- Thiophene-benzimidazolium Polymer Salts: Synthesis, Characterization, Antimicrobial Activities, and Evaluation in Terms of Occupational Health
Savas Kanbur, Gulcin Ozcan Ates, Sinem Altinisik*, Ulas Cinar**, and Fatma Baycan***,†

Department of Medical Services and Techniques, Canakkale Onsekiz Mart University, Canakkale, Turkey
*Department of Chemical Engineering, Faculty of Engineering, Canakkale Onsekiz Mart University, Canakkale, Turkey
**Occupational Health and Safety Education Application and Research Center, Canakkale Onsekiz Mart University, 17100 Canakkale, Turkey
***Department of Chemistry, Faculty of Arts and Sciences, Canakkale Onsekiz Mart University, Canakkale, Turkey- Thiophene-benzimidazolium 고분자 솔트: 합성, 특성분석, 항균활동 및 산업건강측면에서의 평가
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
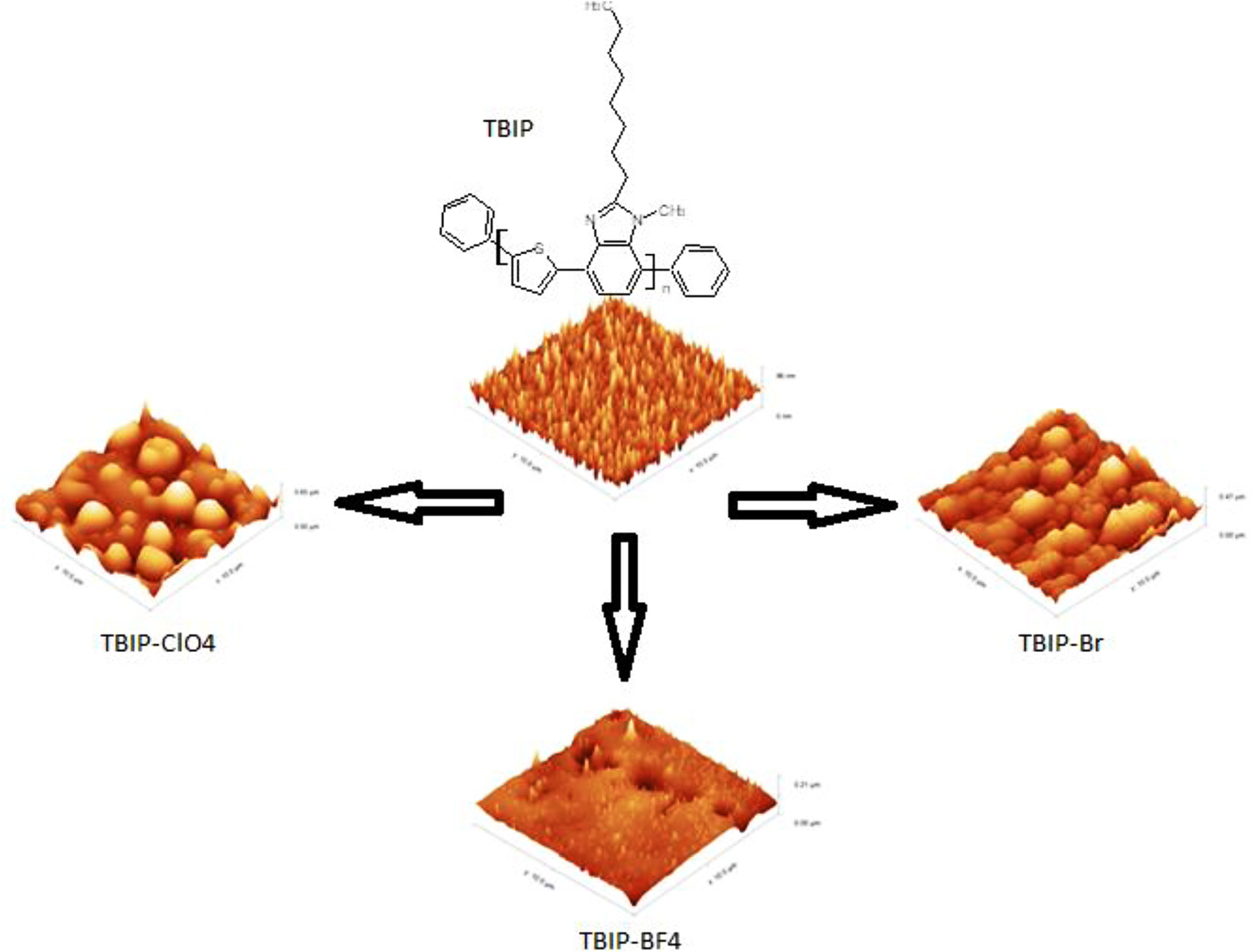
A new thiophene benzimidazole based polymer (TBIP) was synthesized and then the TBIP-Br, TBIP-BF4 and TBIP-ClO4 polymer salts were obtained in water. All polymer salts can be dissolved in environmentally friendly solvents such as water or ethanol. Electrochemical and optical properties of the polymers were elucidated with cyclic voltammetry (CV) and ultraviolet-visible absorption (UV-Vis) spectroscopy. By using CV, the highest occupied molecular orbital (HOMO) values were found as approximately 1.70 eV. According to the atomic force microscopy (AFM) images of the thin film of TBIP, there are homogeneously distributed and smaller particles on the film surface, while the surface of TBIP-Br and TBIP-ClO4 thin films is rougher due to -Br and -ClO4 counter ions being bigger than -BF4. Additionally, the antimicrobial activity of TBIP polymers was also investigated. All TBIP and its salts were showed antimicrobial activity against S. aureus ATCC 6538 and no antimicrobial activity against E. coli ATCC 1301 and ATCC 25922.
Thiophene benzimidazole-based polymer (TBIP) polymer containing benzimidazole and thiophene units was synthesized for the first time and its salts (-ClO4, -BF4 and -Br) that can be dissolved in environmentally friendly solvents were obtained. The structures of this polymer and its salts were characterized and their surface morphologies were investigated. At the same time, the antimicrobial properties of the polymers were examined.
Keywords: benzimidazole polymer, thiophene, antimicrobial activity, polymer salts, occupational health.
Information is available regarding the synthesized TBIP polymer and its salts’ structural characterization. The materials are available via the Internet at http://journal.polymer-korea.or.kr.
PK_2021_045_06_817_Supporting_Information_template.pdf (317 kb)
Supplementary Information
Benzimidazole, a heteroaromatic compound, is formed by the fusion of benzene and imidazole rings and shows weak basic properties. Therefore it usually dissolves in dilute acids. The pK value of benzimidazole is known as pKa1=5.30 and pKa2=12.3.1 Benzimidazole compounds generally show acidic properties in aqueous basic solutions. The fact that benzimidazoles have acidic properties, such as imidazoles, is due to the stabilization of the ion by resonance.2 Benzimidazole compounds (i.e. imide nitrogen) containing a hydrogen atom in the 1-position are generally readily soluble in hot water as they are soluble in polar solvents. However, it does not dissolve in benzene and ligroin, which are apolar solvents. Therefore, the solubility in non-polar solvents is increased by adding non-polar substituents at various locations of the benzimidazole core.3
When polar substituents are added to the benzimidazole core, the solubility in polar solvents is increased; for example, 2-aminobenzimidazole is soluble in water.4 In addition, due to the high stability of the benzimidazole ring, oxidation separates the benzene ring of benzimidazole only under strong conditions. The benzimidazole ring is highly resistant to the reduction so it cannot be reduced except for a small number of catalytic reduction methods. The benzimidazole compounds mainly react via 1 and 3-position nitrogens. In addition, they can form salt by interacting with acids such as monopicrate, mononitrate, monohydrochloride, monoacetate.5,6
N-containing heteroaromatic compounds are important structural motifs found in many natural and synthetic compounds. Among them, benzimidazole and its derivatives are decisive nucleus structures used in drugs and materials.7 In 1962 benzimidazole was first discovered as tiabendazole and was found to show an anthelmintic property. In this heterocyclic scaffold, the formic acid carbon between the two nitrogen atoms is the most reactive region for possible sub- stitution(s).8 As a result of intensive research, it has been found that the main structure of benzimidazole has a number of pharmacological properties.9 First, researchers suggested that benzimidazole exhibited physiological effects similar to purines.10 A benzimidazole derivative with similar activity to vitamin B12 was synthesized by examining the degradation products of vitamin B12.11 Combining synthetic versatility and biodiversity, these unique compounds have many applications and significant potential. Combining synthetic versatility and biodiversity, these unique compounds have many applications and significant potential. Benzimidazole derivatives have recently been used medicinally to cure many diseases.
The ability of microbial agents to operate on surfaces in the work environment and to be included in the environment through surfaces that are permeable to the external environment poses a great threat to employees. Since biological risk factors cause different infections in terms of employee health, the control of these factors is vital for occupational hygiene.
Especially after 2000, research activities related to the development of antimicrobials from benzimidazole seeds have increased. By combining 2-alkylthiobenzimidazole with the-lactam ring, molecules with strong antibacterial and antifungal activities were produced.12 Güven et al. showed that 1-substituted benzimidazole compounds showed weak antimicrobial properties.13 It has been reported by Halloway et al. that the 2-iminobenzimidazole compound has a high antibacterial potential by inhibition of trypanothione reductase.14 By binding heterocyclic compounds such as chroman, -lactam, thiadiazole and oxadiazole to a benzimidazole core, potent antibacterial and/or antifungal new compounds were obtained.15
In this study, thiophene benzimidazole based polymer (TBIP) was synthesized by using Suzuki reaction between 2,5-thiophene bisboronic acid and 4,7-dibromo-1-methyl-2-octyl-1H-benzimidazole with the reaction yield of 65%. Then, the TBIP polymer salts were prepared by the reaction of excess ethyl bromide in acetonitrile (ACN). Finally, the -Br- ion on TBIP was also easily replaced with the -BF4- and -ClO4- by ion exchange reaction in water. The polymer salts can be soluble in ethanol and water as environmentally friendly solvents. Remarkable changes were observed in the Fourier-transform infrared spectroscopy (FTIR) spectrum of TBIP polymer and its salts. The molecular weight of the polymer salts determined by Gel permeation chromatography (GPC) was found as about 3-4 K corresponding to 5-6 repeating units. In the cathodic scan of cyclic voltammograms (CV), the reduction peak was observed at TBIP salts arising from the cationic form of the benzimidazole unit. According to the UV-Vis spectrum in the methanol solution and thin film on the glass surface, TBIP and its salts can absorb until 400 nm corresponding to the bandgap of 3, 10 eV. Atomic force microscopy (AFM) measurements of thin film revealed that porous film can be obtained from the TBIP salts. We determined that the 10% solutions of the TBIP polymer (prepared in 96% alcohol) were showed antimicrobial activity against Staphylococcus aureus (Gram positive), but no antimicrobial activity was showed against Esherichia coli (Gram negative). In addition, only TBIP-BF4 was showed antimicrobial activity against Candida albicans (yeast).
Intrumentation. All chemicals were supplied from Aldrich Chemical and used without further purification. 4,7-dibromo-1-methyl-2-octyl-1H-benzimidazole was synthesized according to previously published procedures.16 FTIR spectra were obtained using Perkin Elmer Spectrum One device (with ATR system) (4000-650 cm-1). 1H-NMR spectra of TBIP and its salts were used by Bruker Avance DPX-400. Measurements were recorded at 25 °C using deuterated CHCl3 (for TBIP), deuterated dimethylsulfoxide (DMSO, for TBIP salts) and tetramethylsilane. GPC analyses of the TBIP polymer and salts were conducted with an Agilent 1260 HPLC instrument. Electrochemical characterizations of TBIP salts were carried out with CH Instruments 617D electrochemical workstation out under argon atmosphere. All electrochemical measurements were carried out in a cell containing a support electrolyte and a triple electrode system (1- Working electrode-platinum disk, 2- Reference electrode-Ag wire and 3- Counter electrode- Pt wire). All electrochemical measurement was performed containing 0.1 M TBAPF6 ACN solution was used as the supporting electrolyte. The potentials were calibrated according to the ferrocene redox couple Eo(Fc/Fc+) = +0.41 V vs. Ag/Ag+. Analytic Jena Speedcord S-600 diode-array spectrophotometer was used for UV-Vis absorption spectra of the TBIP solutions and films. The TBIP polymer and its derivatives morphologies were investigated by the AFM technique (Nanosurf Naio).
Synthesis of TBIP and Its Salts. In a two-necked 100 mL balloon, 2,5-Thiophenediylbisboronic acid (1) (0.855 g, 5 mmol) and 4,7-dibromo-1-methyl-2-octyl-1H-benzimidazole (2) (2.01 g, 1 mmol) were dissolved in 20 mL of Toluene and 5 mL of 2.0 M K2CO3, and the solution was purged with argon for 15 min. Pd(PPh3)4 (0.231 g, 0.2 mmol) was added to the reaction mixture at room temperature under an argon atmosphere. The mixture was stirred at 110 oC under argon for 24 h. The colorless solution turned to brown after an hour. After 24 h, bromo benzene (0, 15 g, 1.0 mmol) was added to the mixture. After that 2 h, benzene boronic acid (0.12 g, 1.0 mmol) was added, and the mixture was further stirred for another 2 h to complete the end-capping reaction. The polymer was precipitated in hexane and filtered and finally dried under vacuum at 60 °C overnight to afford TBIP polymer (TBIP: 1.062 g; yield 64%). In the second step, The TBIP polymer (800 mg, 2.4 mmol) was dissolved in the 50 mL of Acetonitrile. An excess amount of bromoethane (2.592 g, 24 mmol) was added to the solution. The mixture was stirred at 80 oC for 18 h. After the cooling of the reaction mixture, the solvent was evaporated to obtain the TPIB-Br salt. Finally, TPIB-Br (0.22 g, 0.5 mol) was dissolved in the 20 mL of ethanol. The mixture poured in to 0.025 M 20 mL NaClO4 or NaBF4 solution. The precipitated TBIP-ClO4 or TBIP-BF4 polymer salts were filtered and finally dried under vacuum at 60 °C overnight (TBIP-Br: 0.920 g; yield 94%, TBIP-ClO4: 0.21 g; yield 82%, TBIP-BF4: 0.22 g; yield 93%) (Scheme 1).
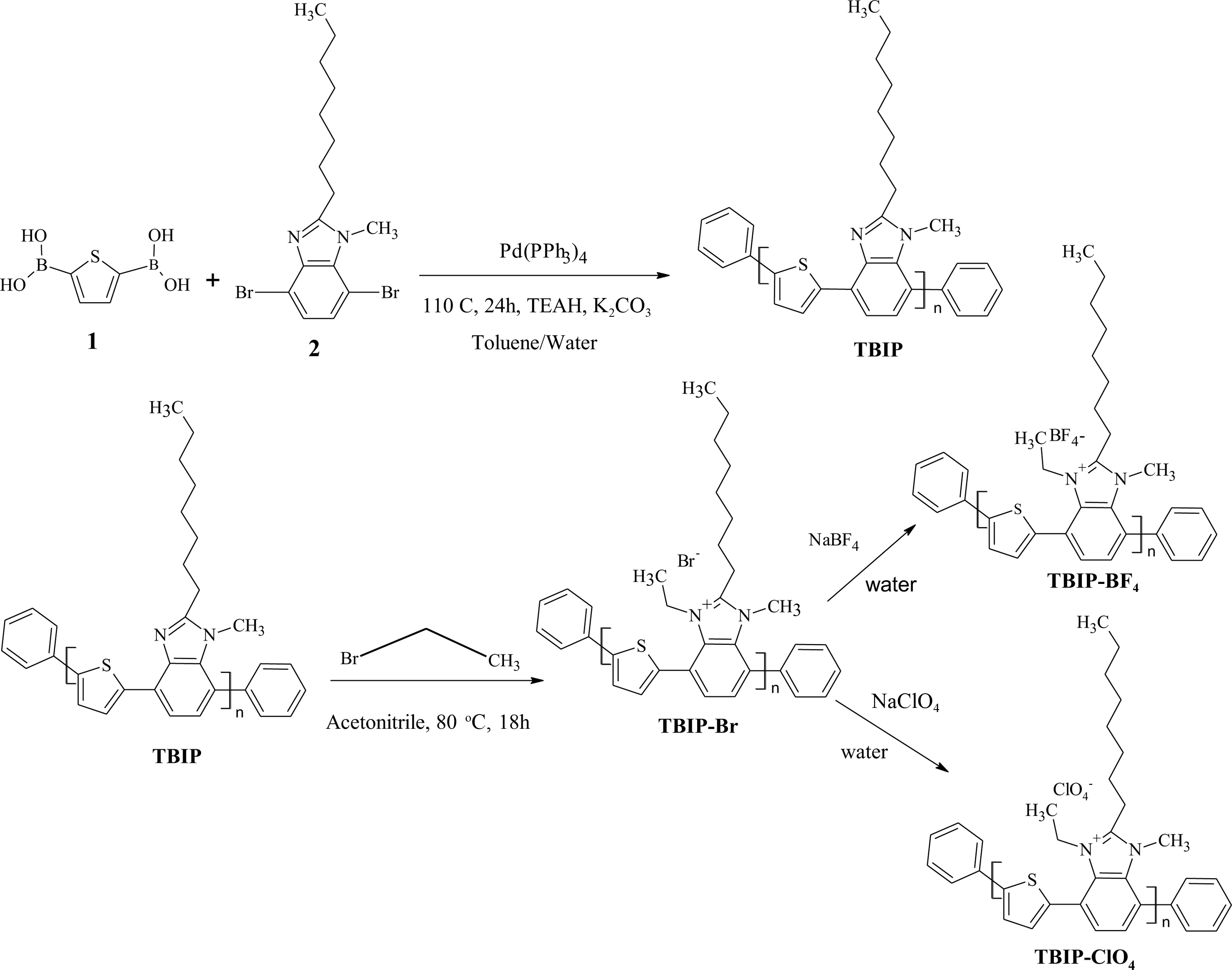
Scheme 1. Synthetic route to TBIP polymer and its salts.
Thiophene-Benzimidazole Polymer (TBIP): (Yield, 64%), 1H NMR (CHCl3-d, 400 MHz): δ, ppm = 8.18-7.14 (4H, m, C-H aromatic), 3.74 (2H, t, -CH2 aliphatic), 3.61 (2H, m, -CH2 aliphatic), 2.92 (2H, m, -CH2 aliphatic), 1.94 (2H, m, -CH2 aliphatic), 1.62 (s, 3H, N-CH3 aliphatic); 1.25 (6H, m, -CH2 aliphatic), 0.89 (t, 3H, -CH3 aliphatic). FTIR (ATR) v [cm-1] 3072 (C-H aromatic); 2914, 2844 and 2786 (C-H aliphatic); 1450 (C-N).
Thiophene-Benzimidazole Polymer-Br Salt (TBIP-Br): (Yield, 94%), 1H-NMR (DMSO-d6, 400 MHz): δ, ppm = 8.15-7.13 (4H, m, C-H aromatic), 3.54 (2H, m, N-CH2), 3.21 (3H, t, N-CH2-CH3), 2.87 (2H, m, -CH2 aliphatic), 2.68 (2H, m, -CH2 aliphatic); 2.33 (2H, m, -CH2 aliphatic), 1.77 (2H, m, -CH2 aliphatic); 1.28-1.06 (2H, m, -CH2 aliphatic); 1.16 (3H, s, -CH3 aliphatic); 0.89 (t, 3H, -CH3 aliphatic). FTIR (ATR) v [cm-1] 3071 (C-H aromatic); 2915, 2844 and 2787 (C-H aliphatic); 1451 (C-N); 1225 and 1100 (salt counter ion peaks).
Thiophene-Benzimidazole Polymer-BF4 Salt (TBIP- BF4): (Yield, 93%), 1H-NMR (DMSO-d6, 400 MHz): δ, ppm = 8.15-7.01 (4H, m, C-H aromatic), 3.54 (2H, m, N-CH2), 3.21 (3H, t, N-CH2-CH3), 2.87 (2H, m, -CH2), 2.66 (2H, m, -CH2 aliphatic); 2.33 (2H, m, -CH2 aliphatic), 1.77 (2H, m, -CH2 aliphatic); 1.28-1.06 (2H, m, -CH2 aliphatic); 1.16 (3H, s, N-CH3 aliphatic); 0.87 (t, 3H, -CH3 aliphatic). FTIR (ATR) v [cm-1] 3071 (C-H aromatic); 2915, 2844 and 2787 (C-H aliphatic); 1449 (C-N); 1120 and 1080 (salt counter ion peaks).
Thiophene-Benzimidazole Polymer-ClO4 Salt (TBIP-ClO4): (Yield, 82%), 1H-NMR (DMSO-d6, 400 MHz): δ, ppm = 7.97-7.37 (4H, m, C-H aromatic), 3.54 (2H, m, N-CH2), 3.21 (3H, t, N-CH2-CH3), 2.86 (2H, m, -CH2), 2.67 (2H, m, -CH2); 2.33 (2H, m, -CH2 aliphatic), 1.77 (2H, m, -CH2 aliphatic); 1.29-1.06 (2H, m, -CH2 aliphatic); 1.16 (3H, s, N-CH3 aliphatic); 0.87 (t, 3H, -CH3 aliphatic). FTIR (ATR) v [cm-1] 3071 (C-H aromatic); 2915, 2844 and 2787 (C-H aliphatic); 1449 (C-N); 1121 and 1050 (salt counter ion peaks).
Determination of Antimicrobial Activity. Antimicrobial activity was determined using Kirby Bauer agar disc diffusion method. Antimicrobial activity was investigated against microorganisms (Esherichia coli ATCC 1301 and ATCC 25922 (Gram-negative bacteria), Staphyloccoccus aureus ATCC 6538 (Gram-positive bacteria), and Candida albicans ATCC 10231 (yeast)). 10% alcohol solutions of chemical substances were prepared. Initially, cultures were revived with 5 mL of Tryptic Soybean Broth (TSB) overnight at 37 °C. After incubation, in 5 mL TSB medium at 37 °C, E. coli, S. Aureus,and C. albicans were re-incubated for 90, 180, and 180 min, respectively. After incubation, the cultures were spread over Muller Hinton Agar (MHA) to be at the level 6 log cfu/mL. 6 mm discs containing 15 µL of solution were placed on MHA. Then, petri dishes incubated in 24 h at 37 °C. Inhibition zone diameters were measured in mm at the end of incubation. Cycloheximide (0.1%) and gentamicin (10 µg) were used as positive controls for yeast and bacteria, respectively. 96% alcohol was used as a negative control. The analysis repeated 3 periods. Inhibition zones were expressed as mean ± standard deviation.17,18
Synthesis and Characterization. TBIP polymer was synthesized in three steps. 4,7-dibromo-2-octyl-1H-benzimidazole was prepared according to the published procedure.19 Then the N-H group on the benzimidazole unit was alkylated by using iodomethane in presence of KOH in Toluene. Finally, the Suzuki type polymerization process was applied to obtain TBIP polymer. In the further process, TBIP polymer salts were obtained from the TBIP polymer. The obtained bromine salt of TBIP was converted to -BF4 and -ClO4 via ion exchange process on the polymer subunit. After completing the synthetic works, the chemical structures of TBIP polymer and its salts were identified by FTIR and 1H NMR spectroscopy (Figure S1-S5 in the supporting information). FTIR and 1H-NMR techniques were used in the structural characterization of the products. The triple signals at 2786, 2844, and 2914 cm-1 attributed to, aliphatic C-H group were observed in the FTIR spectrum of TBIP and its polymer salts. However, the main difference in the FTIR spectra of TBIP and its salts was observed in the 800-1200 cm-1 region. The broad peaks in this region were due to vibrations of counter ions on the TBIP. On the other hand, 1H-NMR spectrum of TBIP (Figure S1), the aromatic peaks were detected between 8.18 and 7.14 ppm. While the CH2 protons on the octyl side chain was observed at 3.74 ppm, -N-CH3 protons on the benzimidazole unit were observed at 1.62 ppm. The other aliphatic protons were detected from 1.25 to 0.89 ppm. In the 1H-NMR spectra of TBIP polymer salts (Figure S2, S3, and S4), the protons attributed to the ethyl group added to the TBIP structure were observed at 1.77 and 1.16 ppm. The obtained results clearly indicated that the TBIP polymer and its salts have been synthesized successfully.
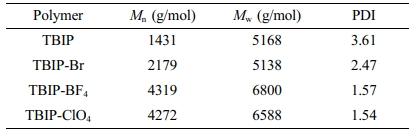
GPC was used to determine both the molecular weight and polydispersity index (PDI) of TBIP polymers and salts (Table 1) and the curves obtained are given in the supporting information (Figures S6-S9). While the PDI values of the polymers ranged from 1.54 to 3.61, the number average molecular weight (Mn) values were found to be 1431 (TBIP), 2179 (TBIP-Br), 4319 (TBIP-BF4) and 4272 (TBIP-ClO4) g/mol, respectively.
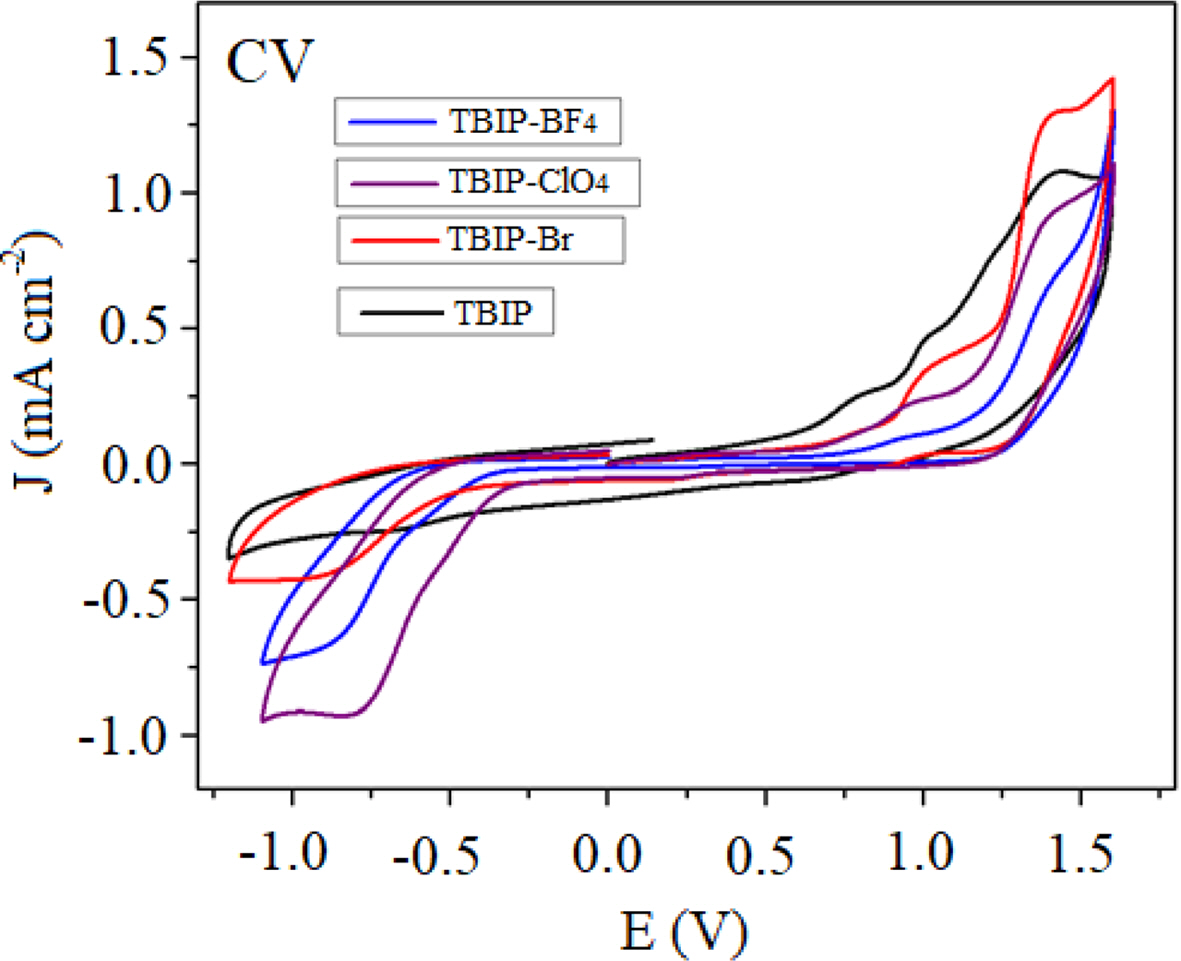
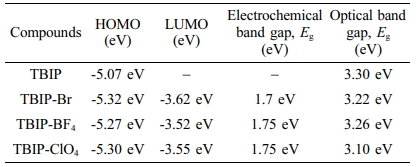
Electrochemical redox behaviors of TBIP polymer and its salts were investigated by using the cyclic voltammetry (CV) technique (Figure 1). Multiple oxidations starting from the 0.75 V (vs. Ag wire) was observed at the anodic scan of all samples. These multiple oxidations are attributed to methyl benzimidazole subunit and thiophene based conjugated main chain, respectively. The first oxidation peak at about 0.82 V slightly shifted to higher potential upon occurred benzimidazolium salt of TPIB polymer (TBIP-Br, TPIB-BF4, TPIB-ClO4). This positive shift is due to less electron nature of the benzimidazole subunit when it was converted positively charged form. On the other hand, there are no reduction waves at the cathodic scan of TBIP. After converting the TBIP to benzimidazolium salts, the reduction peaks were observed between -0.75 and -0.9 V attributed to form the cationic form on the benzimidazolium unit. Due to the higher electronegativity of -ClO4 counter ion, TBIP-ClO4 needed a less negative potential for the reduction of the polymer system when compared to TBIP-Br and TBIP-BF4. The oxidation and reduction potential initials obtained from DPV results were used to calculate the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) levels of TBIP and its salts (Table 2). Since TBIP salts are in active salt form, electrochemical and optical band gap values are not compatible with each other.
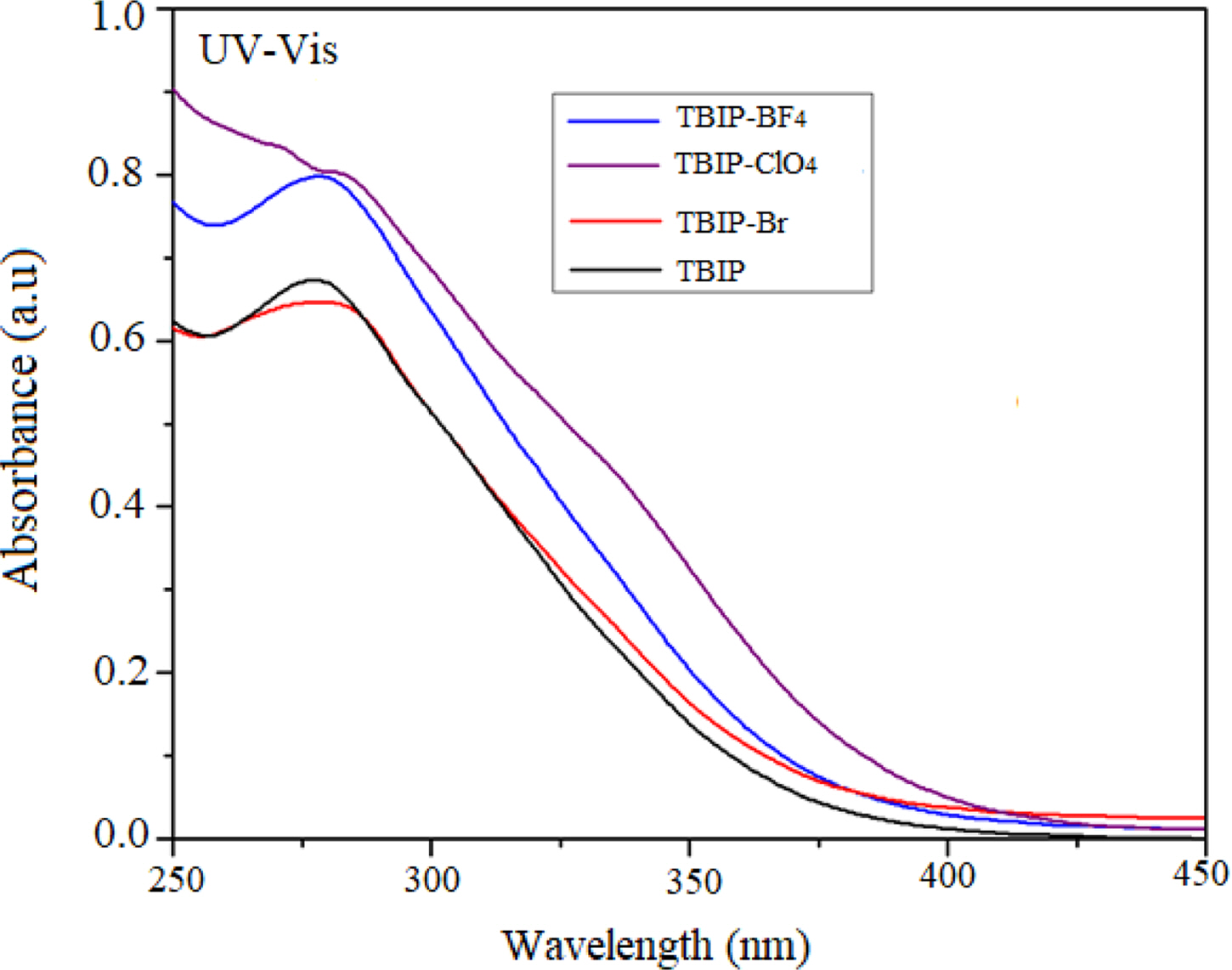
UV-Vis absorption spectra of TBIP and its polymer salts were recorded in 2×10-4 M solution of methanol (Figure 2). The absorption band of TBIP centered at about 280 nm was slightly shifted to a higher wavelength due to converted to a charged form of benzimidazolium unit. A more positive shift was observed at TBIP-ClO4 when compared to the other polymer salts. This positive shift can be due to higher electronegativity of the -ClO4 salt as a counter anion than the others. Finally, optical band gaps were calculated from the absorption band edges.
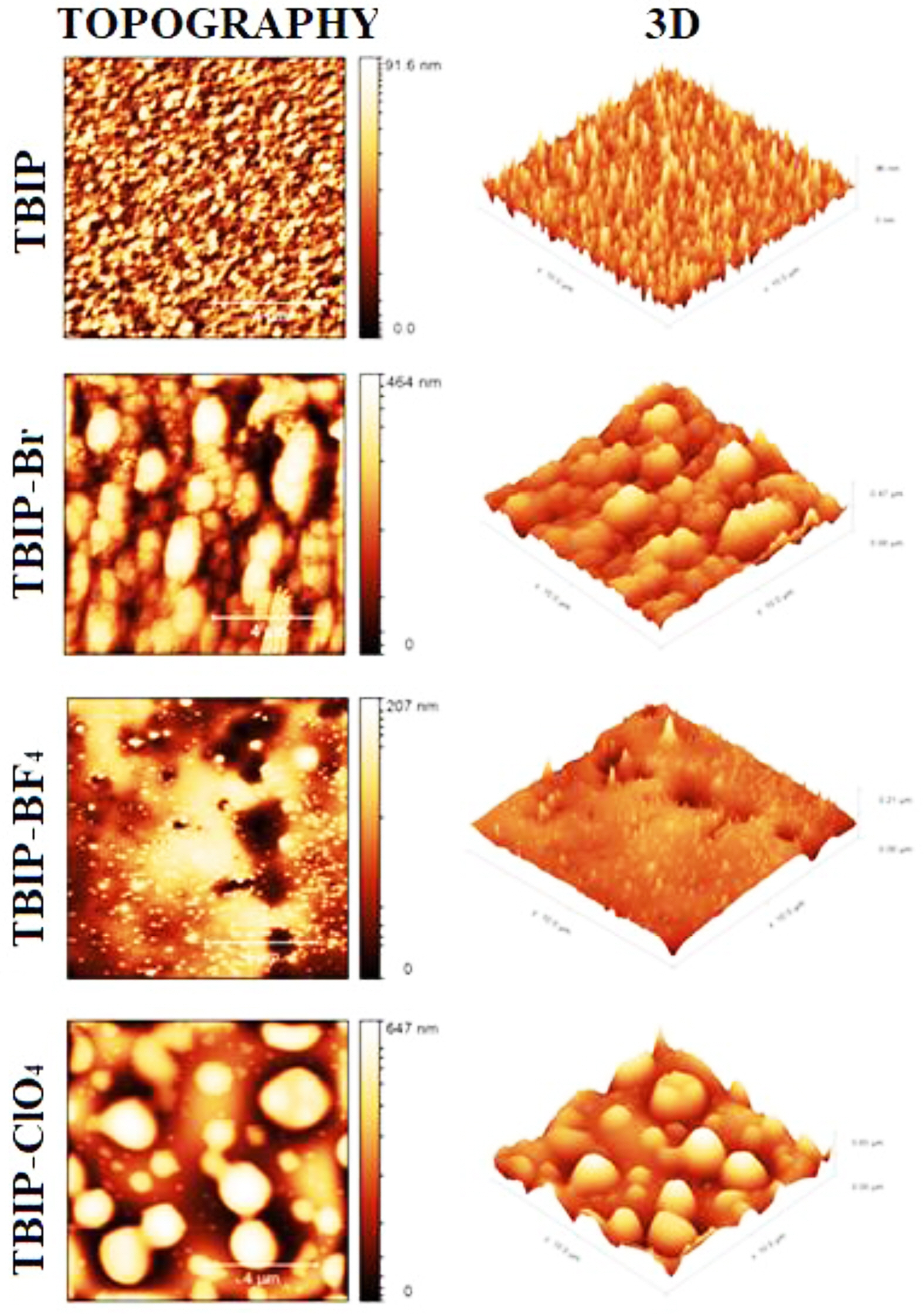
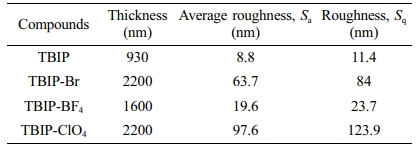
Surface Morphologies. The thin films of TBIP and its salts were prepared by spray coating technique on the glass surface. The sample solution was prepared by using a 50 mg polymer sample in 20 mL methanol. After the polymer solution was put into the spray gun, polymer thin films were prepared by spraying vertically on the ITO/glass surface. In order to determine the surface characterization of TBIP and its salts, the particle size and surface roughness (RMS) of polymeric thin films were examined in non-contact mode by using AFM. AFM images of TBIP exhibited that homogeneously dispersed and smaller particles were observed on the film surface. When the roughness parameters of TBIP and its salts on the film surface were investigated, TBIP-Br and TBIP-ClO4 have more rough surface than the other polymer films (Figure 3). This can be due to the -Br and -ClO4 counter ions are bigger than that of -BF4. Because of this reason, TBIP-BF4 exhibits a low average particle diameter when compared to other polymer salts on the film surface. Finally, roughness and thickness parameters of all TBIP derivatives can be seen in Table 3.
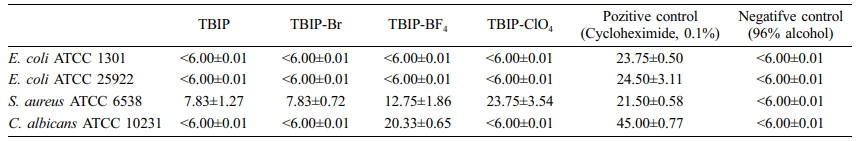
Antimicrobial Activity. The Kirby Bauer agar disc diffusion method in Mueller Hinton Agar was used to assess the antimicrobial activity of compounds. All TBIP and derivates were screened against four bacterial and one yeast strains. Gentamycin and Cycloheximide were used as positive controls, 96% alcohol was used as a negative control. The results of antimicrobial activity presented in Table 4.
TBIP and its salts were showed antimicrobial activity against S. aureus ATCC 6538 and no antimicrobial activity against E. coli ATCC 1301 and ATCC 25922. Interestingly, TBIP-ClO4 was showed better antimicrobial activity compare to the positive control against S. aureus ATCC 6538. Only TBIP-BF4 was showed antimicrobial activity against C. albicans ATCC 10231.
Benzimidazoles are highly effective compounds due to their inhibitory activities and high selectivity. There are various studies in the literature on the biochemical and pharmacological properties of benzimidazoles.19-21 It has been determined that benzimidazole molecules are especially effective on different types of microorganisms. Benzimidazoles are considered bioactive heterocyclic compounds (a component of vitamin B12), and their biological activities are important.It was determined that the heterocyclic ring system showed antioxidant, antiparasitic, anthelmintic, antiproliferative, anti-HIV, anticonvulsant, anti-inflammatory, antihypertensive, antineoplastic and antitrichinellosis activities. Because of the different biological activities and importance of benzimidazoles, studies by adding new side groups are very important.19 In our study, it is thought that the side groups affected the antimicrobial activity of the heterocyclic ring, therefore, the antimicrobial activity could not be determined against all microorganisms tested. There is a need for further research using very different microorganisms and different methods on the subject.
|
Figure 1 CV voltammograms of TBIP and its polymer salts. |
|
Figure 2 UV-Vis Absorption spectra of TBIP and its polymer salts. |
|
Figure 3 AFM images of TBIP, TBIP-Br, TBIP-ClO4, TBIP-BF4 films coated on the glass substrate (all area is 10 mm×10 mm). |
|
Table 2 HOMO and LUMO Energy Levels, Electrochemical and Optical Band Gaps (Eg) Values of TBIP and Its Salts |
In this work, a new thiophene benzimidazole based polymer (TBIP) was synthesized by using Suzuki reaction between 2,5-thiophene bisboronic acid and 4,7-dibromo-1-methyl-2-octyl-1H-benzimidazole. Then, the TBIP polymer salts TBIP-Br, TBIP-BF4 and TBIP-ClO4 were obtained, respectively. According to UV-Vis spectra, the absorption band of TBIP centered at about 280 nm was slightly shifted to a higher wavelength due to converting a charged form of benzimidazolium unit. Thus, multiple oxidation peaks starting from the 0.75 V (vs. Ag wire) are attributed to methyl benzimidazole subunit and thiophene based conjugated the main chain, respectively, slightly shifted to higher potential upon occurred benzimidazolium salt of TPIB polymer. While the AFM images of TBIP exhibited that homogeneously dispersed and smaller particles were observed on the film surface, TBIP-Br and TBIP-ClO4 have rougher surface. In addition, TBIP and its salts were showed antimicrobial activity against S. aureus ATCC 6538 and no antimicrobial activity against E. coli ATCC 1301 and ATCC 25922. The increase in antimicrobial activities against S. aureus ATCC 6538, is an important dataset for the control of biological risk factors and occupational hygiene. “S. aureus” bacteria can reach colonization sites in the human body through respiration or surface contact, causing negative consequences, especially food poisoning. In addition, the antimicrobial effect on “C. albicans” type yeasts is of great importance, especially for female worker health.
- 1. Walba, H.; Isensee, R. W. Acidity Constants of Some Arylimi- dazoles and Their Cations. J. Org. Chem. 1961, 26, 2789-2791.
-

- 2. Singh, P. K.; Silakari, O. Chapter 2-Benzimidazole: Journey from Single Targeting to Multitargeting Molecule. Key Heterocycle Cores for Designing Multitargeting Molecules, Elsevier: Amsterdam, 2018.
-

- 3. Wright, J. B. The Chemistry of the Benzimidazoles Research Laboratories, The Upjohn Company: Kalamazoo, 1951.
-

- 4. Hunter, L.; Marriott, J. A. The Associating Effect of the Hydrogen Atom. Part VIII. The N-H-N Bond. Benziminazoles, Glyoxalines, Amidines, and Guanidines. J. Chem. Soc. 1941, 139, 777-786.
-

- 5. Husain, A.; Varshney, M. M.; Rashid, M.; Mishra, R.; Akhter, A. Benzimidazole: A Valuable Insight into The Recent Advances and Biological Activities. J. Pharm. Res. 2011,4, 413-419.
- 6. Kalidhar, U.; Kaur, A. An Overview on Some Benzimidazole and Sulfonamide Derivatives with AntiMicrobial Activity. Research J. Pharm. Biol. Chem. Sci. 2011, 2, 11-17.
- 7. Kuldipsinh, P. B.; Stoyanka, N.; Illiyan, I.; Manjunath, D. G. Novel Research Strategies of Benzimidazole Derivatives: A Review. Mini-Rev. Med. Chem. 2013, 13, 1421-1447.
- 8. Chanda, K.; Rajasekhar, S.; Maiti, B.; Musuvathi, B. Synthesis and Medicinal Applications of Benzimidazoles: An Overview. Curr. Org. Synth. 2016, 13, 1-11.
- 9. Narasimhan, B.; Sharma, D.; Kumar, P. Benzimidazole: A Medicinally Important Heterocyclic Moiety. Med. Chem. Res. 2012, 21, 269-283.
-

- 10. Woolley, D. W. Some Biological Effects Produced by Benzimidazole and Their Reversal by Purines. J. Biol. Chem. 1944, 152, 225-232.
-

- 11. Wuerges, J.; Geremia, S.; Randaccio, L. Structural Study on Ligand Specificity of Human Vitamin B12 Transporters. Biochem. J. 2007, 403, 431-440.
-

- 12. Desai, K. G.; Desai, K. R. Green Route for the Heterocyclization of 2-mercaptobenzimidazole into β-Lactum Segment Derivatives Containing -CONH- Bridge with Benzimidazole: Screening in vitro Antimicrobial Activity with Various Microorganisms. Bioorg. Med. Chem. 2006, 14, 8271-8279.
-

- 13. Holloway, G. A.; Baell, J. B.; Fairlamb, A. H.; Novello, P. M.; Parisot, J. P.; Richardson, J.; Watson, K. G.; Street, I. P. Discovery of 2-iminobenzimidazoles as a New Class of Trypanothione Reductase Inhibitor by High-throughput Screening. Bioorg. Med. Chem. Lett. 2007, 17, 1422-1427.
-

- 14. Guven, O. O.; Erdogan, T.; Goker, H.; Yildiz, S. Synthesis and Antimicrobial Activity of Some Novel Phenyl and Benzimidazole Substituted Benzyl Ethers. Bioorg. Med. Chem. Lett. 2007, 17, 2233-2236.
-

- 15. Ansari, K. F.; Lal, C. Synthesis and Evaluation of Some New Benzimidazole Derivatives as Potential Antimicrobial Agents. Eur. J. Med. Chem. 2009, 44, 2294-2299.
-

- 16. Harris, J. D.; Liu, J.; Carter, K. R. Synthesis of π-Bridged Dually-Dopable Conjugated Polymers from Benzimidazole and Fluorene: Separating Sterics from Electronics. Macromolecules 2015,48, 6970-6977.
-

- 17. Burt, S. A.; Reinders, R. D. Antibacterial Activity of Selected Plant Essential Oils Against Escherichia coli O157: H7. Lett. Appl. Microbiol. 2003, 36, 162-167.
-

- 18. Özcan, G.; Demirel Zorba, N. N. Combined Effect of Ultrasound and Essential Oils to Reduce Listeria Monocytogenes on Fresh Produce. Food Sci. Technol. Int. 2016, 22, 353-362.
-

- 19. Ansari, K. F.; Lal, C. Synthesis, Physicochemical Properties and Antimicrobial Activity of Some New Benzimidazole Derivatives. Eur. J. Med. Chem. 2009, 44, 4028-4033.
-

- 20. Emadi, A. Schiff Benzimidazole Derivatives: Synthesis, Properties and Antimicrobial Activity. Intl. J. Farm. Alli. Sci. 2020, 9, 72-82.
- 21. Padalkar, V. S.; Borse, B. N.; Gupta, V. D.; Phatangare, K. R.; Patil, V. S.; Umape, P. G.; Sekar, N. Synthesis and Antimicrobial Activity of Novel 2-substituted Benzimidazole, Benzoxazole and Benzothiazole Derivatives. Arab. J. Chem. 2016, 9, S1125-S1130.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2021; 45(6): 817-823
Published online Nov 25, 2021
- 10.7317/pk.2021.45.6.817
- Received on Feb 25, 2021
- Revised on Jul 22, 2021
- Accepted on Jul 27, 2021
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Supporting Information
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Fatma Baycan
-
Department of Chemistry, Faculty of Arts and Sciences, Canakkale Onsekiz Mart University, Canakkale, Turkey
- E-mail: fatmabaycan@comu.edu.tr
- ORCID:
0000-0003-0133-9869









 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.