- Castor Oil and HER Based Polyurethanes: Synthesis, Characterization and Structure-Thermal Stability Relation
Thangamani Rajkumar†
 , Venugopalan Rajasudha*, Arunjunai Raj Mahendran**, and Chinnaswamy Thangavel Vijayakumar***
, Venugopalan Rajasudha*, Arunjunai Raj Mahendran**, and Chinnaswamy Thangavel Vijayakumar***Department of Chemistry, Rajah Serfoji Government College (Affiliated to Bharathidasan University), Thanjavur - 613005, Tamilnadu, India
*Department of Chemistry, Bon Secours College for Women, Thanjavur - 613006, Tamilnadu, India
**Wood K Plus-Competence Center for Wood Composites and Wood Chemistry, Altenberger Strasse 69, A-4040 Linz, Austria
***Department of Polymer Technology, Kamaraj College of Engg. & Tech., S.P.G.C. Nagar,
K. Vellakulam Post - 625701, Tamilnadu, India- Castor 오일과 HER 기반 폴리우레탄: 합성, 분석, 구조-열 안정성 상관관계
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
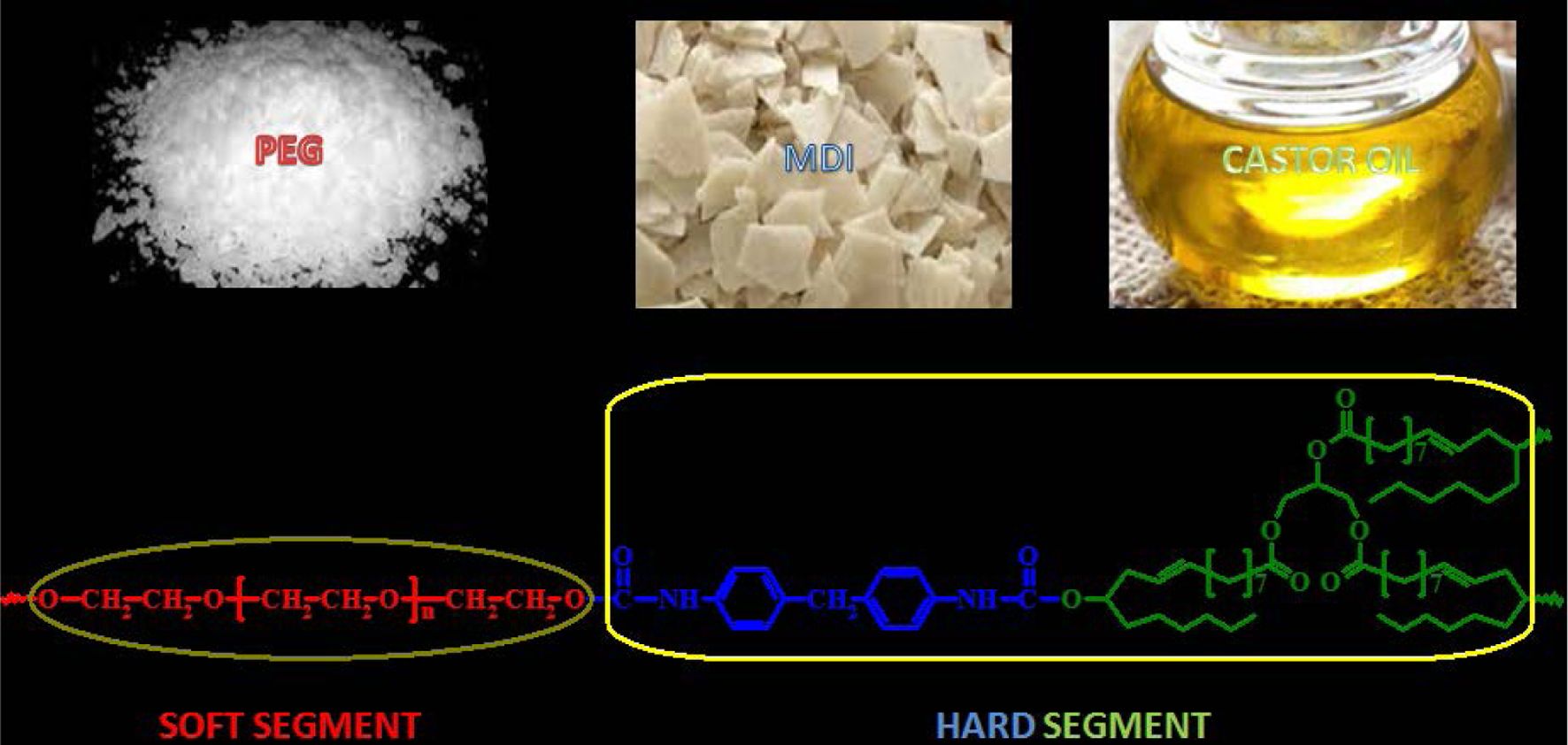
Polyurethanes (PUs) were prepared from solvent and catalyst free reaction mixture containing polyethylene glycol (PEG), 4,4’-methylene diphenyl diisocyanate (MDI) and 1,3-bis(2-hydroxyethoxy) benzene (HER) and/or castor oil (CAO). PUs were characterized using the Fourier-transform infrared spectroscopy (FTIR) and proton nuclear magnetic resonance (1H NMR) spectroscopic methods. Thermal studies on PUs using differential scanning calorimetric (DSC) and thermogravimetric analysis coupled with FTIR (TG/FTIR) proved that CAO-extended PUs were thermally more stable than HER extended PUs. The partial and complete replacement of HER content in PUs using CAO enhanced the thermal stability by shifting degradation temperature to higher temperature (5-15 °C) and enhancing the char residue. The parameters derived from DSC traces, thermogram and differential thermogram confirmed that PUs synthesized using PEG 1500 are thermally more stable than the PUs prepared using PEG 4000. The probable degradation mechanism to study the role of β-hydrogen on the thermal stability was proposed and discussed based on the results of TG/FTIR.
Thermally stable and bio-degradable polyurethanes were prepared using 1,3-bis(2-hydroxy- ethoxy) benzene (HER) and castor oil as polyols. Here, we studied thermal stability using thermogravimetric analysis and proposed degradation mechanisms to support the role of β-hydrogen on the structure-thermal stability relationship.
Keywords: β-hydrogen, mechanism, polyurethane, synthesis, thermal degradation.
The authors wish to thank the Principal of Rajah Serfoji Government College (Autonomous), Thanjavur, Bon Secours College for Women, Thanjavur and Kamaraj College of Engg. and Tech., Virudhunagar for their encouragement and providing facilities to carry out this work successfully. This research did not receive any specific funding.
Polyurethane (PU) based material applications have increased significantly compared to other polymer materials. Utilization of thermoplastic PUs (TPUs) for every day purpose includes barrier film, breathable clothing, medical garment, cable jacketing, tubing, athletic equipment, medical devices, automotive molded parts, durable elastomeric wheels and tires, drive belts, synthetic fibers, adhesives, seals, gaskets, etc. PUs find applications in engineering purposes because of their mechanical properties like toughness and durability dependent on the soft and hard segments in the molecular backbone.1,2
PUs are traditionally prepared by reacting di- or polyisocyanate with a polyol. Petroleum based diacids and diols and/or polyols are the conventional raw materials used in the manufacture of PUs. PUs made from 1,3-bis(2-hydroxyethoxy)- benzene (HER) show improved stability with respect to its resistance to the absorption of moisture than conventional PUs based on polyether and polyester polyols.3 Resorcinol based chain extenders like HER and 1,4-bis(2-hydroxyethoxy)benzene (HQEE) have been used to improve hardness and tear resistance properties of PUs.4 Further, HER extended polyether MDI PU showed higher rebound resilience properties than polyester system. Opera prepared PUs having improved thermal and mechanical properties using resorcinol derivatives as chain extenders and studied the effect of chemical structure on the enhanced properties of PU elastomers.5 Studies on the effect of aliphatic and aromatic chain extenders on the thermal stability have also been reported.6-10 Aromatic moieties in the soft and/or hard segments of the PU impart thermal stability by enhancing high inter-chain cohesion energy. Hence, PUs based on hydrophobic backbones are suitable for forming golf equipment.11
The toxicity issues of isocyanate minimize the uses of isocyanate based PUs and necessitate non-isocyanate PUs (NIPU).12-15 The NIPU is obtained by reacting non-toxic polycyclic carbonates with aliphatic or cyclo aliphatic polyamines having primary amino groups. In addition, NIPU is not sensitive to the moisture present in surrounding environment. Nowadays TPU consumers especially, electronics, automotive, footwear, carpet, furniture and apparel industries are looking for greener polymers. Hence, raw materials made from plant based and renewable materials are used for making bio-based PUs.16-19
Castor oil (CAO) extended PUs have been used in making products, ranging from coatings to foams and the use of CAO derivatized with propylene oxide in PU foam for mattresses, adhesives, automotive seats and so on is well established. Gonzaga et al. attempted to synthesize new PU resins derived from CAO having stable physical and chemical properties for application in the medical field.20 They studied electrical and mechanical properties of these materials also.
Das et al. in his article reviewed and highlighted recent trends and development in CAO based PU and its nanocomposite for coating application.21 They also suggested CAO and palm oil based isocyanate as potential substitutes for conventional petroleum based materials in the preparation of PUs with improved thermal, mechanical and coating properties.
Tran et al. synthesized and used novel 7,10,12‐trihydroxy‐8(E)‐octadecenoic acid (TOD), the microbially modified CAO in the preparation of bio-based PUs.22 The studies performed using differential scanning calorimetry and thermogravimetry confirmed that TOD based PU is having improved thermal stability.
Zhang et al. synthesized PU elastomers using CAO polyols without petroleum based polyols.23 They performed analyses using differential scanning calorimetry, thermogravimetry and dynamic mechanical analyzer to study the effect of CAO on the properties of PUs. They claimed hydrogen bonding in hard segment is responsible for the properties associated with PUs. The initial weight loss of about 30-35% and residual mass after 650 °C are independent of molar ratio of CAO. The weight loss (above 35%) responsible for soft segment degradation decreases with increase in molar ratio of CAO.
Literature survey shows CAO based PU elastomers are bio-compatible and bio-degradable.24 They find applications not only in industry but also in health care systems and in tissue engineering. A complete survey of literature reveals that researchers used vegetable oils like CAO, linseed oil, etc. in the preparation of PUs instead of petroleum based materials without compromising the thermal property. No such work has been attempted to study chemistry behind the role of hard segment in imparting thermal stability to PUs. Hence it is intended to synthesize CAO and HER based PUs and to investigate the effect of β-hydrogen on the thermal stability of PUs.
Materials and Methods. Polyethylene glycol (PEG) of 1500 and 4000 g mol-1 supplied by LOBA Chemie Pvt. Ltd., Mumbai, India and 4,4’-methylene diphenyl diisocyanate (MDI) supplied by SIGMA-ALDRICH Chemie GmbH, Germany were dried at 50 °C in a vacuum oven. The chain extenders, 1,3-bis(2-hydroxyethoxy)benzene (HER) and castor oil (CAO) were obtained as gift samples and used without any further purification.
The Fourier-transform infrared (FTIR) spectra for the synthesized PUs were recorded on a SHIMADZU-8400S infrared spectrophotometer (Japan) using KBr pellet technique. The absorption bands in the FTIR spectrum were used to study the structural aspects of the materials. Proton nuclear magnetic resonance (1H NMR) spectra for the PUs were recorded in CDCl3 on a Bruker (400 MHz, USA) instrument with tetramethyl silane (TMS) as an internal standard.
Physical changes in synthesized PUs while heating was analyzed using Metler Toledo DSC 3 differential scanning calorimeter (USA). The sample and the reference cells were purged with nitrogen at flow rate of 40 mL min-1 and heated at a rate of 10 °C min-1 with individual heater. Glass transition temperature (Tg) measurement was carried out from -80 to 100 °C. Parameters like the crystallisation temperature (Tc), the melt temperature (Tm) and the degradation or decomposition temperature (Td) were measured by heating the samples from 25 to 280 °C.
Thermogravimetric analysis (TGA) coupled with FTIR studies were performed under nitrogen flow (25 mL/min) on TA Instruments TGA Q5000IR (USA) connected to Thermo Electron Corporation Nicolet 380 FTIR (USA). The measurements were carried out at a heating rate of 20 °C min-1 from ambient to 650 °C. FTIR spectra of evolved volatile degradation products were recorded at a resolution of 14 scans per every 20 s. The sample size was about 5 mg.
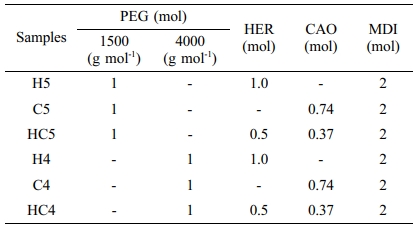
Synthesis of PUs.25 A weighed quantity of vacuum dried polyol, PEG of 1500 or 4000 g mol-1 (1 mol) and the chain extender, HER and/or CAO of different proportions were taken in a round bottom flask and heated to 60 °C in an oil bath with constant stirring under reduced pressure to expel trapped gas. To this molten mixture, MDI (2 mol) was added in portions for 30 min with constant stirring. The temperature of the homogeneous mixture obtained was raised to 80 °C and kept for a further period of 1 h with constant stirring (until the reaction mixture becomes highly viscous). Finally, the highly viscous homogeneous mixture was transferred to a stainless steel bowl and post cured at 110 °C in a hot air oven for 24 h. The sample codes of PUs prepared and the composition of the raw materials used are listed in Table 1. The schemes for the preparation of PUs are presented in Scheme 1.
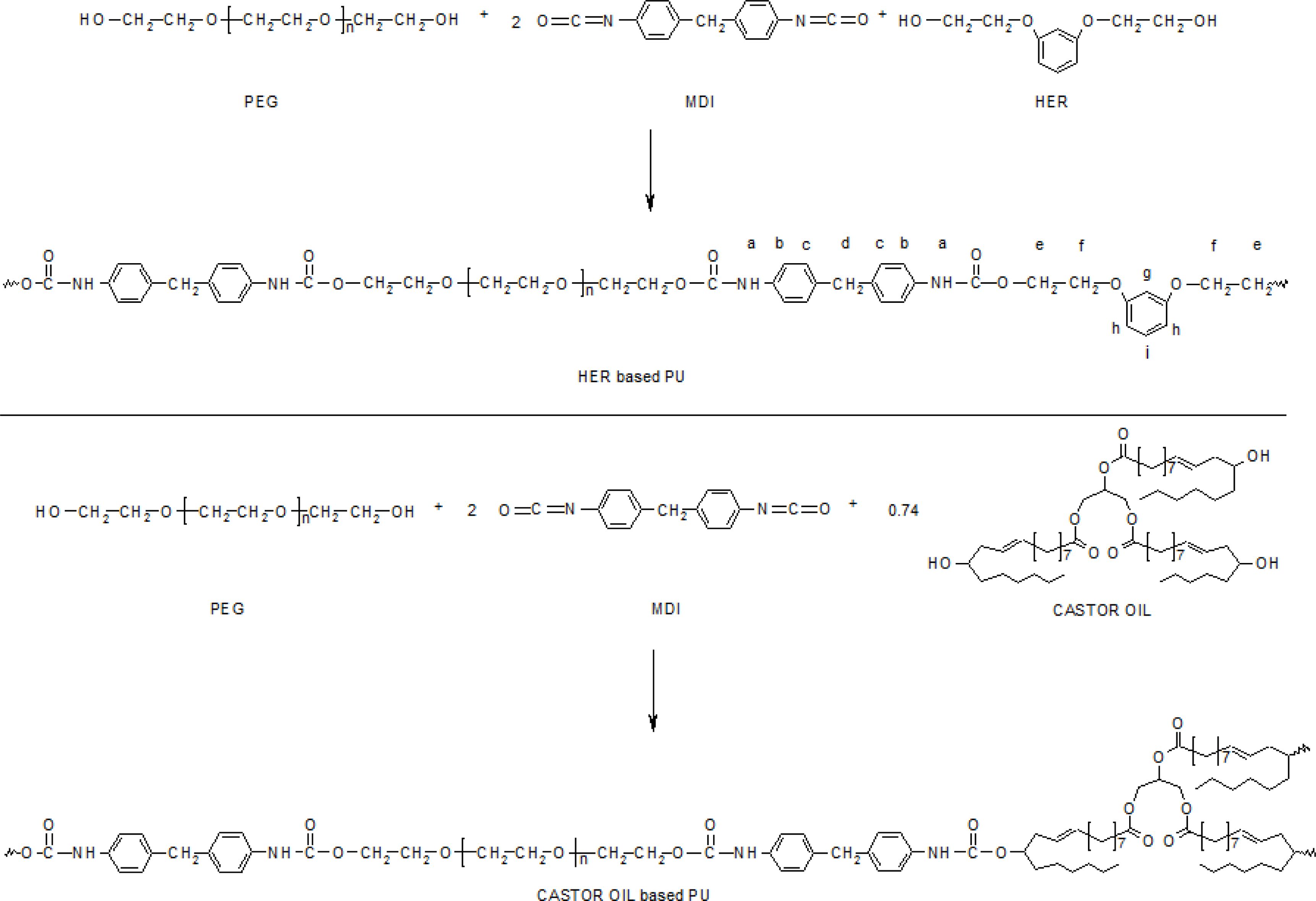
Scheme 1. Synthetic route for the preparation of PUs.
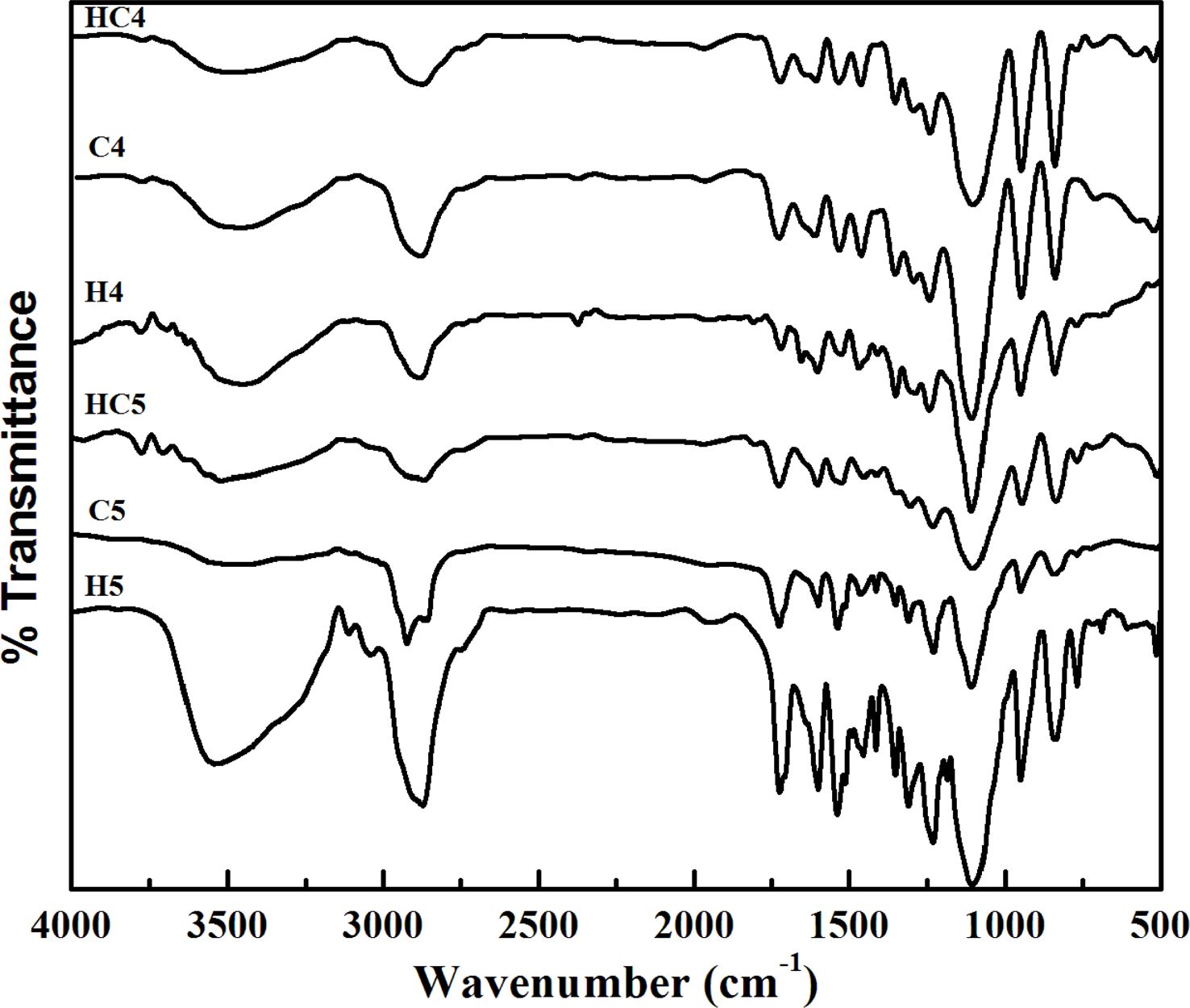
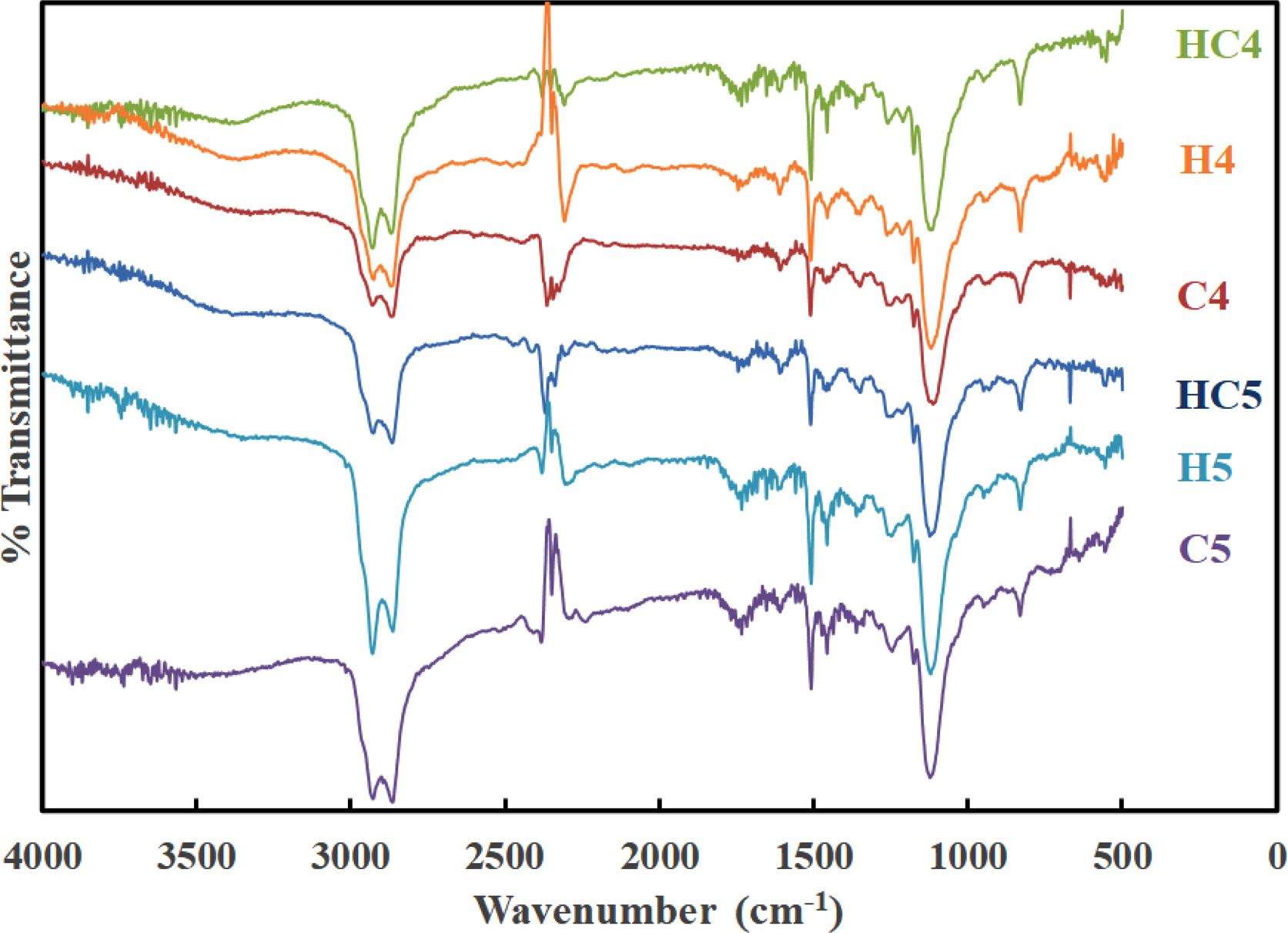
Spectral Studies. FTIR spectra of the synthesized PUs are presented in Figure 1. Irrespective of the chain extender (HER and/or CAO) used, the nature and the position of absorption bands are same because of the overlapping of the absorption bands responsible for PEG with the absorption bands of chain extenders.
One can find the absence of absorption band responsible for the stretching vibration of -NCO (isocyanate) group at around 2280 cm-1 and the occurrence of bands at 1570-1515 cm-1 (N-H bending) and 3290 cm-1 (N-H stretching).26 The broad absorption band in the region 3700-3400 cm-1 is due to the free -OH group.27 This indicates the completion of polymerization reaction and the absence of unreacted MDI in the polymerized material. In addition to the above, the absorption bands at around 2900 cm-1 (aromatic C=C-H stretching), 1740 cm-1 (C=O stretching) and 1240 cm-1 (C-N stretching) are also seen. Thus, the presence of urethane linkage (-NHCOO-) in all the synthesized PUs was confirmed.28
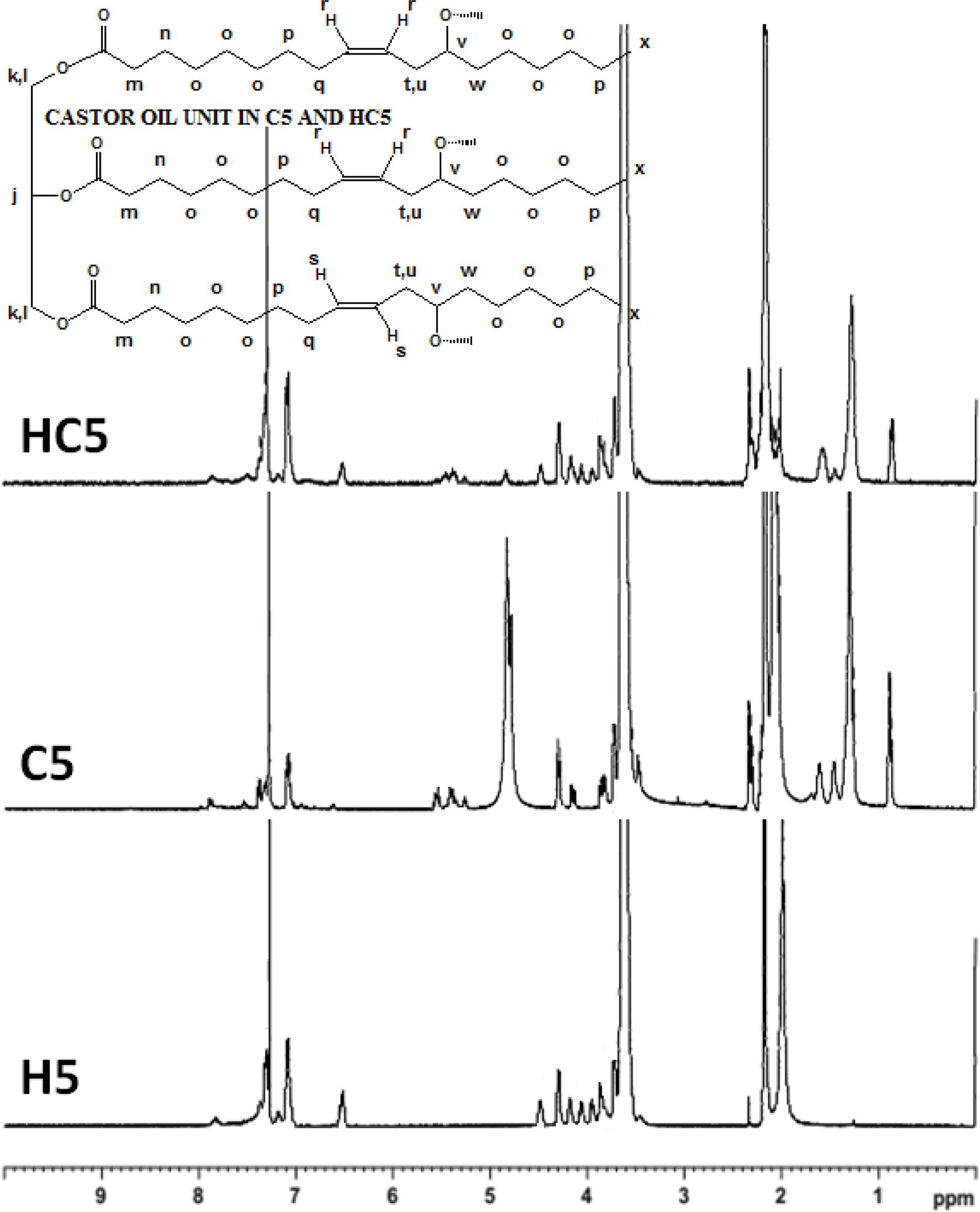
The 1H NMR spectra of the synthesized PUs (H5, C5 and HC5) using PEG 1500 as a polyol recorded in CDCl3 are presented in Figure 2. The 1H NMR (400 MHz, CDCl3) spectral assignments21-27 for the PUs synthesized: δ 7.85 (s, 1H, Ha), 7.38 (d, 2H, Hb), 7.10 (d, 2H, Hc), 7.17 (s, 2H, Hd), 4.48 (t, 2H, He), 3.95 (t, 2H, Hf), 6.51 (s, 1H, Hg), 6.52 (d, 2H, Hh), 7.16 (t, 1H, Hi), 5.25 (quin, 1H, Hj), 4.30 (d, 2H, Hk), 4.15 (d, 2H, Hl), 4.80 (quin, 6H, Ho), 5.42 (q, Hr), 5.55 (q, Hs), the chemical shifts (δ) shown within the range of 0.88-2.30 are responsible for aliphatic protons (Hm, Hn, Hp, Hq, Ht, Hu, Hv and Hw) present in CAO, 0.88 (t, 3H, Hx), 3.55 (methylene proton in PEG unit). The presence of peak at 7.85 ppm responsible for >NH proton confirms the formation of urethane linkage. One cannot able to follow changes in the area under the peak responsible for -OH protons that fall in the aliphatic proton region.
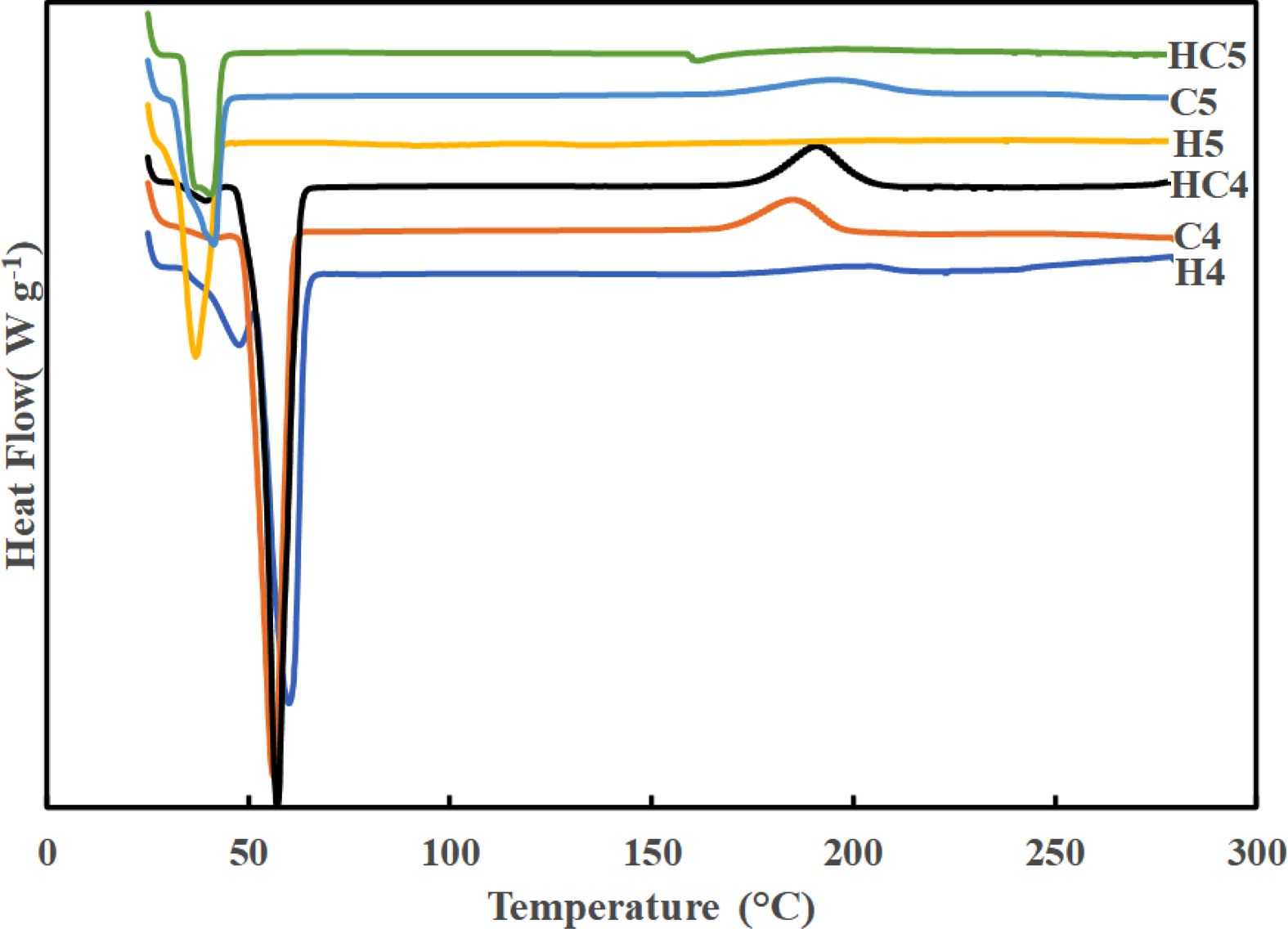
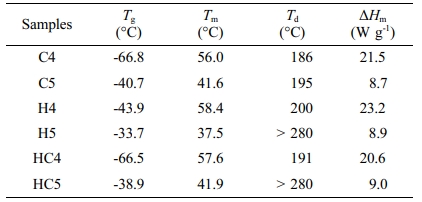
DSC Studies. DSC traces of synthesized PUs recorded at a heating rate of 10 °C min-1 are presented in Figure 3. The parameters derived from DSC traces are given in Table 2. Endothermic step seen between -40 and -70 °C reflects glass transition. Irrespective of the chain extender in the hard segment and molecular weight of PEG used, PUs show endothermic melting and exothermic decomposition in nitrogen atmosphere. All the PUs had glass transition temperature (Tg) around -70 to -45 °C. PUs prepared using PEG 4000 (H4, HC4 and C4) exhibited higher melting temperature and higher heat of melting than PUs prepared using PEG 1500 (H5, HC5 and C5). As observed by Chen et al., melting point decreases with increasing number of methylene units in the repeating unit.29 Partial or complete replacement of CAO with HER has no effect on melting. The temperature responsible for degradation is not much affected by replacing CAO with HER. Td values showed that PUs prepared using PEG 1500 and HER are thermally more stable.
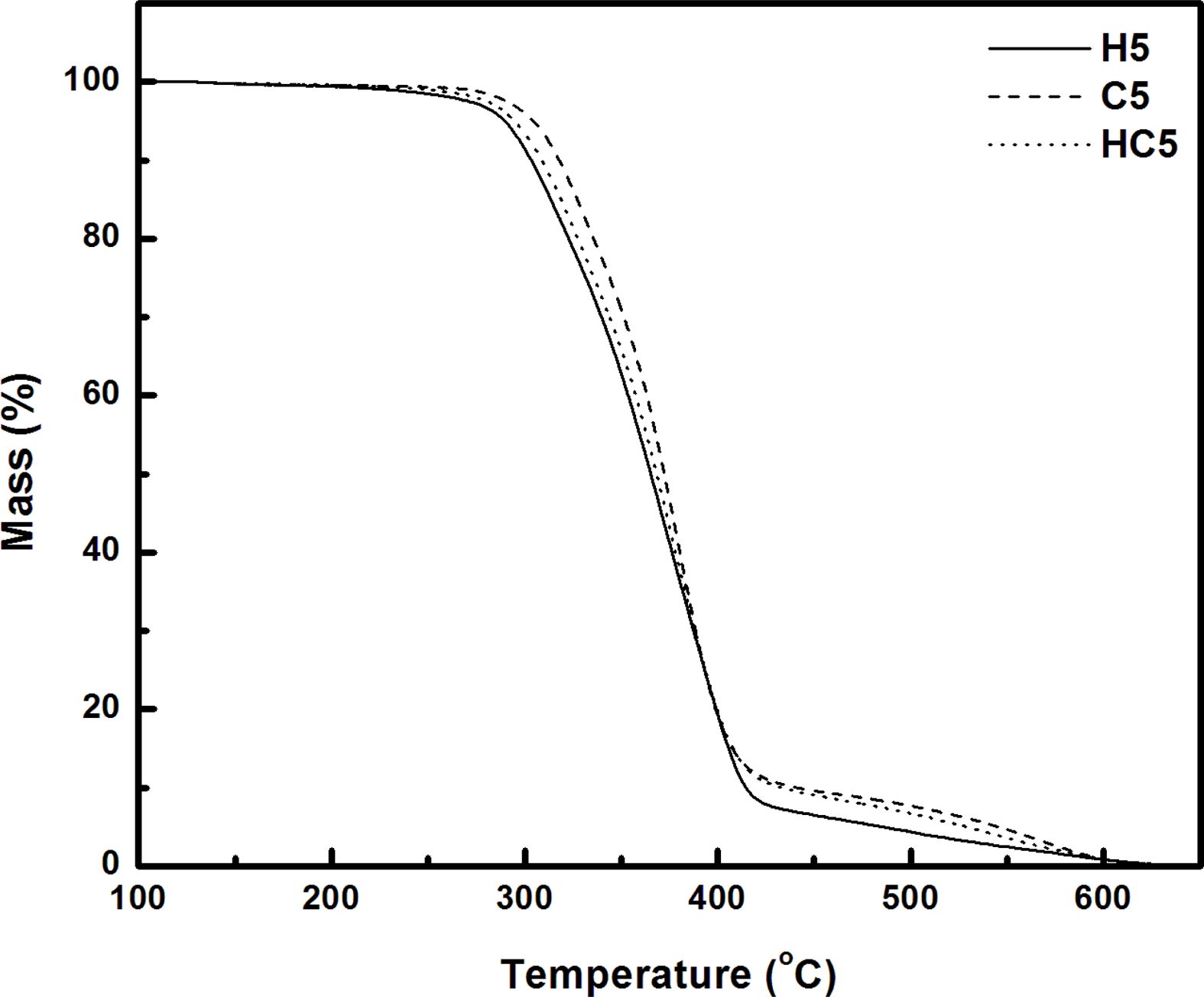
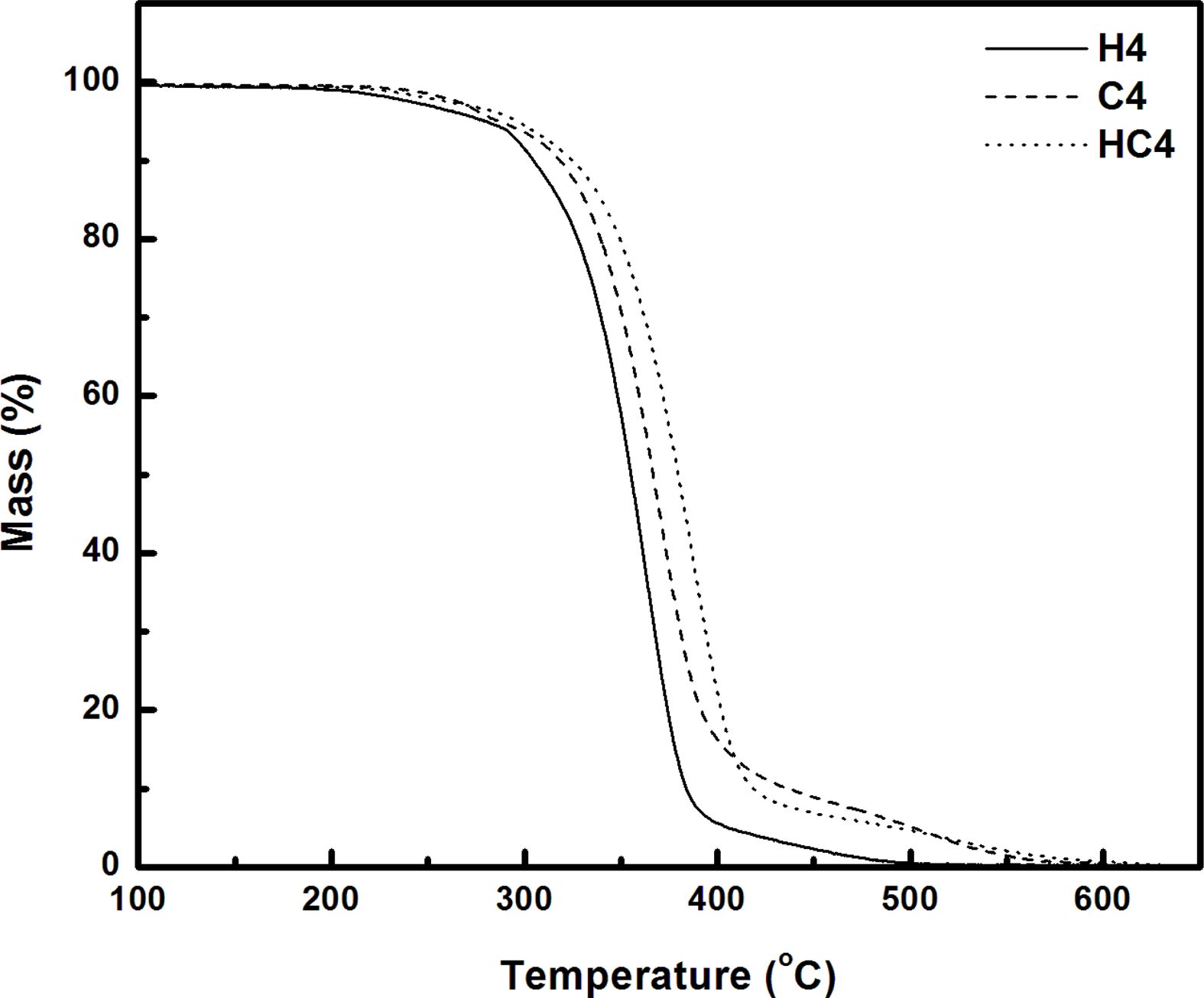
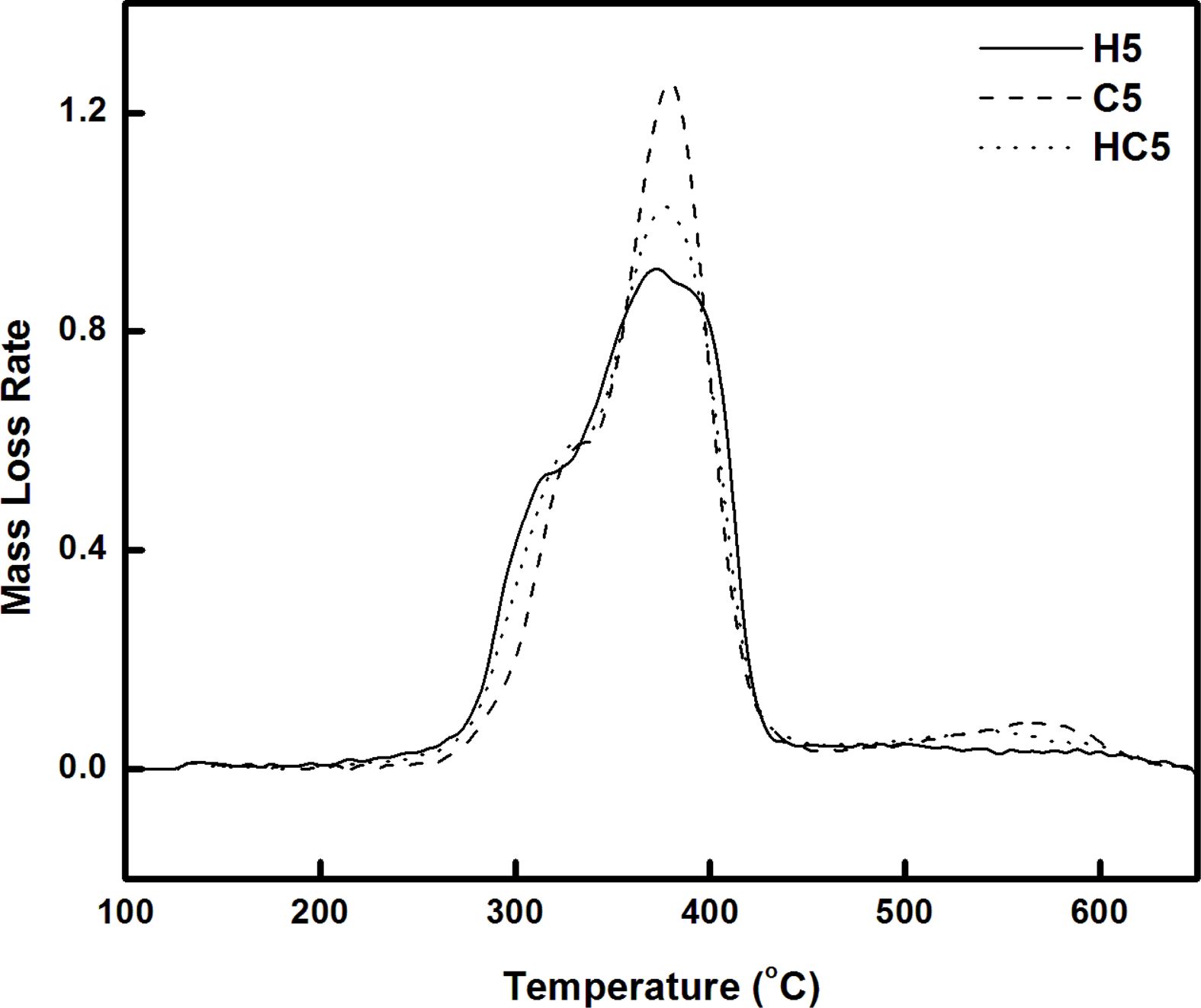
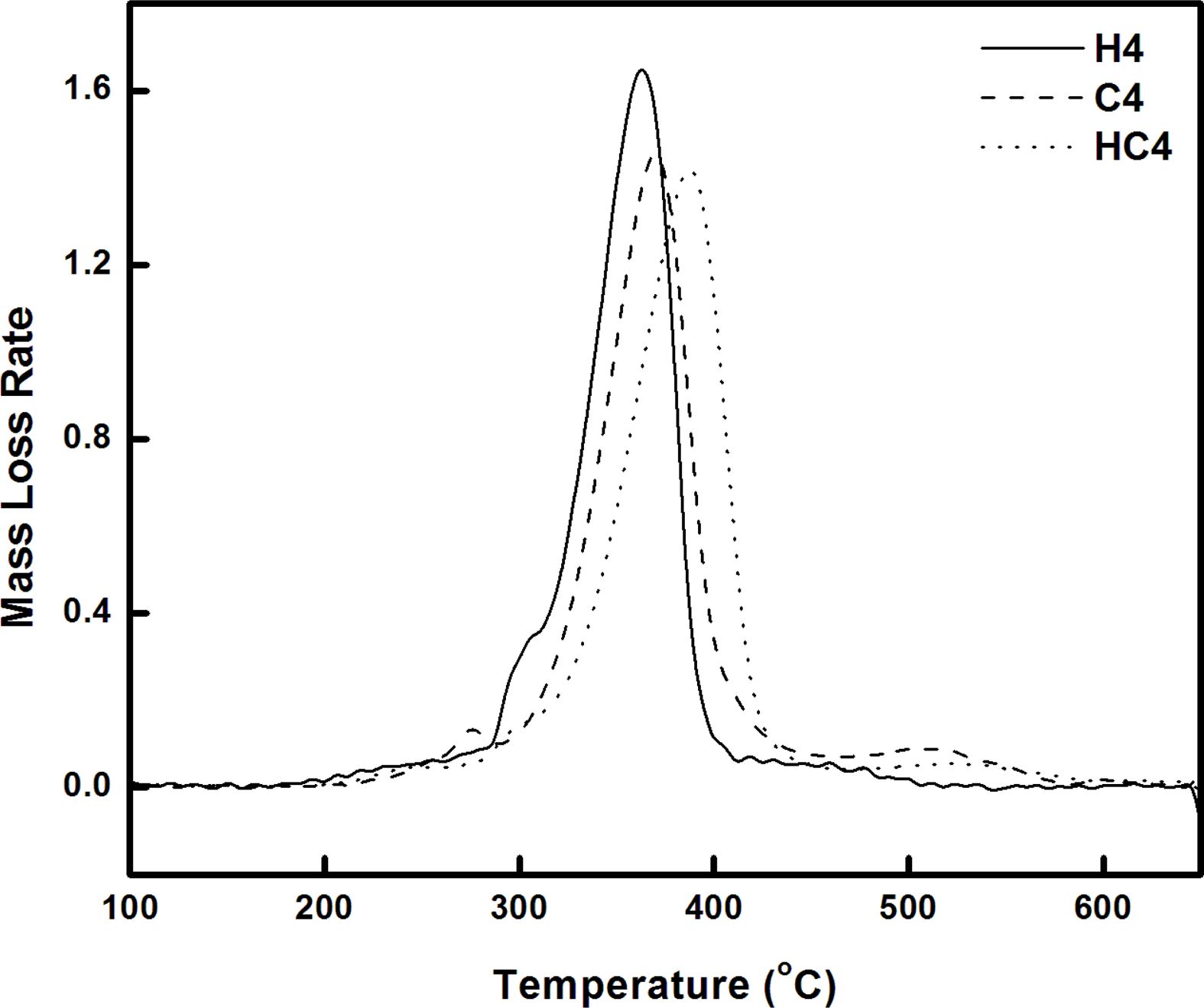
TGA/FTIR Studies. The response of material to temperature is essential for processing and to predict the temperature at which the products from these materials undergo deformation and degradation. Hence it is intended to study the thermal stability and the degradation pattern of the synthesized bio and resorcinol based thermoplastic PUs using thermogravimetric analysis. Thermograms (TGs) of PUs (H5, C5 and HC5; H4, C4 and HC4) recorded in nitrogen atmosphere at a heating rate of 20 oC min-1 are given in Figures 4 and 5. Differential thermograms (DTGs) are presented in Figures 6 and 7.
Thermogravimetric analysis shows H5 and H4 undergo two stages of degradation and the other four TPUs, C5, HC5, C4 and HC4 undergo three stages of degradation. Thus the absence of the third degradation stage in the PUs (H5 and H4) prepared using 1,3-bis(2-hydroxyethoxy) benzene (HER) as the chain extender allows one to conclude that third degradation seen in the temperature region from 450 to 630 oC is concerned with the degradation of branched chain networks formed by CAO units in the bio-based PU and the degradation temperature of HER units falls in the degradation region of PEG.17 Hence the PUs, H5 and H4 prepared using HER degrade at a faster rate and they are found to be the least stable among the PUs discussed here.
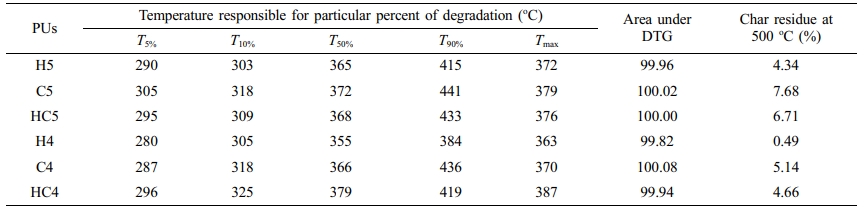
One can clearly predict the thermal stability by comparing the parameters derived from the TG and DTG curves. The temperatures corresponding to PU degradation of beginning (T5%), T10%, half (T50%), ending (T90%) and maximum degradation (Tmax) are presented in Table 3. It is observed that all the PUs exhibit a pronounced weight loss in the temperature range 275-420 oC. The presence of CAO in the bio-based PU shifts the degradation pattern as a whole to higher temperature (temperature responsible for inception and half of degradation by 5-10 oC and end of degradation by 40-60 oC for PUs prepared using PEG 1500 and about 90 oC for PUs prepared using PEG 4000). Though the variation in the area under the DTG (Figures 6 and 7) is not seen, the abrupt shift in the temperature responsible for maximum degradation (Tmax) to higher temperature is seen. Irrespective of the molecular weights of PEG used in the synthesis of PU, the presence of CAO alone or along with HER in the hard segment of PU makes the bio-based material thermally stable.
The length of the hard segment in the polymer network influences phase separation. PUs with longer chain extender (a part of hard segment) have higher thermal stability.30 Resorcinol based PU prepared using HER (diol) is linear chain polymer but bio-based PU prepared using CAO (triol) is branched one. Secondly, longer hard segment provided by CAO unit makes PU thermally more stable. It is observed that branched bio-based PU is thermally more stable than the linear resorcinol based PU. Percentage of char residue measured at 500 °C (Table 3) is in the order C5 > C4 > HC5 > HC4 > H5 > H4. This experimental result evidences that CAO based PUs are having higher char forming capacity than HER based PUs and also supports the above findings.
Molecular weight of PEG used in the preparation of PU also determines the thermal stability. The inception of degradation for PUs prepared using PEG 1500 (H5 and C5) is about 10 oC higher than PUs prepared using PEG 4000 (H4 and C4). The inception temperatures for degradation of PUs, HC5 and HC4 are same. The temperature responsible for the end of degradation (T90%) is also higher by about 40-90 oC for PUs prepared using PEG 1500 than PUs prepared using PEG 4000. The trend in the variation of T50% is also same but for PUs HC5 and HC4, the trend is reverse. The char residue measured at 500 oC showed that PUs prepared using PEG 1500 (the material left for further weight loss reaches zero at 600 oC) are more stable compared to PUs prepared using PEG 4000 (the char residue reaches almost zero at 550 oC).
One can conclude that soft segment (PEG) with a greater number of repeating units present in the PUs is more prone to chain scission and makes the material as a whole thermally unstable. Hence PUs prepared using PEG 1500 g mol-1 are thermally more stable than PUs prepared using PEG 4000 g mol-1.
FTIR spectra of the thermal degradation products evolved during thermal analysis under nitrogen atmosphere are super imposable images of each other. FTIR spectra of the thermal degradation products evolved at maximum degradation temperature (temperature corresponding to the peak maximum of DTG curve) are given in Figure 8. The major functional groups identified are free -OH (3700-3400 cm-1), C-H (2950- 2850 cm-1), -N=C=O (2234 cm-1), C=O stretching (1733 cm-1) and alkane C-H bending (1453 cm-1).31 The stretching vibration responsible for N-H in PU at 3320 cm-1 is missing. The resemblance in FTIR spectral data confirmed that all the synthesized PUs degrade through the same mechanism.
Degradation Mechanism. According to the early works reviewed by Saunders et al. urethanes undergo thermal degradation through several mechanisms in an inert atmosphere.32,33 The degradation mechanism is dependent on the curatives used in the preparation of urethane. PUs prepared using curatives (diol and/or triol) having β-hydrogen undergo degradation through β-scission which results in the formation of vinyl and acid terminated compounds.34,35 The acid terminated nitrogen containing compound formed undergoes secondary degradation into primary amine and carbon dioxide.36-38
On the other hand, the absence of β-hydrogen in the curatives forced the polyurehane to undergo degradation through different path which proceeds through depolyaddition into the starting materials. In the present work, bio and resorcinol based PUs are prepared using β-hydrogen containing curatives (HER and CAO) and hence all the PUs discussed here may undergo thermal degradation through β-scission in to amine and vinyl terminated compounds (Scheme 2). Number of β-hydrogen available for β-scission process with resorcinol based PU is six and for bio-based PU is eight and hence bio-based PU undergoes β-scission at a faster rate than resorcinol based PU. Branched chain in the bio-based PU makes the material thermally more stable than resorcinol based PU though the former containing more number of β-hydrogen available for β-scission process than the latter.
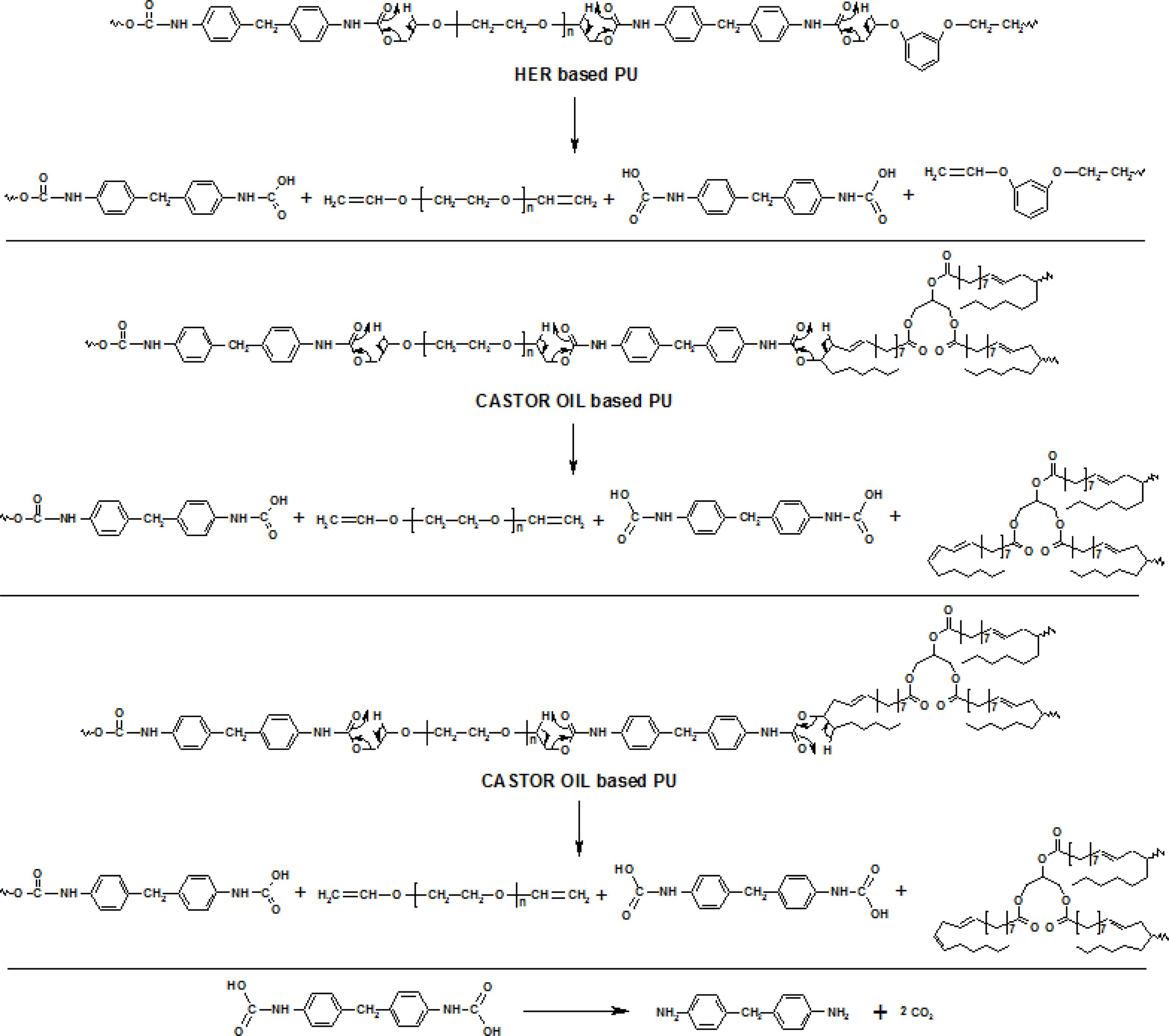
Scheme 2. Mechanistic route for the thermal degradation of PUs via β-scission.
|
Figure 1 FTIR spectra of PUs. |
|
Figure 2 Proton NMR spectra of PUs prepared using PEG 1500. |
|
Figure 3 DSC traces of PUs recorded under nitrogen atmosphere. |
|
Figure 4 TG curves of PUs prepared using PEG 1500. |
|
Figure 5 TG curves of PUs prepared using PEG 4000 |
|
Figure 6 DTG curves of PUs prepared using PEG 1500. |
|
Figure 7 DTG curves of PUs prepared using PEG 4000. |
|
Figure 8 FTIR spectra of volatiles evolved from PUs on thermal degradation. |
|
Table 3 Parameters Derived from the Thermograms and Differential Thermograms of PUs Recorded in Nitrogen Atmosphere at a Heating Rate of 20 oC min-1 |
Bio and resorcinol based PUs were prepared from PEG of molecular mass 4000 or 1500 g mol-1, MDI and the chain extender (HER and/or CAO). The disappearance of absorption band (2280 cm-1) responsible for -NCO stretching and the appearance of peak at 3200 cm-1 for N-H stretching confirmed the completion of polymerization and the product formed was PU. The 1H NMR spectral data of the synthesized materials also confirmed the PU formation. DSC showed that PUs prepared using PEG 4000 are having higher heat of melting and PUs prepared using PEG 1500 and HER are thermally more stable. The PUs prepared using PEG 4000 were more prone to chain scission through unzipping because of having higher degree of polymerization than in PUs synthesized using PEG 1500. The maximum degradation temperature and residual mass left at 500 °C obtained from thermogram confirmed that PUs synthesized using PEG 1500 are thermally more stable than the PUs prepared from PEG 4000. Evolved gas analysis using TG/FTIR proved that thermal degradation of PUs under inert atmosphere proceeds through β-scission. The branching due to CAO unit in the PU made the material thermally stable by shifting the whole degradation pattern to higher temperature and enhancing the char formation. This is the first attempt in predicting the thermal stability of PU with number of β-hydrogen available for β-scission.
- 1. Handbook of Elastomers: New Developments and Technology, 1st ed.; Polamanteer, K.; Bowmic, A. K.; Stephens, H. L., Eds.; Marcel Decker: New York, USA, 1988.
- 2. Wirpsza, Z. Polyurethane Chemistry, Technology and Applications; Kemp, T. J., Translator; Ellis Harwood PTR Prentice Hall: New York, USA, 1993.
- 3. Wu, S.; Bulpett, D. A.; Harris, K. M.; Lutz, M. E.; Rajagopalan, M. Golf Ball Comprising Water Resistant Polyurethane Elastomers and Methods of Making the Same. US Patent 6,435,986, 2002.
- 4. Durairaj, R. B. Resorcinol: Chemistry, Technology and Appli- cations; Springer-Verlag Berlin Heidelberg: Berlin, Germany, 2005.
- 5. Opera, S. Effect of Resorcinol-based Chain Extenders Chemical Structure on the Enhanced Properties of Polyurethane Elastomers. High Perform. Polym. 2012, 24, 389-397.
-

- 6. Wang, H. H.; Yuen, U. E. Synthesis of Thermoplastic Polyurethane and its Physical and Shape Memory Properties. J. Appl. Polym. Sci. 2006, 102, 607-615.
-

- 7. Mattia, J.; Painter, P. A. A Comparison of Hydrogen Bonding and Order in a Polyurethane and Poly(urethane-urea) and their Blends with Poly(ethylene glycol). Macromol. 2007, 40, 1546-1554.
-

- 8. Rogulska, M.; Kultys, A.; Podkoscielny, W. Studies on Thermo- plastic Polyurethanes Based on New Diphenylethanederivative Diols. II. Synthesis and Characterization of Segmented Polyure- thanes from HDI and MDI. Eur. Polym. J. 2007, 43, 1402-1414.
-

- 9. Kausar, A.; Zulfiqar, S.; Ahmad, Z.; Sarwar, M. I. Studies on Novel Thermally Stable Segmented Polyurethanes Based on Thiourea-Derivative Diols. Polym. Degrad. Stab. 2010, 95, 2281-2288.
-

- 10. Kultys, A.; Rogulska, M.; Głuchowska, H. The Effect of Soft Segment Structure on the Properties of Novel Thermoplastic Polyurethane Elastomers Based on an Unconventional Chain Extender. Polym. Int. 2011, 60, 652-659.
-

- 11. Wu, S. Polyurethane Golf Ball with Improved Resiliency. US Patent 6,210,294 B1, 2002.
- 12. Delebecq, E.; Pascault, J. P.; Boutevin, B.; Ganachaud, F. On the Versatility of Urethane/Urea Bonds: Reversibility, Blocked Iso- cyanate, and Non-isocyanate Urethane. Chem. Rev. 2013, 113, 80-118.
-

- 13. Helou, M.; Carpentier, J. F.; Guillaume, S. M. Poly(carbonate-urethane): an Isocyanate-Free Procedure from α,ω-di(cyclic carbonate) Telechelic Poly(trimethylene carbonate)s. Green Chem. 2011, 13, 266-271.
-

- 14. Javni, I.; Hong, D. P.; Petrovic, Z. S. Polyurethanes from Soybean Oil, Aromatic, and Cycloaliphatic Diamines by Non-Isocyanate Route. J. Appl. Polym. Sci. 2013, 128, 566-571.
-

- 15. Diakoumakos, C. D.; Kotzev, D. L. Non-Isocyanate-Based Polyurethanes Derived upon the Reaction of Amines with Cyclocarbonate Resins. Macromol. Symp. 2004, 216, 37-46.
-

- 16. Orgilés-Calpena, E.; Arán-Aís, F.; Torró-Palau, A. M.; Orgilés-Barcelo, C. Biodegradable Polyurethane Adhesives Based on Polyols Derived from Renewable Resources. Proc. Inst. Mech. Eng. L 2014, 228, 125-136.
-

- 17. Desroches, M.; Escouvois, M.; Auvergne, R.; Caillol, S.; Boutevin, B. From Vegetable Oils to Polyurethanes: Synthetic Routes to Polyols and Main Industrial Products. Polym. Rev. 2012, 52, 38-79.
-

- 18. Lligadas, G.; Ronda, J. C.; Galiá, M.; Cádiz, V. Plant Oils as Platform Chemicals for Polyurethane Synthesis: Current-State of-the-art. Biomacromolecules 2010, 11, 2825-2835.
-

- 19. Howard, G. T. Biodegradation of Polyurethane: a Review. Int. Biodeter. Biodegrad. 2002, 49, 245-252.
-

- 20. Gonzaga, D. P.; Murakami, C. R.; Chierice, G. O.; Altafim, R. A. C. In Electrical Characterization of Castor-oil Resins, Conference Record of the 1998 IEEE International Symposium on Electrical Insulation, Arlington, VA, June 7-10, 1998; p 181.
-

- 21. Das, S.; Pandey, P.; Mohanty, S.; Nayak, S. An Insight on Castor Oil Based Polyurethane and Nanocomposites: Recent Trends and Development. Polym. Plast. Technol. Eng. 2017, 56, 1556-1585.
-

- 22. Tran, T. K.; Kumar, P.; Kim, H. R.; Hou, C. T.; Kim, B. S. Bio‐Based Polyurethanes from Microbially Converted Castor Oil. J. Am. Oil Chem. Soc. 2019, 96, 715-726.
-

- 23. Zhang, J.; Yao, M.; Chen, J.; Jiang, Z.; Ma, Y. Synthesis and Properties of Polyurethane Elastomers Based on Renewable Castor Oil Polyols. J. Appl. Polym. Sci. 2019,136, 47309.
-

- 24. Arévalo-Alquichire, S.; Valero, M. Castor Oil Polyurethanes as Biomaterials. In Elastomers; Cankaya, N., Ed.; IntechOpen: London, UK, 2017; pp 137-157.
- 25. Lévesque, S.; Rodrigue, D.; Vermette, P.; Gunatillake, P. Biomedical Applications of Polyurethanes, In: Vermette, P.; Griesser, H. J.; Laroche, G.; Guidoin, R. Eds.; Landes Bioscience-Eurekah.com Publishers: Texas, USA, 2001, pp 22-54.
- 26. Huang, K. S.; Chen, S. W.; Lu, L. A.; Min, R. R. Polyrethane/Carboxymethyl Cellulose Blended Polymer. Cellul. Chem. Technol. 2007, 41, 113-117.
- 27. Li, X. X.; Jin, K. H.; Cho, U. R. Synthesis and Analysis of Bio-based Polyurethanes with Different Polyester Polyols. Polym. Korea 2019, 43, 595-601.
-

- 28. Li, X. X.; Jin, K. H.; Cho, U. R. Changes in the Properties of Bio-Based Thermoplastic Polyurethanes with Different Chain Extenders. Polym. Korea 2019, 43, 621-628.
-

- 29. Chen, Z.; Nikos, H.; Feng, X.; Gnanou, Y. Poly(urethane-carbonate)s from Carbon Dioxide. Macromol. 2017, 50, 2320-2328.
-

- 30. Conceiḉão, M. M.; Fernandes, V. J.; Araújo, A. S.; Farias, M. F.; Santos, I. M. G.; Souza, A. G. Thermal and Oxidative Degradation of Castor Oil Biodiesel. Energy Fuels 2007, 21, 1522-1527.
-

- 31. Wong, C.; Badri, K. Chemical Analyses of Palm Kernel Oil-Based Polyurethane Prepolymer. Mater. Sci. Appl. 2011, 3, 78-86.
-

- 32. Saunders, J. H. The Reactions of Isocyanates and Isocyanate Derivatives at Elevated Temperatures. Rubber Chem. Tech. 1959,32, 337-345.
-

- 33. Saunders, J. H.; Backus, J. K. Thermal Degradation and Flamm- ability of Urethane Polymers. Rubber Chem. Tech. 1966, 39, 461-480.
-

- 34. Vijayakumar, C. T.; Sivasamy, P.; Rajkumar, T. Synthesis and Characterization of 1,3-bis(2-hydroxyethoxy)benzene Based Saturated and Unsaturated Polyesters. Eur. Polym. J. 2007, 43, 3028-3035.
-

- 35. Sivasamy, P.; Palaniandavar, M.; Vijayakumar, C. T.; Lederer, K. The Role of b-hydrogen in the Degradation of Polyesters. Polym. Degrad. Stab. 1992, 38, 15-21.
-

- 36. Backus, J. K.; Darr, W. C.; Gemeinhardt, P. G.; Saunders, J. H. Thermal Decomposition of Rigid Urethane Foams. J. Cell. Plast. 1965, 1, 178-186.
-

- 37. Mead, J. L. Mechanical Degradation of a Polyurethane Elastomer. Ph.D. Thesis, Massachusetts Institute of Technlogy, USA, 1986.
- 38. Pabbo, M.; Levin, B. C. A Review of the Literature on the Gaseous Products and Toxicity Generated from the Pyrolysis and Combustion of Rigid Polyurethane Foams. Fire Mater. 1987, 11, 1-29.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2021; 45(4): 511-519
Published online Jul 25, 2021
- 10.7317/pk.2021.45.4.511
- Received on Jan 11, 2021
- Revised on Apr 17, 2021
- Accepted on Apr 22, 2021
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Thangamani Rajkumar
-
Department of Chemistry, Rajah Serfoji Government College (Affiliated to Bharathidasan University), Thanjavur - 613005, Tamilnadu, India
- E-mail: rajkumar_t@rsgc.ac.in
- ORCID:
0000-0002-5815-3769









 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.