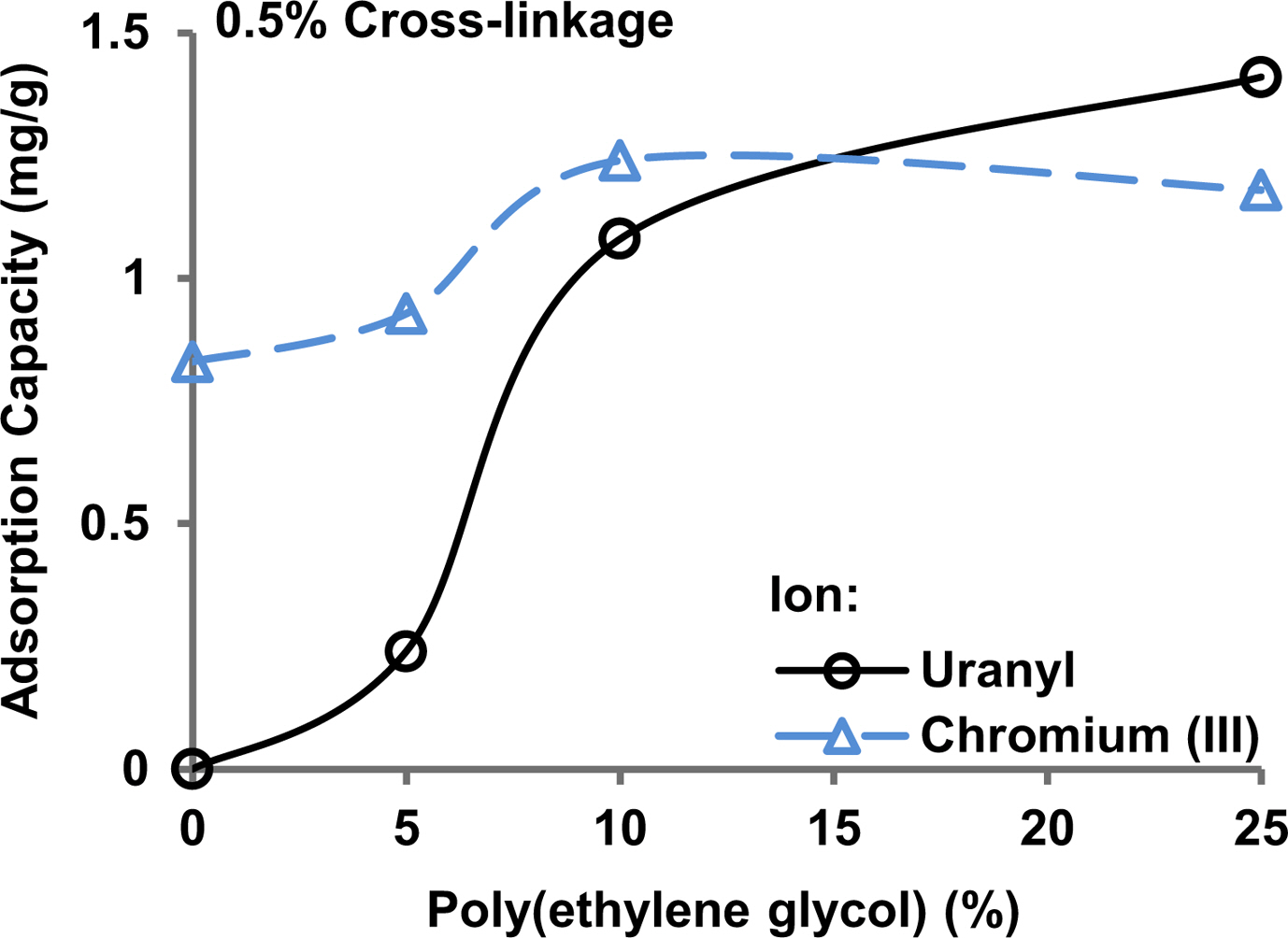
Removal of Chromium(III) and Uranyl Ion Pollutants from Water Samples. Figure 6 shows the adsorption of chromium(III) and uranyl ions by the hydrogels having 0.5% cross-linking and a different proportion of added poly(ethylene glycol). Uranyl ions were not adsorbed in the absence of poly(ethylene glycol) in the hydrogel. The adsorption capacity of the hydrogel for the uranyl ion increased gradually with an increase in poly(ethylene glycol) content in the hydrogel. Chromium(III) ions were adsorbed in the absence of the poly(ethylene glycol) and the adsorption capacity increased with an increase in the poly(ethylene glycol) content in the hydrogel.
The results are explained on the basis of the fact that ion-exchangers usually have a higher selectivity for trivalent ions, such as chromium(III), compared to divalent ions, such as uranyl ions.29 Heavy metal ions, like chromium(III) and uranyl ions, can also be adsorbed through co-ordinate covalent bond formation with chelating groups in the adsorbents. Ether groups in the poly(ethylene glycol) are chelating agents that explains the increasing adsorption of chromium(III) and uranyl ions with an increase in the poly(ethylene glycol) content. The maximum adsorption capacity, i.e., 2.2 and 2.9 mg/g for uranyl and chromium(III) in this set of experiments, is comparable to a chelating ion-exchanger carrying ethylendiaminetetraacetic acid groups grafted on poly(acrylamide)3 or a natural product chitosan having chelating groups added to a poly(acrylamide) hydrogel.21
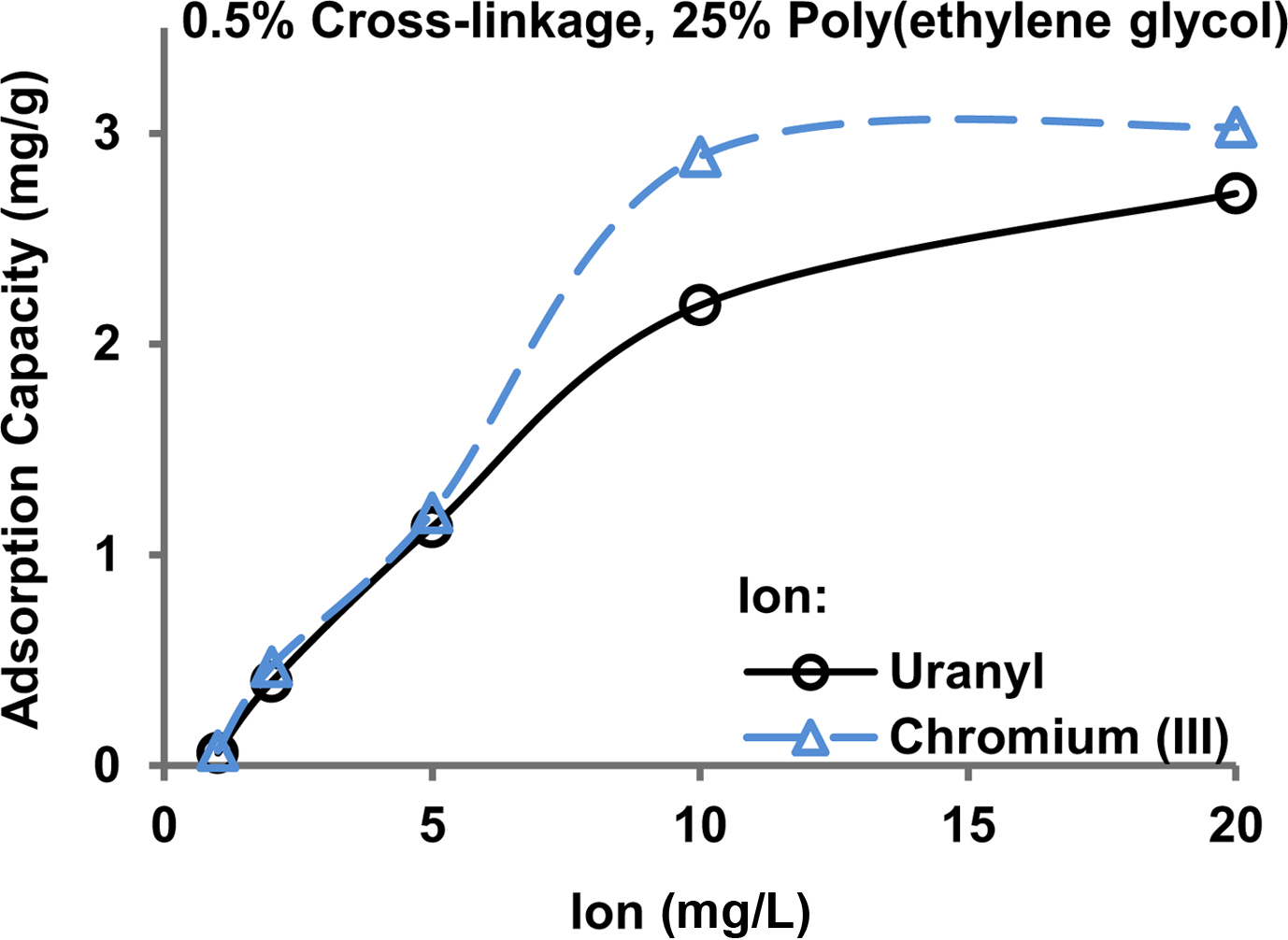
The adsorption capacity of the pollutant ions is usually dependent on the ion concentration, particularly in a dilute solution. A 0.5% cross-linked hydrogel with the addition of 25% poly(ethylene glycol) was employed to adsorb uranyl and chromium(III) ions having a dose range of 1-20 mg/L. Figure 7 shows that the adsorption capacity increased with an increase of dose up to 10 mg/L. There was only a slight increase in the capacity from 10 to 20 mg/L doses indicating that the saturation level reached in this dose range.
In the following experiments, adsorption of uranyl and chromium(III) ions from a 1-to-1 mixture of chromium(III) and uranyl ions was studied. Poly(ethylene glycol) content in the hydrogel was gradually varied from 0 to 25%. Results in Figure 8 shows that chromium(III) was selectively adsorbed in the absence of poly(ethylene glycol). Both uranyl and chromium(III) ions were adsorbed in the presence of poly(ethylene glycol). The adsorption capacity for both the ions increased with an increase in the poly(ethylene glycol) content. The selectivity remained higher for chromium(III) compared to uranyl in all cases studied for up to 10% poly(ethylene glycol) and changed in favor of uranyl ions for 25% poly(ethylene glycol) in these experiments. These results show that the addition of poly(ethylene glycol) provided another tool to tailor the characteristics of the hydrogel, i.e., the chelating ability that allows adsorption and removal of more toxic heavy metal ions, like uranyl ions.
The hydrogel exhausted after the adsorption was regenerated with 2 M HCl and repeatedly re-used for about five cycles with no damage noticed in the hydrogel. This observation indicated that the hydrogel has reasonable stability and is re-usable in practical applications.
|
Figure 1 FTIR spectra of the hydrogel with 0% polyethylene glycol (1) and 25% polyethylene glycol (2). |
|
Figure 2 Swelling curves of poly(acrylamide-co-potassium methacrylate)/poly(ethylene glycol) hydrogels at (a) 0.5% cross-linker; (b) 1.0% cross-linker at pH 7.1. |
|
Figure 3 Kinetic curves of poly(acrylamide-co-potassium methacrylate)/poly(ethylene glycol) hydrogels for (a) 0.5% cross-linking; (b) 1.0% cross-linking, with different amounts of added poly(ethylene glycol), at pH 7.1. |
|
Figure 4 Effect of pH on the swelling of poly(acrylamide-co-potassium methacrylate)/poly(ethylene glycol) hydrogels containing differing amounts of poly(ethylene glycol) |
|
Figure 5 Swelling of the hydrogel versus the poly(ethyelene glycol) amount curves for different salt solutions (0.9% salt solution of KI at pH 6.9, KCl at pH 7.2 and CaCl2·2H2O at pH 6.6) at room temperature. |
|
Figure 6 Adsorption of uranyl and chromium(III) ions on the hydrogels composed of different amount of poly(ethylene glycol). Feed amount of the ions was 10 ppm in each experiments at pH 7. |
|
Figure 7 Adsorption of uranyl and chromium(III) ions on the hydrogel at pH 7.1. |
|
Figure 8 Adsorption of uranyl and chromium(III) ions from a 1:1 mixture of the two on the 0.5% cross-linked hydrogels with different proportions of additional poly(ethylene glycol). The feed amount was 5 ppm each at pH 7.1. |
|
Table 1 Characterization Data of Poly(acrylamide-co-potassium methacrylate)/poly(ethylene glycol) Hydrogels at pH 7.1 |
This study reveals that adding poly(ethylene glycol) to poly(acrylamide-potassium methacrylate) hydrogel provides a new tool to adjust important hydrogel characteristics, like swelling in water, absorption kinetics, and capacity for heavy metal pollutant removal from wastewater. Specifically, the addition of poly(ethylene glycol) to poly(acrylamide-potassium) resulted in:
i) a decreased swelling of the hydrogel in water still increased absorption kinetics, and
ii) significantly increased adsorption capacity for heavy metal pollutants like chromium(III) and uranyl ions from polluted water. Uranyl ion, which is toxic and radioactive, could be removed from the water sample only after adding poly(ethylene glycol) in poly(acrylamide-potassium methacrylate) hydrogel.
- 1. Singha, N. R.; Dutta, A.; Mahapatra, M.; Roy, J. S. B.; Mitra, M.; Deb, M.; Chattopadhyay, P. K. In Situ Attachment of Acrylamido Sulfonic Acid-Based Monomer in Terpolymer Hydrogel Optimized by Response Surface Methodology for Individual and/or Simultaneous Removal(s) of M(III) and Cationic Dyes. ACS Omega 2019,4, 1763-1780.
-

- 2. TaŞar, Ş.; Orhan, R. Temperature-responsive Poly(acrylamide-co-N-isopropyl acrylamide) Hydrogels: Synthesis, Characterization and Sorption Application. Polym. Korea 2020, 44, 49-60.
-

- 3. İnam, R.; Çaykara, T.; Kantoĝlu, Ö. Polographic Determination of Uranyl Adsorption onto Poly(acrylamide-g-ethylenediamine- tetraacetic acid) Hydrogels in the Presence of Cadmium and Lead. Nucl. Instrum. Methods Phys. Res. Sect. B 2003, 208, 400-404.
-

- 4. Wongjaikham, W.; Wongsawaeng, D.; Hosemann, P. Synthesis of Amidoxime Polymer Gel to Extract Uranium Compound from Seawater by UV Radiation Curing. J. Nucl. Sci. Technol. 2019, 56, 541-552.
-

- 5. Çaykara, T.; İnam, R.; Özyürek, C. Radiation Synthesis and Uranyl Ion Adsorption of Poly(2-hydroxyethyl methacrylate/maleic acid) Hydrogels. J. Polym. Sci. A Polym. Chem. 2001, 39, 277-283.
-

- 6. Angar, N.-E.; Aliouche, D. An Enhanced Immobilization of BSA Biomolecule on Anionic Hydrogels: Swelling and Adsorption Modelling. Chem. Pap. 2017, 71, 1389-1397.
-

- 7. Bajpai, S. K.; Dubey, S. In Vitro Dissolution Studies for Release of Vitamin B12 from Poly(N-vinyl-2-pyrrolidone-co-acrylic acid) Hydrogels. React. Funct. Polym. 2005, 62, 93-104.
-

- 8. Heidari, S.; Esmaeilzadeh, F.; Mowla, D.; Ghasemi, S. Synthesis of an Efficient Copolymer of Acrylamide and Acrylic Acid and Determination of its Swelling Behaviour. J. Pet. Explor. Prod. Technol. 2018, 8, 1331-1340.
-

- 9. Saraydɪn, D.; Ișɪkver, Y.; Karadaǧ, E. Adsorption of Phenazine Dyes Using Poly(Hydroxamic Acid) Hydrogels from Aqueous Solutions. Polym. Eng. Sci. 2018, 58, 310-318.
-

- 10. Oucif, A.; Haddadine, N.; Zakia, D.; Bouslah, N.; Benaboura, A.; Beyaz, K.; Guedouar, B.; El-Shall, M. S. Poly(hydroxyethyl methacrylate-co-hydroxyethyl acrylate) Soft Contact Lenses for Acetazolamide Release. Polym. Bull. [Online early access]. DOI: 10.1007/s00289-021-03573-5. Published Online: Feb 8, 2021 (accessed Feb 8, 2021).
-

- 11. Yuan, D.; Jacquier, J. C.; O’Riordan, E. D. Entrapment of Proteins and Peptides in Chitosan-Polyphosphoric Acid Hydrogel Beads: A New Approach to Achieve Both High Entrapment Efficiency and Controlled In Vitro Release. Food Chem. 2018, 239, 1200-1209.
-

- 12. Tay, J.-W.; Choe, D.-H.; Mulchandani, A.; Rust, M. K. Hydrogels: From Controlled Release to a New Bait Delivery for Insect Pest Management. J. Econ. Entom. 2020, 113, 2061-2068.
-

- 13. Ali, S. W.; Zaidi, S. A. R. Synthesis of Copolymeric Acrylamide/Potassium Acrylate Hydrogels Blended with Poly(Vinyl Alcohol): Effect of Crosslinking and the Amount of Poly(Vinyl Alcohol) on Swelling Behaviour. J. Appl. Polym. Sci. 2005, 98, 1927-1931.
-

- 14. Omidian, H.; Hashemi, S. A.; Askari, F.; Nafisi, S. Swelling and Crosslink Density Measurements for Hydrogels. Iranian J. Polym. Sci. Technol. 1994, 3, 115-119.
- 15. Khare, A. R.; Peppas, N. A. Swelling/deswelling of Anionic Copolymer Gels. Biomaterials 1995, 16, 559-567.
-

- 16. Castel, D.; Ricard, A.; Audebert, R. Swelling of Anionic and Cationic Starch‐Based Superabsorbents in Water and Saline Solution. J. Appl. Polym. Sci. 1990, 39, 11-29.
-

- 17. Hosseinzadeh, H.; Alijani, D. Synthesis, Characterization and Swelling Properties of Chitosan/Poly(acrylic acid-co-crotonic acid) Semi-Interpenetrating Polymer Networks. Polym. Korea 2014, 38, 588-595.
-

- 18. Mahdavinia, G. R.; Pourjavadi, A.; Hosseinzadeh, H.; Zahuriaan, M. J. Modified Chitosan 4. Superabsorbent Hydrogels from Poly(acrylic acid-co-acrylamide) Grafted Chitosan with Salt-and pH-Responsiveness Properties, Eur. Polym. J. 2004, 40, 1399-1407.
-

- 19. Liu, Y.; Cao, X.; Hua, R.; Wang, Y.; Liu, Y.; Pang, C.; Wang, Y. Selective Adsorption of Uranyl Ion on Ion-Imprinted Chiatosan/PVA Cross-Linked Hydrogel. Hydrometallurgy 2010, 104, 150-155.
-

- 20. Ali, A. EI-H.; Shawky, H. A.; El-Rehim, H. A. A.; Hegazy, E. A. Synthesis and Characterization of PVA/AAC Copolymer Hydrogel and its Applications in the Removal of Heavy Metals from Aqueous Solution. Eur. Polym. J. 2003, 39, 2337-2344.
-

- 21. Akkaya, R.; Ulusoy, U. Adsorptive Features of Chitosan Entrapped in Polyacrylamide Hydrogel for Pb2+, UO22+, and Th4+. J. Hazard. Mater. 2008, 151, 380-388.
-

- 22. Kam, E.; Taşdelen, B.; Osmanlioglu, A. E. Uranyl Ion Uptake Capacity of Poly(isopropylacrylamide/malic Acid) Copolymeric Hydrogels Prepared by Gamma Rays. Radiat. Phys. Chem. 2012, 81, 618-621.
-

- 23. CRC Handbook of Chemistry and Physics, 69th Edition; Weast, R. C., Ed.: CRC Press Inc.: Boca Rosa, 1988.
- 24. Hennink, W. E.; Van Nostrum, C. F. Novel Crosslinking Methods to Design Hydrogels. Adv. Drug. Deliv. Rev. 2002, 54, 13-36.
-

- 25. Guilherme, M. R.; Aouada, F. A.; Fajardo, A. R.; Martins, A. F.; Paulino, A. T.; Davi, M. F. T.; Rubira, A. F.; Muniz, E. C. Super- absorbent Hydrogels Based on Polysaccharides for Application in Agriculture as Soil Conditioner and Nutrient Carrier: A Review. Eur. Polym. J. 2015, 72, 365-385.
-

- 26. Rička, J.; Tanaka, T. Swelling of Ionic Gels: Quantitative Performance of the Donnan Theory. Macromolecules 1984, 17, 2916-2921.
-

- 27. Zhou, X.; Weng, L.; Chen, Q.; Zhang, J.; Shen, D.; Li, Z.; Shao, M.; Xu, J. Investigation of pH Sensitivity of Poly(acrylicacid‐ co‐acrylamide) Hydrogel. Polym. Int.2003, 52, 1153-1157.
-

- 28. Kiatkamjornwong, S.; Mongkolsawat, K.; Sonsuk, M. Synthesis and Property Characterization of Cassava Starch Grafted Poly [acrylamide-co-(maleic acid)] Superabsorbent via g-Irradiation. Polymer 2002, 43, 3915-3924.
-

- 29. Ion exchangers; Dorfner, K. Ed.: Walter de Gruyter: New York, 1991.
- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2021; 45(6): 832-840
- 10.7317/pk.2021.45.6.832
 Services
Services
Shared
 Correspondence to
Correspondence to
- Syed Wasim Ali
-
Chemistry Division, Pakistan Institute of Nuclear Science and Technology (PINSTECH), PO 45650, Nilore, Islamabad, Pakistan
- E-mail: swasimali@pinstech.org.pk
- ORCID:
0000-0002-4950-8599









 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.