- Synthesis and Characterization of Poly(lactic acid-co-glycolic acid-co-ε-caprolactone) and Its Pressure Sensitive Adhesive Property
School of Material Science and Engineering, East China University of Science and Technology, Shanghai 200237, China
- Poly(lactic acid-co-glycolic acid-co-ε-caprolactone)의 합성, 분석 및 감압 접착 특성
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
Random copolymers of poly(lactic acid-co-glycolic acid-co-ε-caprolactone) (PLGA-CL) based on lactic acid (LA), glycolic acid (GA), citric acid (CA), and e-caprolactone (CL) were synthesized and characterized. 1H NMR spectra showed that the polymerization conversion of various monomers was high under the experimental conditions, and the degree of polymerization of the polycaprolactone (PCL) segment increased with the growth of CL content. The content of CL has a remarkable effect on the decrease of glass transition temperature (Tg) of the copolymers. The adhesive properties of random copolymers as pressure-sensitive adhesive (PSA) were evaluated. With the increase of CL content, 180° peel strength, the shear strength and loop tack decreased. When CL content was 16.7 wt%, 180° peel strength was 4.76 N/25 mm, and loop tack was 8.45 N/25 mm.
Random copolymers of poly(lactic acid-co-glycolic acid-co-ε-caprolactone) (PLGA-CL) based on lactic acid (LA), glycolic acid (GA), and e-caprolactone (CL) as raw material, CA as the starting agent and Sn(Oct)2 as the catalyst were synthesized by copolymerization. Copolymers demonstrated the degree of polymerization of polycaprolactone (PCL) segment increased with the growth of CL content. The content of CL was developed with an emphasis on the decrease of glass transition temperature (Tg) of copolymer. The adhesive properties of random copolymers as pressure-sensitive adhesive (PSA) were evaluated.
Keywords: poly(lactic acid-co-glycolic acid), ε-caprolactone, glass transition temperature, pressure-sensitive adhesive property.
Pressure-sensitive adhesive (PSA) is a kind of viscoelastic material which could make the adhesive immediately to bonding any smooth surface within low pressure.1,2 Most traditional press-sensitive adhesives are acrylate polymers. They have been widely used in all walks of life,3,4 and their production accounts for more than 65% of PSA. However, they are polymer materials that cannot be easily biodegraded.
Recently, adhesive based on several biodegradable materials has been extensively studied.5-7 Poly(lactic acid-co-glycolic acid) (PLGA) is a kind of good biodegradable material with high biocompatibility and non-toxicity, which is widely used in medical engineering materials, pharmaceutical, and industrial fields.8-11 Studies have shown that the higher the glycolic acid content of PLGA, the faster the degradation rate of PLGA. When the molar ratio of lactic acid to glycolic acid is about 1:1, the degradation only costs two months.12 In general, the PLGA is in a glassy state at room temperature because its glass transition temperature (Tg) is about 30 °C. Therefore, the performance of PLGA is in urgent need of modification.
Polycaprolactone (PCL) is a semi-crystalline polymer with high biocompatibility, organic polymer compatibility, and biodegradability, which is one of the most widely studied aliphatic polyesters for a wide variety of uses.13-17 Because of its relatively low Tg, it can be used to decrease the Tg of PLGA. Lando18 used trimethylolpropane as the trifunctional central molecule to form a new biodegradable binder with lactic acid (LA) and e-caprolactone (CL). They found that the Tg of the copolymer decreased after CL monomer united into the backbone of poly lactic acid (PLA), but its glass transition temperature was above room temperature. Liu19 synthesized random copolymers of poly(d,l-lactide-co-glycolide-co-e-caprolactone) (PLGC) by the ring-opening polymerization of d,l-lactide (DLLA), glycolic acid (GA), and CL. They copolymerized lactide, glycolide, and e-caprolactone directly to get a copolymer that appeared as rigid materials to rubber. The copolymer is not suitable for PSA because of its high molecular weight and no viscosity at room temperature. Therefore, we hope to synthesize low molecular weight PLGA and add CL to improve its performance, while controlling the content of CL to provide it with appropriate adhesive properties. PCL and PLGA are complementary in terms of adhesive properties and glass transition temperature, and would lead to more effectiveness if combined.
In this paper, low molecular weight PLGA was synthesized from LA, GA, and citric acid (CA). By adding different amounts of CL, the copolymers with low Tg were synthesized. Then we studied the conversion of CL monomer and the degree of polymerization of PCL segment, as well as the glass transition temperature and water contact angle. Finally, 180° peel strength, shear strength, and loop tack of PSA tapes were investigated to reveal their pressure-sensitive bonding properties.
Materials. Glycolic acid (GA, 70%) was supplied by Jiangsu Taixing Water Chemical Co., Ltd (Jiangsu, China). Lactic acid (LA, 88%) was purchased from Henan Jindan Lactic Acid Technology Co., Ltd (Henan, China). Citric acid (CA) was supplied by Shanghai Lingfeng Chemical Reagent Co., Ltd (Shanghai, China). ε-Caprolactone (CL) was purchased from Hunan Juren Chemical New Material Technology Co., Ltd (Hunan, China) and stannous octoate was supplied by Shanghai Zhengui New Materials Technology Co., Ltd (Shanghai, China). All other reagents were of analytical grade and used without further purification.
Synthesis of Poly(lactic acid-co-glycolic acid-co-ε-caprolactone) (PLGA-CL). GA (4.08 mol, 443.3 g), LA (3.35 mol, 342.7 g) and CA (0.41 mol, 78.3 g) were mixed in a three-mouth flask, which was then placed in a heat-collecting magnetic stirrer for oil bath heating and turned on magnetic stirring. Firstly, it was heated at 120 ℃ and kept for 90 min. After that, Sn(Oct)2 (0.041 mmol, 16.8 g) was added in while heating up to 180 ℃. Vacuum to 100 Pa, and the reaction ended in 12 h. The prepared PLGA was placed in a beaker for further characterization.
The prepared PLGA and CL monomer were then mixed with different mass ratios (5:1,5:2,5:3,5:4,5:5), with a total reactant amount of about 100 g. Then placed them in a flask, heated up to 150 ℃ with an absolute pressure of 400 Pa, and the reaction lasted 6 h. PLGA-CL with different mass ratios were obtained.
Characterization. Infrared Spectral Characterization (Fourier Transform Infrared Spectroscopy, FTIR): The Nicolet5700 type infrared spectrometer (USA) was applied to record the FTIR spectra of the polymer films. The range of 4000 to 400 cm-1 and the resolution is 4 cm-1.
Nuclear Magnetic Resonance Spectrometer (1H NMR): An appropriate amount of polymer was dissolved in CDCl3 containing 0.03% TMS, and the polymer was characterized by AVANCE Ⅲ 400 (Bruker, Switzerland).
Gel Penetration Chromatography (GPC): The molecular weight and distribution of the polymers were determined by a GPC (Waters, USA) with a Waters Model 1515. In the test process, the flow rate was 0.5 mL/min with polystyrene (PS) as internal standard at 40 °C. Tetrahydrofuran (THF) was used as the solvent.
Differential Scanning Calorimetry (DSC): The thermal properties of the polymers were recorded with DSC (Model 8500, China) measurements. The heating temperature ranged from -70 °C to 60 °C, at a rate of 10 °C/min in a nitrogen atmosphere.
Water Contact Angle (WCA): The polymer was heated to 55 °C. After softened, the polymer was coated on a 25 mm wide poly(ethyl terephthalate) (PET) film with the wet film preparation device, and a 40 μm thick viscous film was obtained. The water contact angle on the polymer film was determined by an automatic contact angle goniometer (JC2000D2, Shanghai, China). A drop of deionized water, which was used as a testing liquid (5 μL), was dripped on the surface of the polymer film. Measurement result was given as the mean value of WCA.
Pressure-Sensitive Adhesive (PSA) Testing20,21: The polymer was heated to 55 °C and then coated on a 25 mm wide PET film with a wet film preparation device, and a 40 μm thick viscous film was obtained. MTS Exceed E42.503 electronic universal tensile testing machine (China) was used to evaluate 180° peel strength, shear strength and loop tack.
For the 180° peel test, films coated with adhesive were laminated against the stainless steel plate using a rubber roller. After 30 min, peeled from the substrate at the speed of 300 mm/min. The average force required to peel the film was recorded as 180° peel strength.
For shear holding force, the films were laminated against the stainless steel plate using a rubber roller, and the contact area was 25 mm2. After placing for 30 min, the stainless steel plate was vertically fixed on the instrument, and 1 kg load was suspended at the other end. The time when the strip was completely separated from the stainless steel plate was recorded as the shear holding force.
For the loop tack test, the strip was formed into a loop with the adhesive side facing outwards. Inserted both ends of the strip into the upper grip of the tester. A stainless steel plate was placed on the lower grip. The upper grip moved down at the speed of 300 mm/min until the strip had an area of approximately 25 mm2 in contact with the stainless steel plate. Then moving the upper grip upward at the same speed while recording the force required to separate. The maximum separation force was recorded as loop tack.
The average of the five parallel test results was taken as the final experimental result.
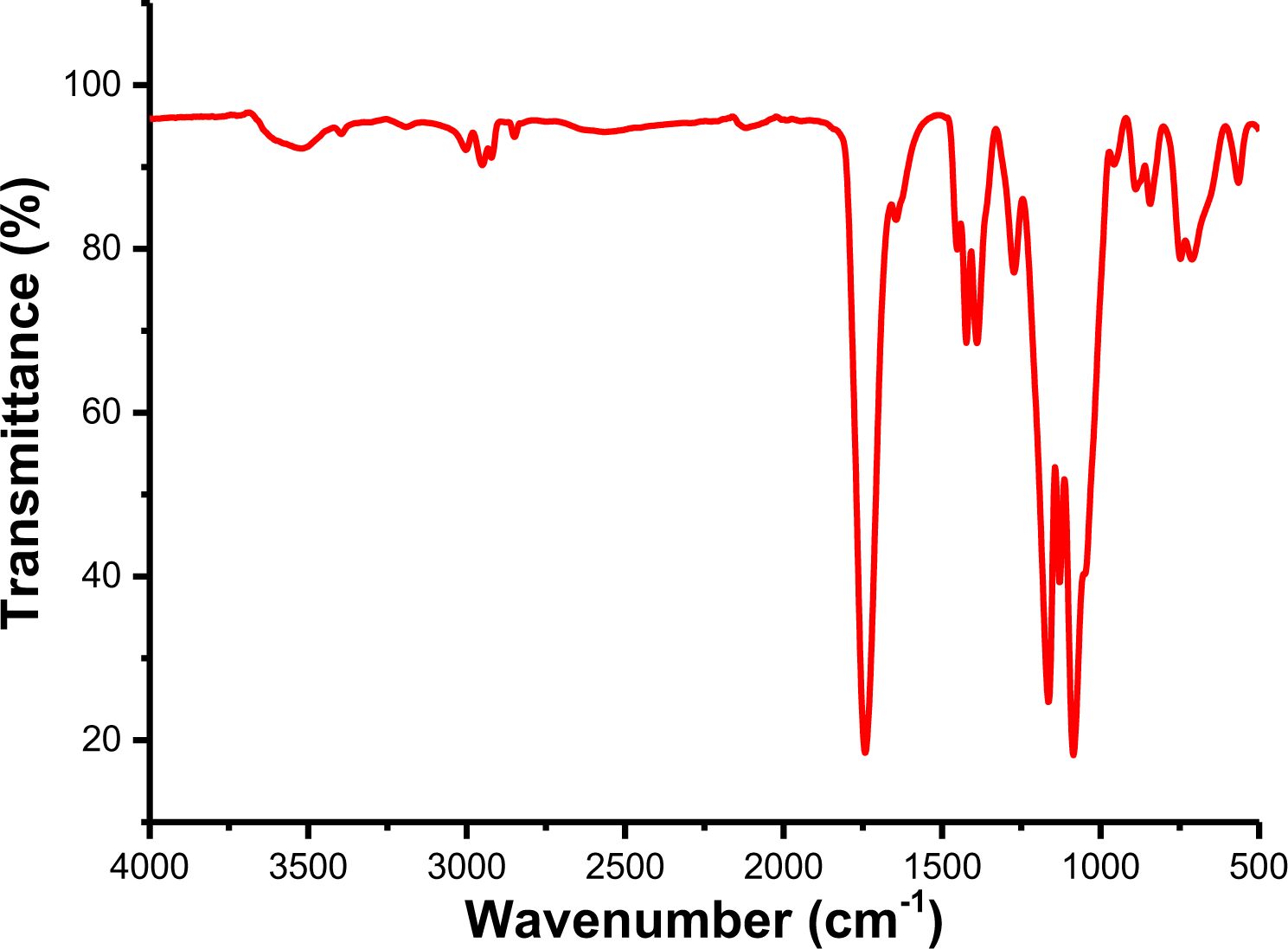
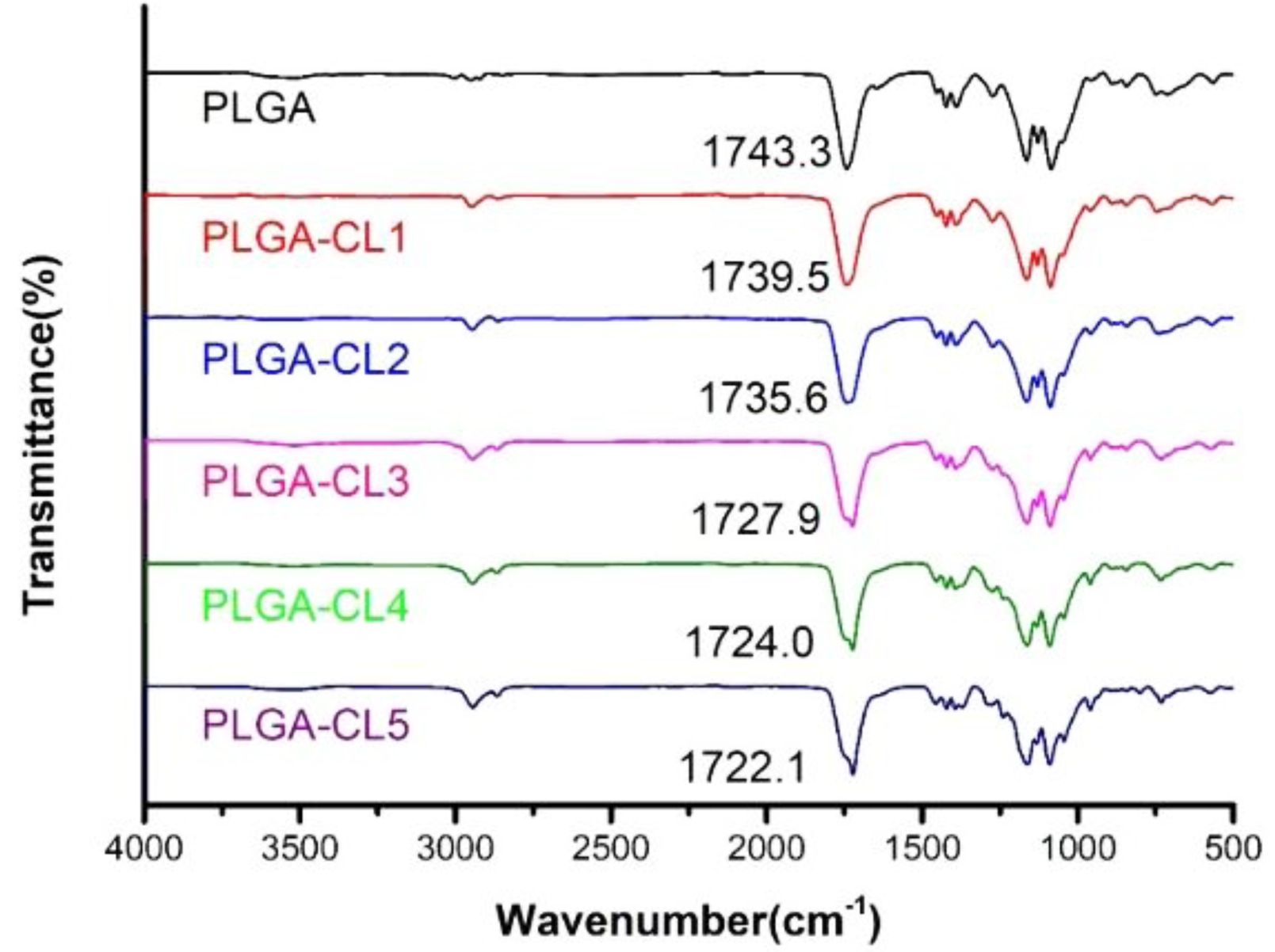
Synthesis and Characterization of PLGA. The chemical structures of PLGA were characterized by FTIR. FTIR spectra of PLGA are shown in Figure 1. It is observed that FTIR spectra exhibit the stretching vibration of -OH and C=O at 3525.2 and 1743.3 cm-1 respectively. The absorption peaks at 2930.4 and 2850.3 cm-1 are the stretching vibration of C-H (CH2). At 1164.8 and 1085.7 cm-1, there are asymmetric and symmetric stretching vibration peaks of C-O bond respectively, indicating the presence of an ester group. The infrared spectra confirmed the synthesis of PLGA.
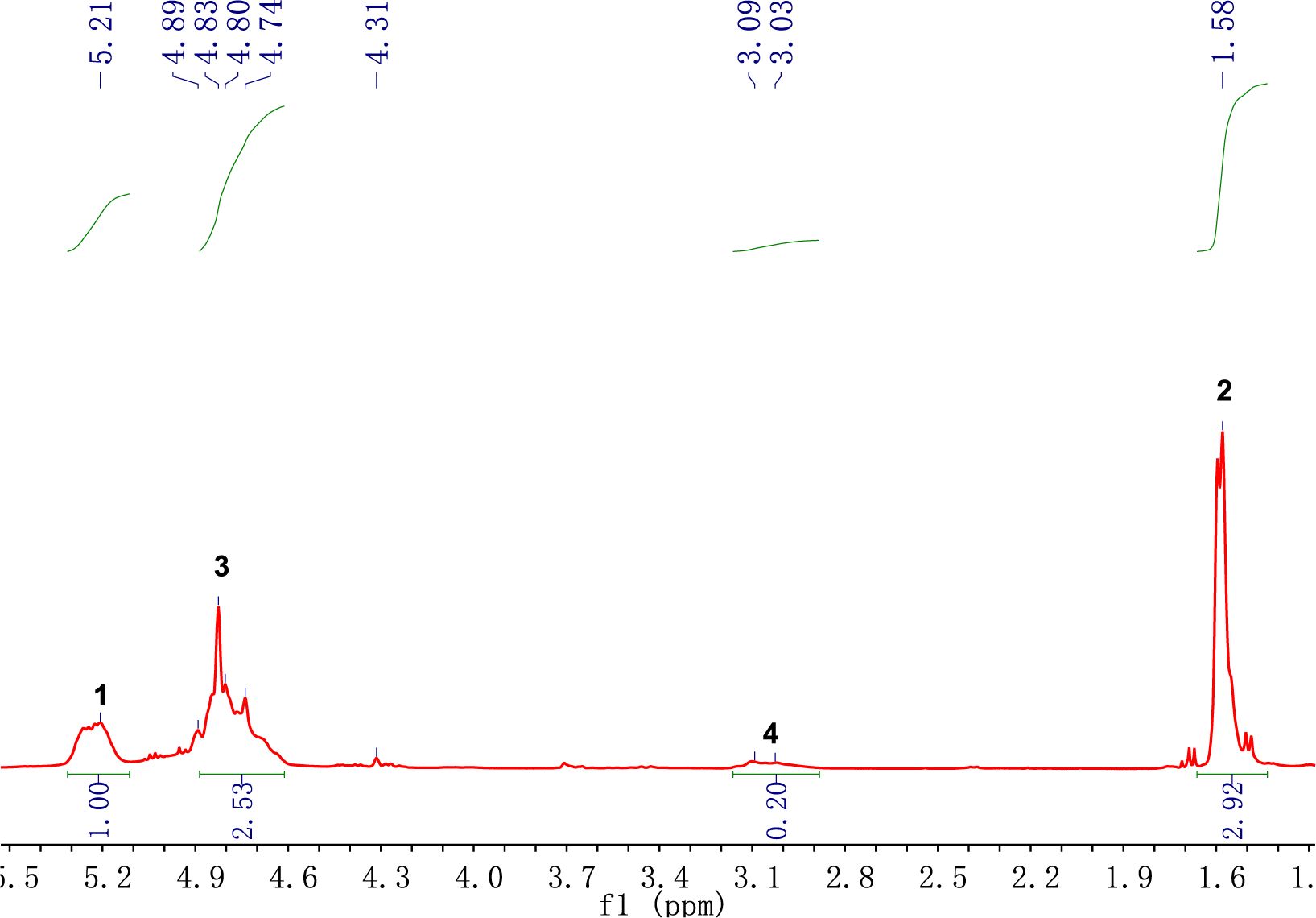
Scheme 1 presents the molecular structure of PLGA and Figure 2 is the 1 H NMR spectra of PLGA. There is the peak at 5.21 ppm, due to the proton of LA’s methine group, the protons of GA’s methylene group, appearing at 4.83 ppm, the protons of the methyl group of LA, at 1.58 ppm and the protons of the methylene group of CA, at 3.03 ppm.
The peak area intensity is shown in Table 1, and we can calculate the copolymerization ratio in the polymer through the peak area. When the feeding ratio [GA]/[LA]/[CA] = 1.22:1: 0.12, the molar ratio of the copolymer is calculated by using the date in the Table 1. [GA]/[LA]/[CA] = [I(3)/2]:[I(1)]:[I(4)/2], the result is about 1.26:1.00:0.10, which is approximately consistent with the feed ratio.
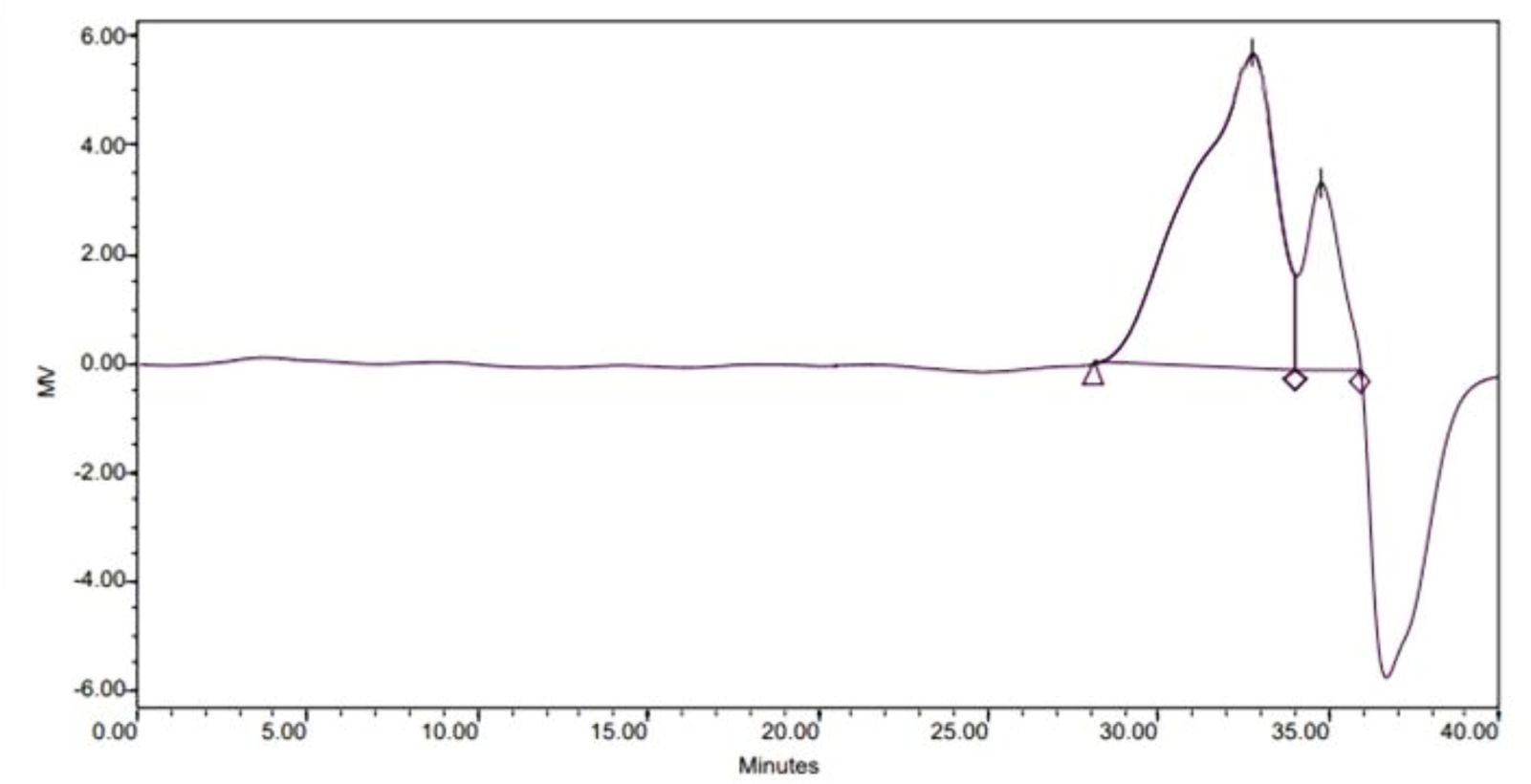
Figure 3 presents GPC chromatograms of PLGA. Two peaks appeared in GPC, which may be due to the uneven degree of polymerization of four functional groups on CA. The polydispersity index is 1.82 and the relative molecular weight of the prepared polymer measured by GPC is 1401.
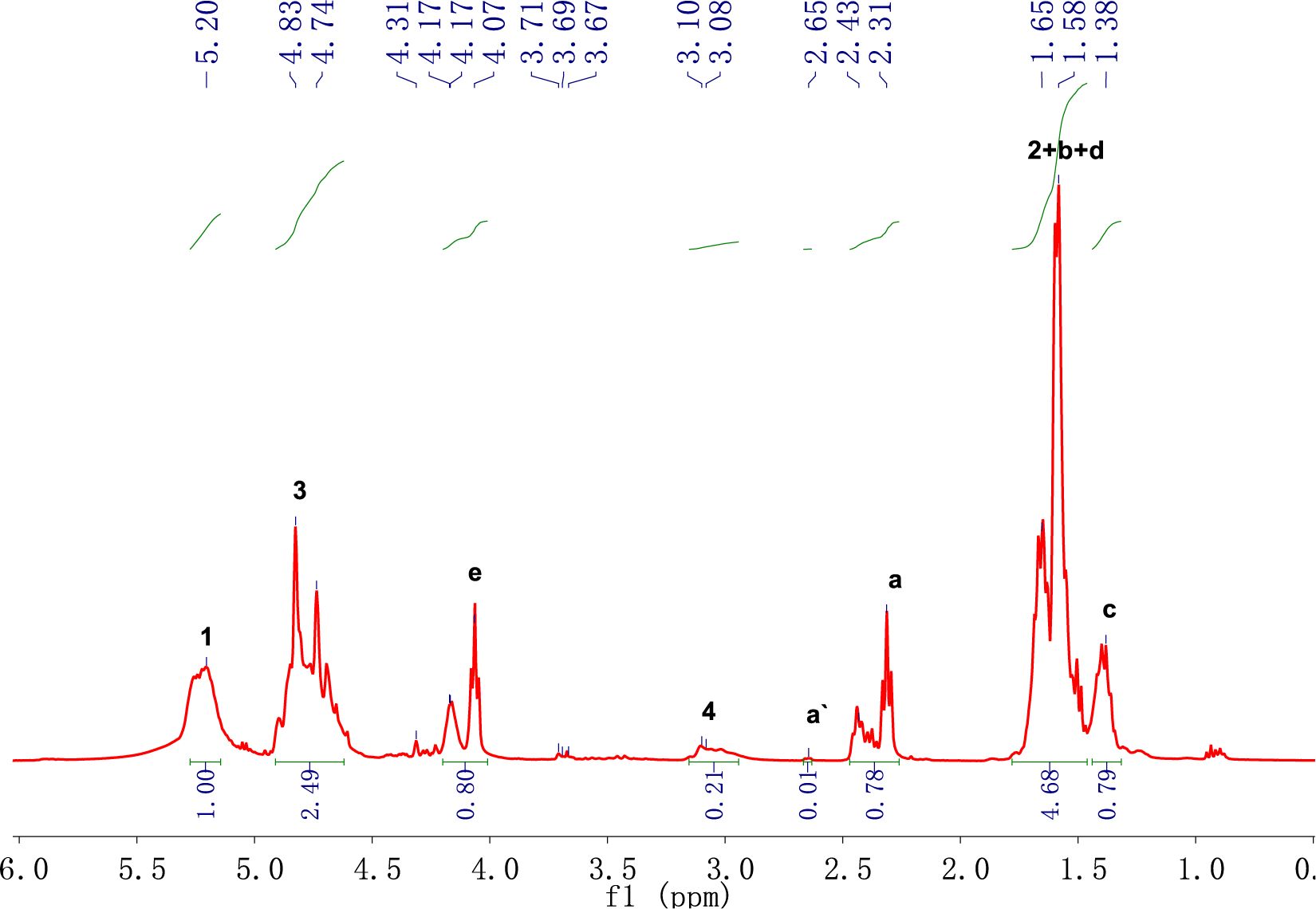
1H NMR and FTIR Analysis of PLGA-CL. A typical 1H NMR spectra is shown in Figure 4 and the molecular structure of PLGA-CL are shown in Scheme 2. The peaks at 4.6-4.9 are the characteristic absorption peaks of GA’s methylene group. The peak at 5.1-5.3 is the absorption peak of the methane group in LA. The characteristic absorption peaks at 4.0-4.2 and 2.3-2.5 are the absorption peaks of -OCH2 and -CH2COO in the structure of CL respectively. The peak area intensity is shown in Table 2.
In order to have a further analysis on the results of 1H NMR spectra, it is necessary to determine the attribution of the peak. The known reactants in the reaction system are PLGA, CL monomer, and Sn(Oct)2, among which the possible existing substances after the reaction are: copolymer, CL monomer, Sn(Oct)2. The chemical shifts of hydrogen atoms in CL and PCL are shown in Table 3 and the molecular structure of CL is shown in Scheme 3. For PSA properties, the monomer conversion during the polymerization process should be as high as possible to prevent small molecules from interfering with the bonding process.22 Therefore, we can calculate the conversion rate of CL by calculating the proportion of the remaining CL and the copolymer composition in the 1H NMR spectra. In Figure 3, PLGA did not have a peak between 2.62-2.66, while the peak at 2.65 of the copolymer in Figure 4 indicated that CL was not completely transformed during the process of copolymer reaction. Theoretically, the conversion rate of CL can be obtained by the ratio of the peak area intensity of CL to the copolymers.23 The formula is: α(CL)=[I(PCL)-I(CL)]/I(PCL) =[I(a)-I(a`)]/I(a); where a is the monomer conversion rate and I is the integrated intensity of each proton peak. The conversion rate of CL in PLGA-CL1 was 98.71%. The other thing to notice is that PCL is a semi-crystalline polymer, when the degree of polymerization is greater than 5, PCL segment will show crystallinity. CA has three carboxyl groups and one hydroxyl group, we can calculate the theoretical degree of polymerization of PCL segment on the single-arm with the molar concentration of PCL and the starting agent CA in the copolymer. The formula is: DP=[PCL]/4[CA]=[I(a)]/4[I(4)]. By calculating, the theoretical degree of polymerization of CL in PLGA-CL1 was 0.93.
1H NMR spectra of PLGA-CL with different content of CL were presented in Figure. 2, which clearly showed that, with the growth of the content of CL, the peak areas of 1.38-1.76, 2.3-2.5 and 4.0-4.2 also increased. After calculating the formula, monomer conversion and theoretical degree of polymerization of PLGA-CL are shown in Table 4. It is worth noting that the conversion rate decreased slightly with the increase of the content of the CL. According to the results, the overall conversion rate change trend was over 94%. All the synthesized copolymers have high monomer conversion levels.
The chemical structure of copolymers was characterized by FTIR spectra. The FTIR spectra of PLGA-CL with different content of CL are shown in Figure 6. As we can see from the figure, all FTIR spectra show similar peaks. However, compared with the spectra of PLGA, the spectra of PLGA-CL copolymers showed a wider peak between 2991.1 cm-1 and 2836.8 cm-1 as the content of CL increased. This is, because with the increase of CL, the number of -CH2 groups in the system increases and C-H produces stronger absorption. In fact, CL reacts with PLGA to form ester bonds after ring-opening, and the number of free C=O in the system decreases. Therefore, compared with PLGA, the carbonyl group peak of PLGA-CL in the copolymer shifts to a lower wave-number.
DSC Analysis. The performance of PSA will be affected by Tg. It is well known that PSA with Tg values below room temperature is preferred and shows good end-use performance.24
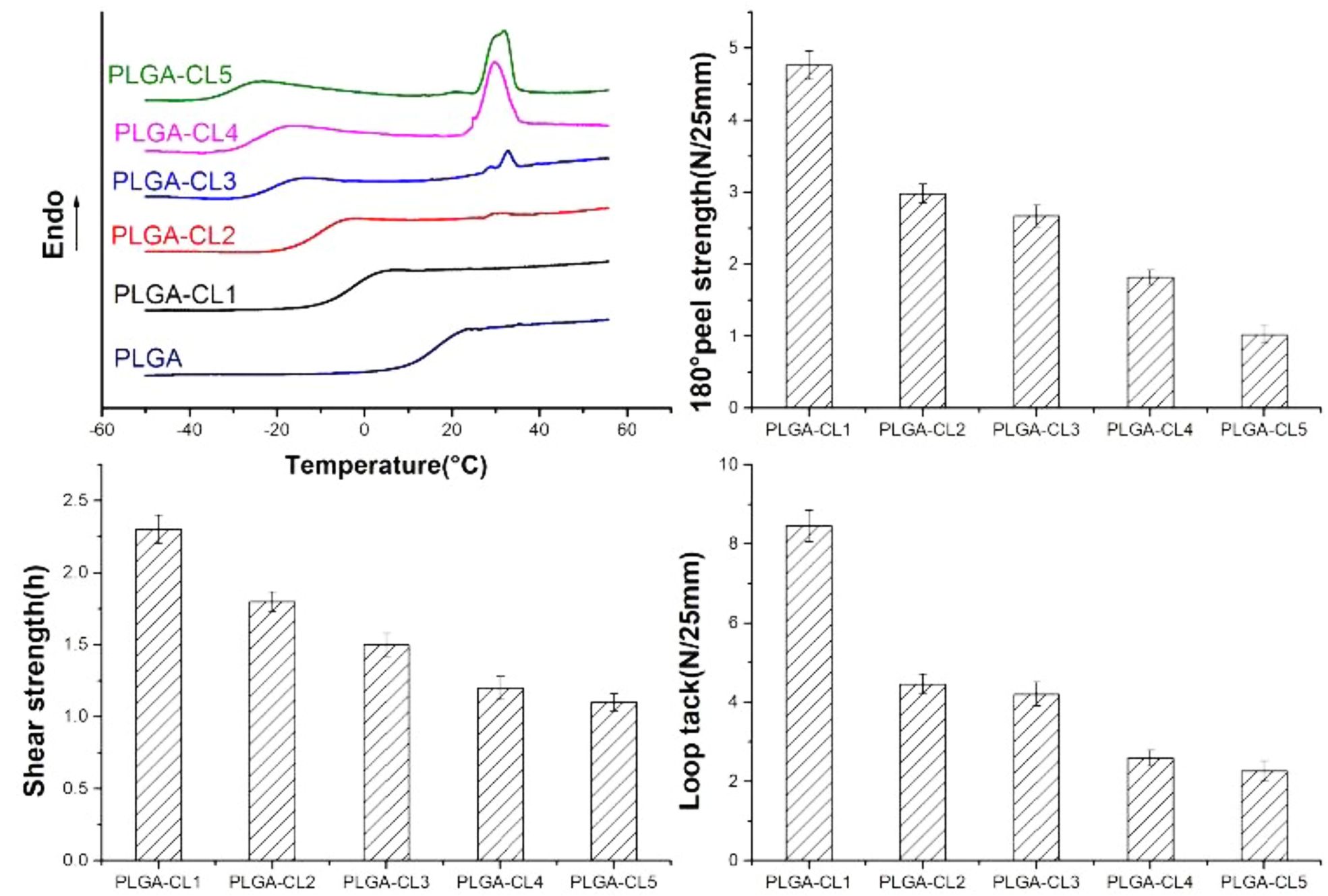
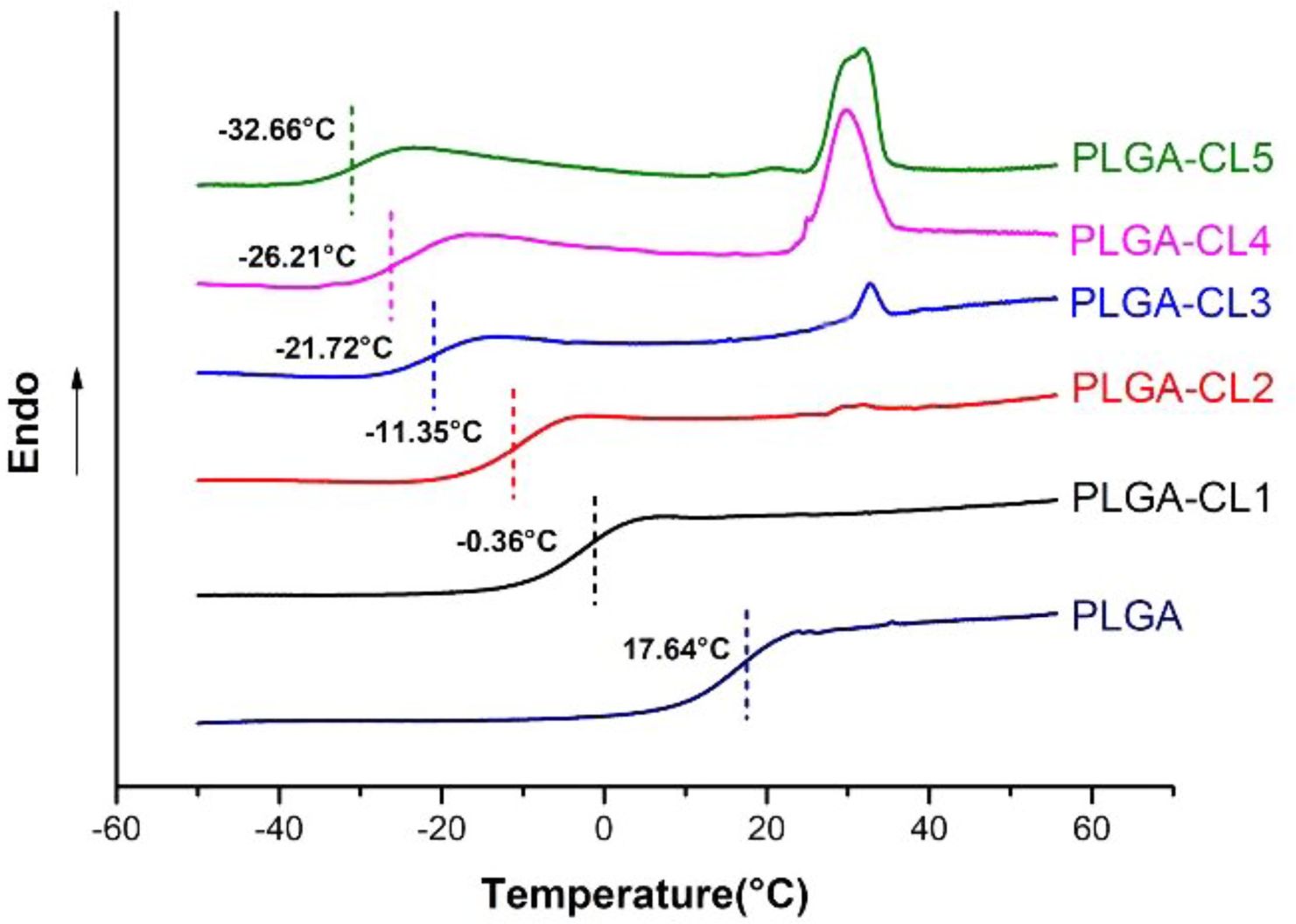
DSC curves of PLGA with different content of CL are compared in Figure 7. DSC curves show that PLGA-CL terpolymer has only one Tg, which indicates that the obtained copolymer is a random copolymer. In general, the Tg of a polymer depends on many factors. The addition of comonomers to the reaction mixture can be used to change the Tg of the polymer. The monomer with a higher Tg value is used to enhance the cohesion of the adhesive, while the monomer with a lower Tg value provides viscosity and flexibility.22 Figure 7 shows that the Tg of PLGA is 17.64 °C. And the Tg of PCL is about -60 °C in general, so Tg of the copolymer can be kept within an ideal range by controlling the feeding amount of CL. In fact, as can be seen from Figure 7, Tg of all PLGA-CL samples are below 0 °C, ranging from -33.66 to -0.36 °C. With the increase of the content of CL, the Tg of the polymer is -0.36, -11.36, -21.72, -26.21, and -32.66 °C respectively, and the number decreases gradually. This can be attributed to the fact that with CL entering into the system for single-ring reaction, the flexible chain segment of the polymer grows, and the incorporation of CL improves the free motion of the polymer chain, thus reducing Tg. Another noteworthy phenomenon is that, with the increase of CL content, the melting point of the copolymer appeared near 32 °C. In the previous 1H NMR spectra calculation, the degree of polymerization of PCL segment on the single-arm in PLGA-CL was all below 5. For PLGA-CL1, the polymer was an amorphous structure, and DSC curve had no crystallization peak. The degree of polymerization of PCL segment in PLGA-CL2 was about 1.42, and a small peak appeared at 32 °C. This might be due to the uneven distribution of chain segments, resulting in the length of some PCL segments exceeding 5. With the growth of CL content, the degree of polymerization of PCL segment in PLGA-CL3, 4, and 5 increased, and the polymers showed obvious crystallization. This phenomenon agreed with the calculation of theoretical degree of polymerization in 1H NMR spectra.
Copolymerization is a reaction in which different compounds come together to form a copolymer. For random copolymers, Fox derived the following eq. from the glass transition temperature of each homopolymer: 1/Tg = W1/Tg1 + W2/Tg2. Where W1 and W2 are the weight fractions of PLGA and CL monomer units in the copolymer, and Tg1 and Tg2 are the Tg(K)s of PLGA and PCL homopolymers. For a typical example, the weight fraction of PLGA-CL1 is W1 = 0.833 and W2 = 0.167. Their Tg values are Tg1 = 17.64 °C (290.64 K) and Tg2 = -63.8 °C (209 K).19 Substituting the Fox eq. the theoretical Tg is -0.52 °C, which is approximately consistent with the actual measured Tg value. The experimental results are in good agreement with the theoretical model, which indicates that the composition of copolymer is consistent with the feed ratio.
Water Contact Angle (WCA) Analysis. Wettability is one of the most important surface properties affecting material properties. It is characterized by the static contact Angle. The hydrophilicity of polymer materials can be evaluated by depositing water droplets on the film surface. The composition of the polymer film will change the value of the contact angle.25 Significantly, the more hydrophilic the membrane surface is, the lower the water contact angle becomes. In the use of PSA, the wettability of PSA should be studied, and in the process of coating, it also needs to determine the wettability of PSA on the substrate surface, which will affect the adhesion of PSA.
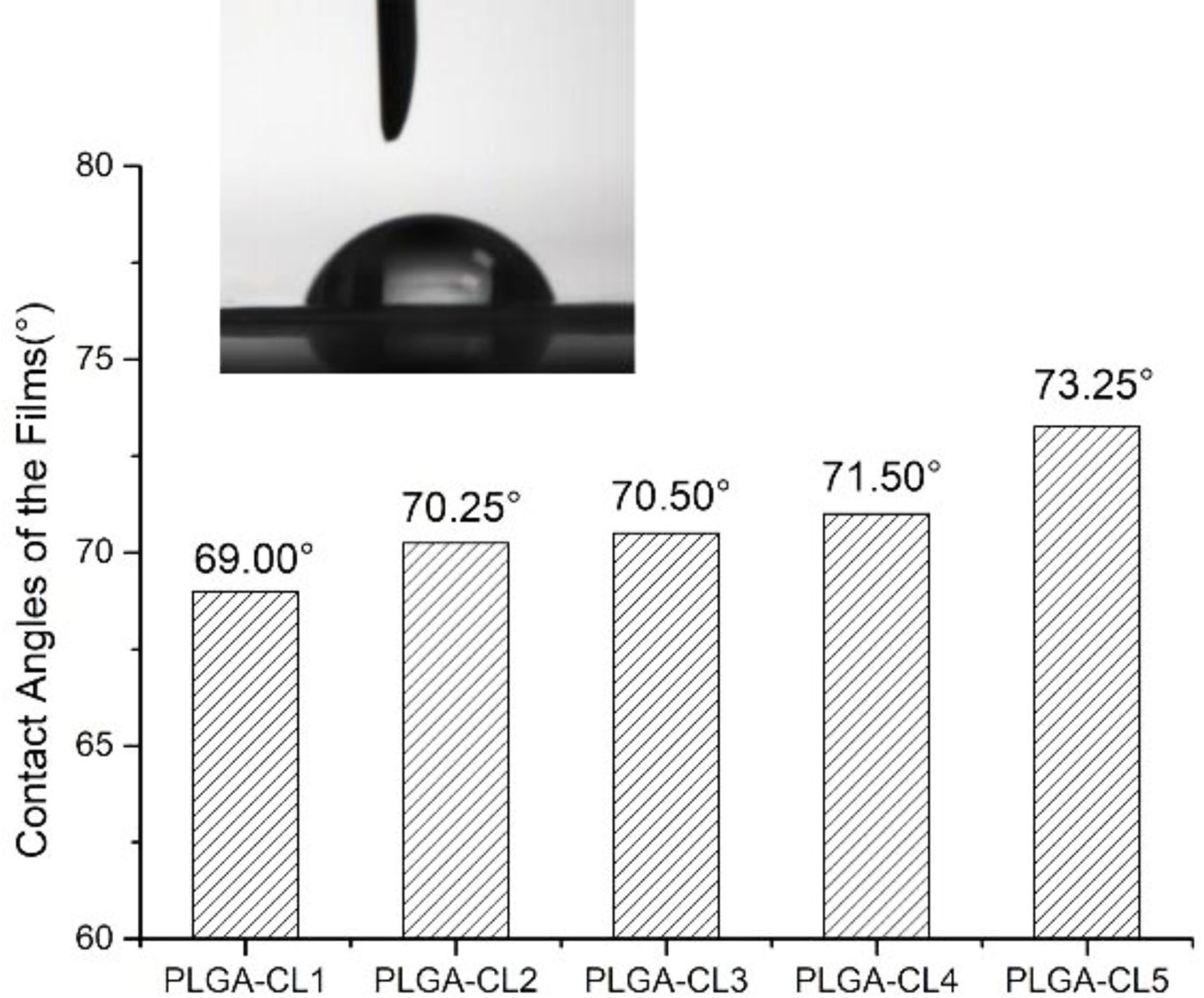
WCA results are shown in Figure 8 by testing the contact angles of polymers with different content of CL, it is obvious that the WCA of the polymers is below 90 °C, indicating that the polymers are hydrophilic. This phenomenon is resulted from the polymerization with CA as the starting agent, and a large number of carboxyl groups at the end of the polymer make the polymer hydrophilic properties. Another noteworthy phenomenon is that with the increase of the content of CL, the WCA of polymer films with different content of CL increased from 69.00 to 73.25°. This is because PCL is a hydrophobic material. The introduction of CL into the ring-opening polymerization resulted in a slight decrease in the hydrophilicity of the polymer.
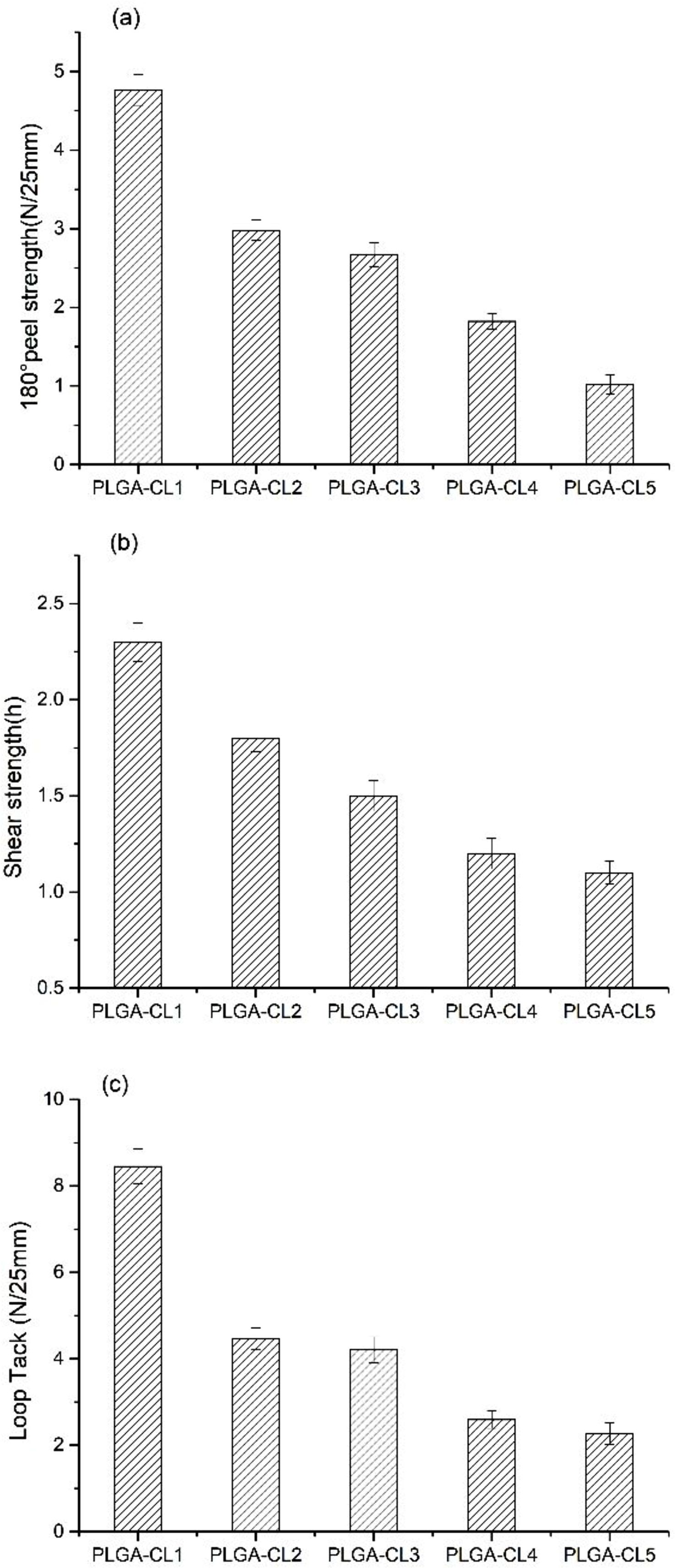
Adhesive Properties Analyses. The experimental results of 180° peel strength, shear strength, and loop tack are shown in Figure 9. For PSA properties, the adhesion and cohesion required by PSA are very important.26 Since PLGA prepared by us is non-viscous at room temperature, PLGA is not pressure-sensitive viscous when the addition of CL is 0. As can be seen in the figure, the copolymers are pressure-sensitive viscous with the addition of CL. This phenomenon is due to the addition of CL into the system makes the polymer's molecular chain flexible and long, while the molecular weight and intermolecular force increase, the Tg of the copolymer decreases. On the other hand, when the CL content is the lowest, the copolymer 180° peel strength, loop tack, and shear strength are the highest. This is, because with the increase of CL content, the degree of polymerization of PCL segment increases, and the copolymer shows crystallinity, which may lead to the decrease of polymer viscosity. Interestingly, it can be concluded from Figure 9(a) that the peel strength of PSA decreases with the increase of CL content. This is because PCL is a semi-crystalline polymer that is not viscous. When the CL content increases, the degree of polymerization of some PCL chain segment gradually exceeds 5 and shows crystallinity, which leads to the decrease of the peel strength. On the other hand, the wettability of materials also affects the adhesive of PSA. With the increase of CL content, the hydrophilicity of the polymer decreases, which leads to the peel strength decreases. As can be seen from Figure 9(b), as expected, the shear strength of PSA gradually decreases with the increase of CL content. This is because, with the increase of CL, the molecular flexible chain segment becomes longer, the cohesion strength of PSA decreases, and the failure type changes into cohesion failure, which leads to the decrease of shear strength. As shown in Figure 9(c), the loop tack is very similar to the peel strength with the change of CL content. Therefore, the above hypothesis explains the peel strength should also be applicable to the change of loop tack. When CL content was 16.7 wt%, 180° peel strength was 4.76 N/25 mm and loop tack was 8.45 N/25 mm. The experimental results showed that loop tack was similar to that of the self-crosslinking fluorinated polyacrylate pressure-sensitive adhesive prepared by Fang,24 and the peel strength was about half of that of the self-crosslinking fluorinated polyacrylate pressure-sensitive adhesive. We found that by controlling the content of CL to control 180° peel strength and shear strength of the copolymer, so as to obtain the appropriate mechanical properties.
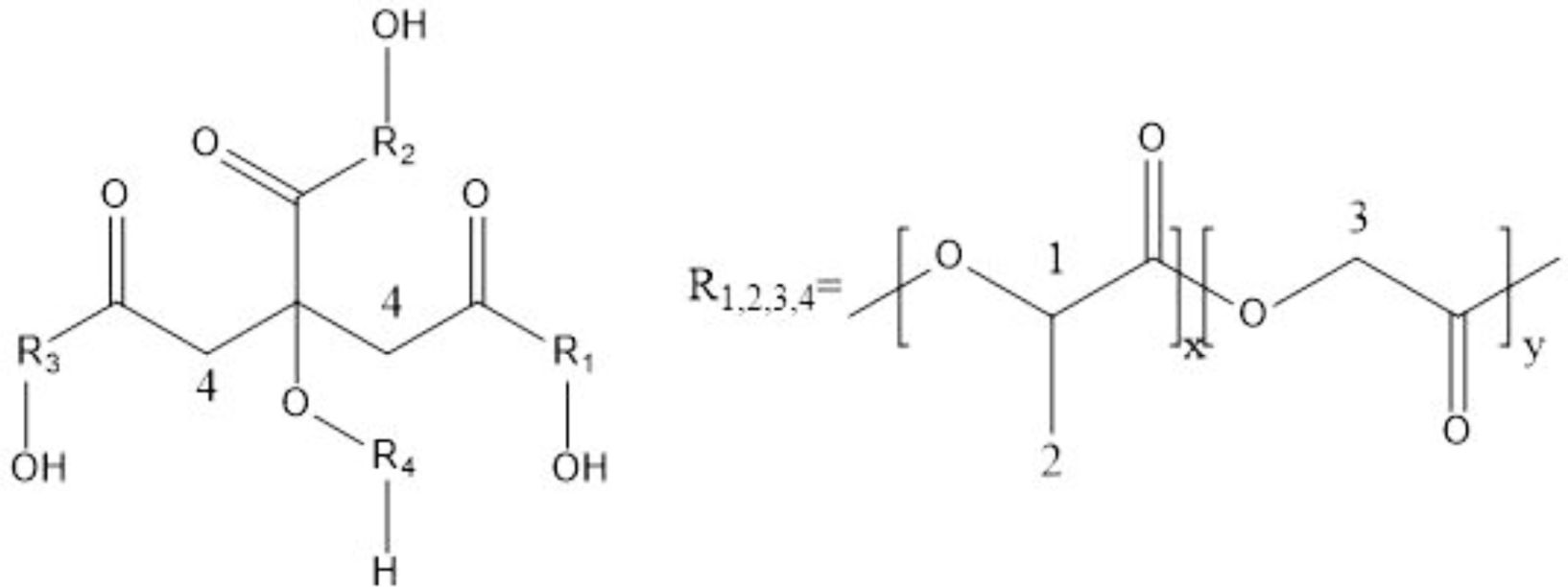
Scheme 1. The molecular structure of PLGA (R1, R2, R3, R4 are cooligomers of LA and GA).
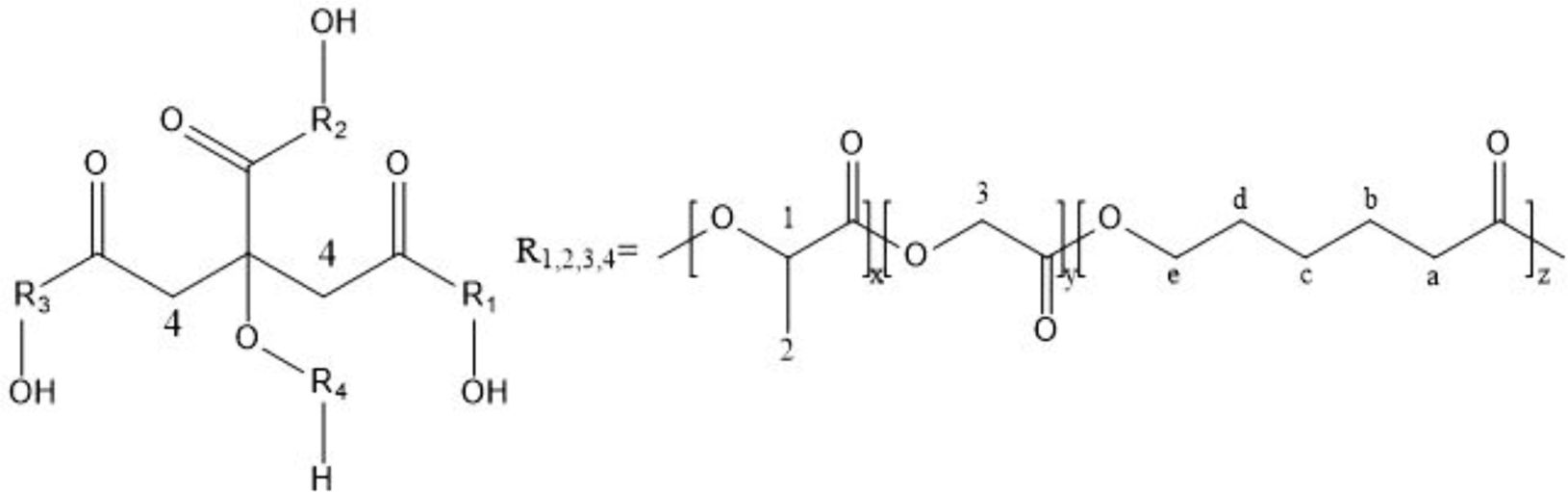
Scheme 2. The molecular structure of PLGA-CL.
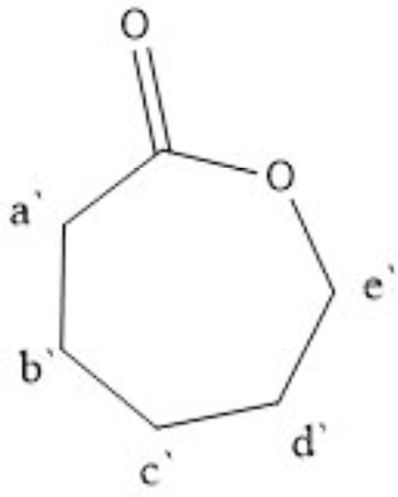
Scheme 3. The molecular structure of CL.
|
Figure 1 FTIR spectra of PLGA. |
|
Figure 2 1H NMR spectra of PLGA |
|
Figure 3 GPC chromatograms of PLGA. |
|
Figure 4 1H NMR spectra of PLGA-CL1. |
|
Figure 6 FTIR spectra of PLGA-CL with different content of CL. |
|
Figure 7 DSC graph of PLGA-CL with different content of CL. |
|
Figure 8 WCA of polymer films. |
|
Figure 9 Adhesive properties of PSA with different content of CL: (a) 180° peel strength; (b) shear strength; (c) loop tack. |
|
Table 1 Chemical Shift and Peak Area of Each Proton Peak in PLGA |
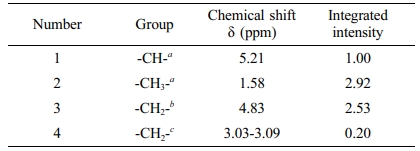
a Lactic acid. b Glycolic acid. c Citric acid. |
|
Table 2 Chemical Shift and Peak Area of Each Proton Peak in PLGA-CL1 |
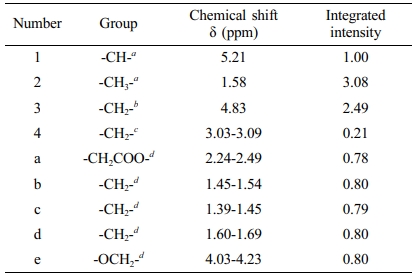
a Lactic acid. b Glycolic acid. c Citric acid. d ε-caprolactone |
PLGA-CL were synthesized by the copolymerization of PLGA and CL. PLGA based on LA and GA as raw material, CA as the starting agent and Sn(Oct)2 as the catalyst was synthesized by copolymerization. The main conclusions are as follows:
(1) FTIR spectra confirmed that the required PLGA was synthesized, and CL participated in polymerization to form copolymers with PLGA. The relative molecular weight of the prepared polymer measured by GPC was 1401. The desired low molecular weight PLGA was obtained.
(2) 1H NMR spectra indicated the synthesis of PLGA-CL. The conversion rate of CL in copolymer is more than 94%, and the degree of polymerization of PCL segment increases with the content of CL increases.
(3) With the increase of the content of CL in the copolymer, Tg of PLGA-CL decreased. Due to the increase in the degree of polymerization of PCL segment, the copolymer gradually showed crystallinity. By controlling the content of CL, the polymer in the ideal Tg range was obtained, which ensured that the material showed good end-use performances at room temperature.
(4) WCA shows that the copolymers synthesized in different proportions are hydrophilic. Moreover, with the increase of the content of CL, the hydrophilicity of the polymer decreases slightly.
(5) With the addition of CL, the copolymer became viscous. Meanwhile, with the increase of CL content, the peel strength, the shear strength, and loop tack decreased gradually. It is found that by controlling the amount of CL, the composition of the polymer can reach a balance and obtain an appropriate peel strength and shear strength.
- 1. Czech, Z.; Butwin, A. Development of Photoreactive UV-Crosslinkable Solvent-free Acrylic Pressure-sensitive Adhesives Coated at Room Temperature and Used for Removable and Repositionable Self-adhesive Materials. Polym. J. Chem. Technol. 2011, 13, 31-34.
-

- 2. Czech, Z. Solvent-based Pressure-sensitive Adhesives for Removable Products. Int. J. Adhes. Adhes. 2006, 26, 414-418.
-

- 3. Creton, C. Pressure-sensitive Adhesives: An Introductory Course. MRS Bull. 2003, 28, 434-439.
-

- 4. Beak, S. S.; Hong, S.; Hwang, S. H. Preparation and Adhesion Performance of Transparent Acrylic Pressure Sensitive Adhesives Containing Biomass-derived Menthol Moiety. Polym. Korea 2016, 40, 678-683.
-

- 5. Cohen, E.; Binshtok, O.; Dotan, A.; Dodiuk, H. Prospective Materials for Biodegradable and/or Biobased Pressure-sensitive Adhesives: A Review. J. Adhes. Sci. Technol. 2013, 27, 1998-2013.
-

- 6. David, S. B.; Sathiyalekshmi, K.; Raj, G. A. G. Studies on Acrylated Epoxydised Triglyceride Resin-co-butyl Methacrylate Towards the Development of Biodegradable Pressure Sensitive Adhesives. Mater. Sci.-Mater. Med. 2009, 20, 61-70.
-

- 7. Riyajan, S. A.; Pheweaw, N. Modification of Skim Rubber Blended With Poly(Vinyl Alcohol) to be Applied as a Bio- degradable Pressure-sensitive Adhesive: Effect of 2,6-di-t-butyl-4-methylphenol and Hydrocarbon Resin. Rubber Chem. Technol. 2012, 85, 547-558.
-

- 8. Lee, Y. M.; Shim, C. R.; Lee, Y.; Kim, H. N.; Jo, S. A.; Song, J. E.; Lee, D.; Khang, G. Effect of Demineralized Bone Particle Gel Penetrated into Poly(lactic-co-glycolic acid) Scaffold on the Regeneration of Chondrocyte; In Vivo Experiment. Polym. Korea 2012, 36, 789-794.
-

- 9. Eom, S.; Yoo, S. C.; Kim, Y. K.; Lee, Y. H.; Lee, E. Y.; Yu, H.; Lee, D.; Khang, G. Effect of Cosurfactants on the Release Behavior of Zaltoprofen-loaded PLGA Microspheres in In Vitro: Preparation and Characterization. Polym. Korea 2010, 34, 333-340.
-

- 10. Kamali, H.; Khodaverdi, E.; Hadizadeh, F. Ring-opening Poly- merization of PLGA-PEG-PLGA Triblock Copolymer in Super- critical Carbon Dioxide. J. Supercrit. Fluids 2018, 137, 9-15.
-

- 11. Wusiman, A.; Xu, S. W.; Ni, H. Y.; Gu, P. F.; Liu, Z. G.; Zhang, Y.; Qiu, T. X.; Hu, Y. L.; Liu, J. G.; Wu, Y.; Wang, D. Y.; Lu, Y. Immunomodulatory Effects of Alhagi Honey Polysaccharides Encapsulated into PLGA Nanoparticles. Carbohydr. Polym. 2019, 211, 217-226.
-

- 12. Vert, M.; Mauduit, J.; Li, S. Biodegradation of PLA/GA Polymers: Increasing Complexity. Biomaterials 1994, 15, 1209-1213.
-

- 13. Liu, J. Y.; Liu, L. J. Ring-opening Polymerization of ε-caprolactone Initiated by Natural Amino Acids. Macromolecules 2004, 37, 2674-2676.
-

- 14. Sudesh, K.; Abe, H.; Doi, Y. Synthesis, Structure and Properties of Polyhydroxyalkanoates: Biological Polyesters. Prog. Polym. Sci. 2000, 25, 1503-1555.
-

- 15. Lenoir, S.; Riva, R.; Lou, X.; Detrembleur, C.; Jerome, R.; Lecomte, P. Ring-Opening Polymerization of a-Chloro-e-caprolactone and Chemical Modification of Poly(a-chloro-e-caprolactone) by Atom Transfer Radical Processes. Macromolecules 2004, 37, 4055-4061.
-

- 16. Hyun, H.; Kim, M. S.; Khang, G.; Rhee, J. M.; Lee, H. B. Synthesis and Characterization of Linear and Branched Copolymers of Poly(ethylene glycol) and Poly(ε-caprolactone). Polym. Korea 2006, 30, 146-151.
- 17. Cho, K. Y.; Lee, K. S.; Park, J. K. Effect of Graft Copolymer Composition on the Compatibility of Biodegradable PCL/PCL-g-PEG Blend. Polymer 2009, 33, 219-223.
- 18. Cohn, D.; Lando, G. Tailoring lactide/caprolactone co-oligomers as tissue adhesives. Biomaterials 2004, 25, 5875-5884.
-

- 19. Liu, Y. X.; Bai, X. Z.; Liang, A. Synthesis, Properties, and In Vitro Hydrolytic Degradation of Poly(d,l-lactide-co-glycolide-co-ε-caprolactone). Int. J. Polym. Sci. 2016, 2016, 1-9.
-

- 20. Council, P. S. T. Test methods for pressure sensitive adhesive tapes; Illinois: Pressure Sensitive Tape Council: Northbrook, 2004.
- 21. Fang, C.; Lin, Z. X. Effect of Propyleneimine External Cross-linker on the Properties of Acrylate Latex Pressure Sensitive Adhesives. Int. J. Adhes. Adhes. 2015, 61, 1-7.
-

- 22. Kajtna, J.; Krajnc, M. Solvent less UV Crosslinkable Acrylic Pressure Sensitive Adhesives. Int. J. Adhes. Adhes. 2011, 31, 822-831.
-

- 23. Li, P.; Zerroukhi, A.; Chen, J.; Chalamet, Y.; Jeanmaire, T.; Xia, Z. Synthesis of poly(ε-caprolactone)-block-poly(n-butyl acrylate) by Combining Ring-opening Polymerization and Atom Transfer Radical Polymerization with Ti[OCH2CCl3]4 as Difunctional Initiator: I. Kinetic Study of Ti[OCH2CCl3]4 Initiated Ring-opening Polymer. Polymer 2009, 50, 1109-1117.
-

- 24. Fang, C.; Zhu, K.; Zhu, X. B.; Lin, Z. X. Preparation and Characterization of Self-crosslinking Fluorinated Polyacrylate Latexes and Their Pressure Sensitive Adhesive Applications. Int. J. Adhes. Adhes. 2019, 95, 102417.
-

- 25. Cui, X. J.; Zhong, S. L.; Wang, H. Y. Emulsifier-free Core-shell Polyacrylate Latex Nanoparticles Containing Fluorine and Silicon in Shell. Polymer 2007, 48, 7241-7248.
-

- 26. Lim, D.; Do, H. U.; Kim, H. J. PSA Performances and Viscoelastic Properties of SIS‐based PSA Blends with H-CPD Tackifiers. J. Appl. Polym. Sci. 2006, 102, 2839-2846.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2021; 45(4): 592-600
Published online Jul 25, 2021
- 10.7317/pk.2021.45.4.592
- Received on Mar 17, 2021
- Revised on Apr 20, 2021
- Accepted on Apr 22, 2021
 Services
Services
Shared
 Correspondence to
Correspondence to
- Zhean Xia
-
School of Material Science and Engineering, East China University of Science and Technology, Shanghai 200237, China
- E-mail: xiazhean@ecust.edu.cn
- ORCID:
0000-0002-3904-6271












 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.