- Tooth Whitening and Protecting Effect Using TiO2/PLCL Biodegradable Polymer Composites
Department of Chemical Engineering, College of Engineering, Dankook University, Jukjeon-dong, Yongin-si, Gyeonggi-do 16890, Korea
- TiO2/PLCL 생분해성 고분자 복합체를 이용한 치아 미백 및 보호효과에 대한 연구
단국대학교 화학공학과
Reproduction, stored in a retrieval system, or transmitted in any form of any part of this publication is permitted only by written permission from the Polymer Society of Korea.
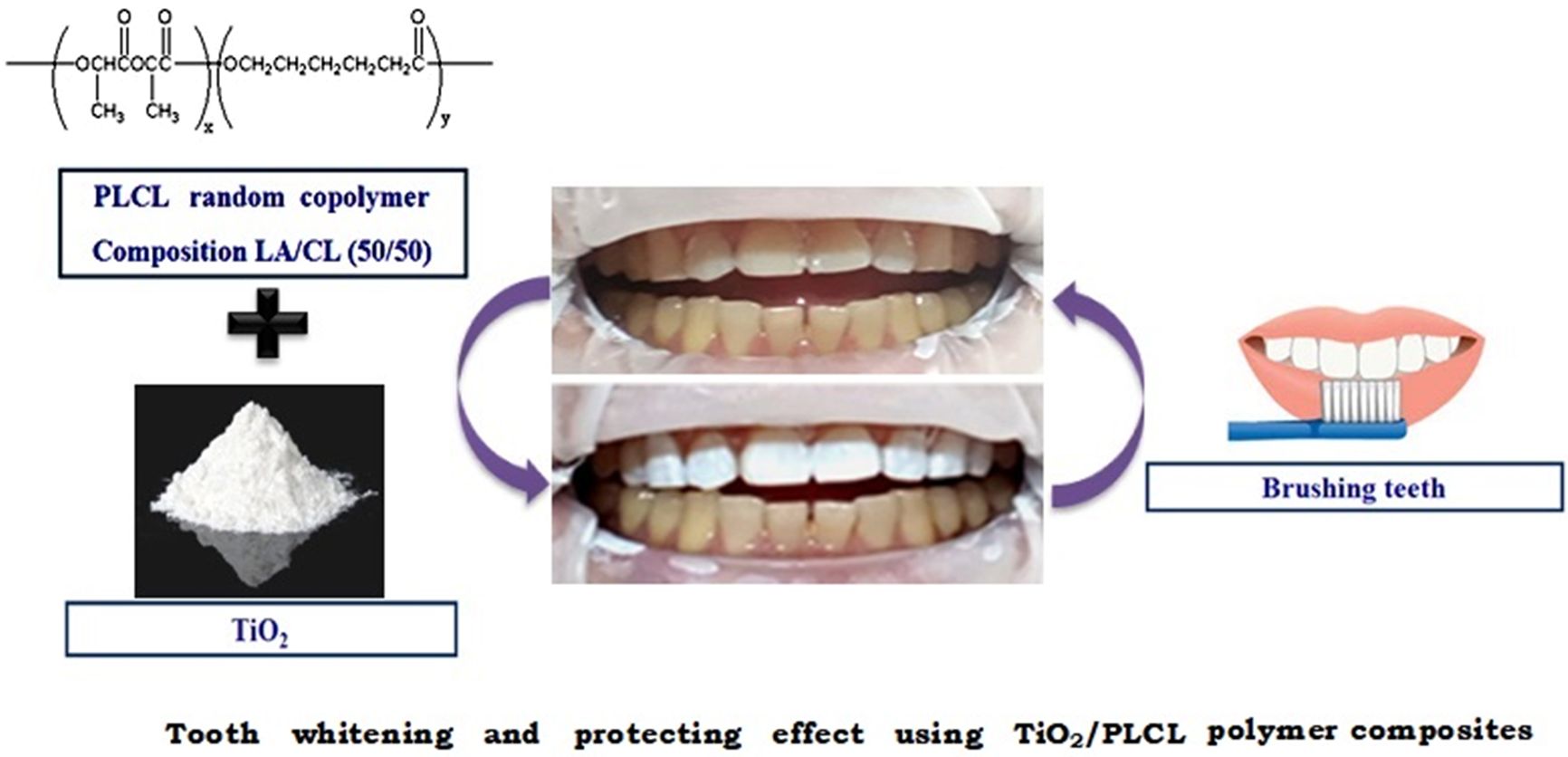
Poly(L-lactide-co-ɛ-caprolactone) (PLCL) was synthesized, and this was mixed with TiO2 for tooth manicure. A mechanical test was conducted using a tensile strength testing machine and pencil hardness tester; these tests indicated that the optimal molar ratio of lactide in PLCL was 50%. Differences in the surface structures of the films with respect to the hydrophobicity and the whitening effect were examined using a digital microscope, a contact angle meter, and colorimeter. The optimal ratio of TiO2 for the composite was determined to be 10%. The composite coated glass plate yielded a good cell adhesion rate in fibroblasts. The TiO2/PLCL composite manufactured by this simple method can be applied to implantable dental materials, used for tooth whitening, and as a protecting agent during tooth manicures
치아매니큐어에 적용하기 위한 베이스 레진으로 poly(L-lactide-co-e-caprolactone)(PLCL)을 합성하였고, 락티드와 카프로락톤의 최적 비율을 결정하였다. TiO2는 미백을 위한 안료로 적용되어졌으며, 최적의 TiO2/PLCL 조성비를 위해 탄성회복률, 연필심 경도 측정, 색차계 시험, 접촉각 측정을 진행하였다. 이를 통해 PLCL을 위한 락티드와 카프로락톤의 최적비율은 50:50이였으며, TiO2의 최적 비율은 10%임을 확인하였다. 세포독성 시험 결과 우수한 세포 부착 및 증식 효과를 확인하였고, 천연 치자색소를 이용하여 다양한 노란색 코팅제를 제조해 보았다. 본 연구결과 TiO2/PLCL 복합체는 치아 미백 및 미용 매니큐어의 재료로 적합한 것으로 판단된다
Poly(L-lactide-co-ɛ-caprolactone) (PLCL) was synthesized, and this was mixed with TiO2 for tooth manicure. It was confirmed that the TiO2/PLCL composite has proper optimal properties for dental manicure. The TiO2/PLCL composite manufactured by this simple method can be applied to implantable dental materials, used for tooth whitening, and as a protecting agent during tooth manicures.
Keywords: tooth manicure, whitening, protecting, poly(L-lactide-co-e-caprolactone), titanium dioxide
This work was supported by the Commercialization Promotion Agency For R&D Outcomes (COMPA) grant funded by the Korea government (MSIT) (1711121239).
Tooth whitening is a popular dental treatment that continues to grow with public demand for aesthetics. For hundred years, various researchers have studied whitening and bleaching materials. There are many reasons for tooth discoloration, such as personal habits, dental problems, or exposure to other substances.1,2
Nowadays, the main active ingredients in tooth whitening products are peroxides, such as hydrogen peroxide3 and carbamide peroxide.4 Although some peroxides, such as peroxy caproic acid (PAP),5 were considered safer, hydrogen peroxide has been the most efficient ingredient of tooth whitening products. However, teeth’s whitening by hydrogen peroxide is harmful to the oral mucosa and dentin because it causes oral mucosa burns and dentin allergies.6,7
Recently, coating resin composite materials, such as shellac, gum, and polyacrylates, have been explored mainly as tooth manicure materials in cases in which instantaneous aesthetic improvement of teeth is a priority. However, these materials have been reported to become unstable because of moisture, resulting in weak mechanical properties.8-10 Various studies have been conducted to overcome this.11,12
In addition, there are other disadvantages, such as feeling of irritation because of the roughness of the coating surface, less glossiness, hypersensitivity, and re-coloration.13,14
In this study, poly(L-lactide-co-ɛ-caprolactone) (PLCL), a synthetic biodegradable polymer, was selected as the optimal base polymer for creating composites for tooth whitening without hydrogen peroxide.
In previous studies, synthetic biodegradable polymers, such as poly(lactic acid) (PLA), poly(glycolic acid) (PGA), poly (lactide-co-glycolide) (PLGA), poly(ɛ-caprolactone) (PCL), poly(glycolide-co-caprolactone) (PGCL), and PLCL were developed for vessel or cartilage tissue engineering.15-17 Among these, PLCL with a molar ratio of 50:50 exhibited unique elasticity; hence, it was used as a tissue engineering scaffold to investigate the effect of mechanical stimulation on tissue regeneration.18,19
TiO2 has been extensively researched over the last few decades because of its outstanding physicochemical properties. It possesses interesting optical, dielectric, and analytical properties that are exploited in industrial applications, such as pigments, filters, and catalyst supports.20,21 In addition, it is generally used as a semiconductor because of its high photoactivity, low cost, high availability, and non-toxicity. White pigment is important in dental manicure. In this study, TiO2, which is not toxic and is used as a food, was selected as a white pigment.
We postulate that the amorphous PLCL at a molar ratio of 50:50 can impart close-up characteristics and elastic properties for tooth whitening and protection in dental manicure.
Therefore, the objectives of this study were to i) determine the optimal molar ratio of PLCL for use as a tooth coating agent; ii) evaluate the whitening and protection efficiency of the TiO2/PLCL composite; and iii) evaluate the cytotoxicity of the composite film on a fibroblast (NIH3T3).
Materials.L-lactide (Purac Biochem, Birmingham, UK) was used to synthesize PLCL. Stannous octoate, e-caprolactone, methanol, chloroform, ethyl acetate, and titanium dioxide (99 wt%) were purchased from Sigma-Aldrich (St. Louis, MO, USA). All chemicals were used without further purification, except ε-caprolactone.
PLCL (molar ratio: 70:30, 50:50, and 30:70; number-average molecular weight: 16×104, 15×104, and 14×104, respectively) was polymerized and analyzed using methods described elsewhere.22
Fabrication of PLCL Films and Solubility Tests.PLCLs of molar ratios 70:30, 50:50, and 30:70 were dissolved in 10% (w/v) chloroform, ethyl acetate, and dichloromethane by magnetic stirring at room temperature for 5 h. The remaining undissolved polymer was weighed. To form the film, resulting solutions of 100 mL as 10% (w/v) of each PLCL solution in chloroform were injected onto a Teflon plate (200×200×3 mm3), dried at room temperature for 2 weeks, and vacuum dried for 48 h. The obtained film was washed with pure water for 1 h and then vacuum dried.
Measurement of Shear Bond Strength.The shear bond strength to dentin was measured as a function of the molar ratio (70:30, 50:50, and 30:70) of lactide and caprolactone to PLCL. To prepare a dentin surface with embedded acryl resin, freshly extracted human molar teeth were polished with 600 grit sand paper using a polishing machine (RotoPol-25; Struers, Ballerup, Denmark). Then, each 10% (w/v) PLCL solution in chloroform was mixed with 10% (w/v) TiO2 and placed in a Teflon mold (diameter = 4 mm, height = 7 mm) laid on a polished dentin surface. After 2 weeks at 40 oC, the mold was removed from the dried composite sample. The specimens were then immersed in distilled water at 37oC for 24 h. The shear bond strength was measured using a universal testing machine.23,24 The crosshead speed was set to 1 mm/min, and the load required to debone the specimen from the dentin was determined.
Fabrication of TiO2/PLCL Composites according to TiO2 Contents.10% (w/v) of PLCL (50:50) solutions were mixed into the PLCL solution to obtain TiO2 concentrations of 0, 5, 10, 15, 20, and 25% (w/v). As described in section 2.2. Films were formed from the mixtures by the aforementioned film casting method and stored at room temperature under vacuum until they were used.
Characterization of TiO2/PLCL Composites.The TiO2/PLCL composite films were dried at room temperature and the water contact angle (Phoenix 150; Surface Electro Optics, Seoul, Korea), surface morphology, and dispersion state of TiO2 were observed. A digitalmicroscope (digital microscope: A-2111; Dino-Lite, Taiwan) was used to observe the surface morphology and dispersion state of TiO2.
To examine recovery from elongation, PLCL films (200× 200×1.5 mm3) were prepared from TiO2 concentrations of 0, 5, 10, 15, 20, and 25%. The recovery test was carried out using a universal testing machine (Instron model 4467, Canton, MA, USA). A 10 N load cell with a crosshead speed of 10 mm/min (strain = 250%) was used. The recovery was calculated as Recovery (%) = 100 - ((L2 - L0)/(L1 - L0)×100), where L0, L1, and L2 indicate the original length, extended length, and final length, respectively, after releasing the stress. In addition, the elongation at break (%) was measured as the elongation length at the broken point.
The surface hardness of the TiO2/PLCL composite film was determined by a hardness test using an Imoto IMC-1552 pencil scratch hardness tester according to the JIS K5600-5-4 standard (equivalent to ISO/DIS 15184).
Meanwhile, the color was measured according to the CIE LAB color scale relative to the standard illuminant D65 in the reflectance mode over a white background (CIE L*=94.3, a*=−0.1, and b*=−0.4) on a spectrophotometer (Color-Eye 7000A, GretagMacbeth Instruments, New Windsor, NY, USA). The aperture size was 3 mm×8 mm. The measurements were repeated three times for each specimen.
The fabricated TiO2/PLCL composites were applied to the upper tooth surface of human body with a sponge-type brush. Only the upper tooth was painted to compare the manicured part with the non-manicured part.
Cytotoxicity tests of the TiO2/PLCL composite were performed using a previously described method.25 For this, the TiO2/PLCL composite films were pre-wetted with a medium [Dulbecco’s modified Eagle’s medium supplemented with 2 mM L-glutamine and 10% fetal bovine serum] and incubated at 37 oC in a 5% CO2 environment. After 12 h, the medium was aspirated, and NIH3T3 cells (ATCC-L929, Manassas, VA, USA) were plated directly on each film in a 200 µL media suspension. The cell density was set at 3×104 cells per well. After another 1 h, 1800 µL medium was added to each well, and the initial adhesion and proliferation were quantified using a WST-8 assay.
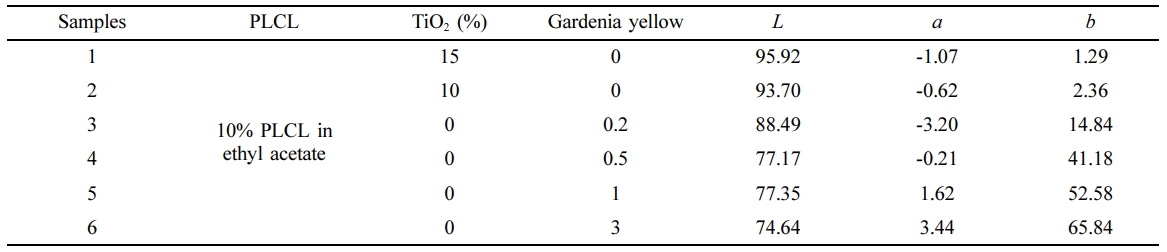
To produce various yellow colors, gardenia yellow and TiO2 were mixed in PLCL, as shown in Table 3. The film was prepared as described earlier, and the color analysis was carried out.
|
Table 3 Composition for Various Colors Producing Using Gardenia Yellow and Analysis of Colors |
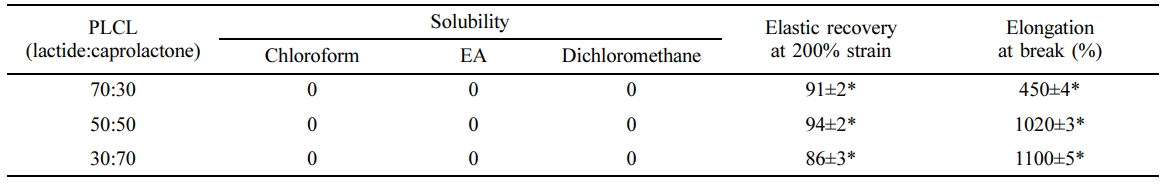
Mechanical Properties for Optimal Molar Ratio of PLCL. The results of the characterization of the synthesized PLCL (molar ratio: 70:30, 50:50, and 30:70) are shown in Table 1. No difference in solubility was observed in chloroform, ethyl acetate, or dichloromethane. Solvents are important in the application of biomaterials. Chloroform and dichloromethane have been reported as highly toxic and carcinogenic,26,27 whereas ethyl acetate, a natural substance that is relatively less toxic, is usually used in food additives, perfume, and solvent for dental vanish as a Fluor protector (Ivoclar vivadent AG, Swiss).28,29 Therefore, ethyl acetate was selected as the optimal solvent for dental manicure. The elastic recovery and elongation at break of the PLCL films were measured and results are shown in Table 1. A high elastic recovery and good elongation properties were observed in the PLCL film at a molar ratio of 50:50. Elastic recovery and elongation properties are important factors that determine the adhesion properties on the tooth surface for low impact strength and tough tensile properties.
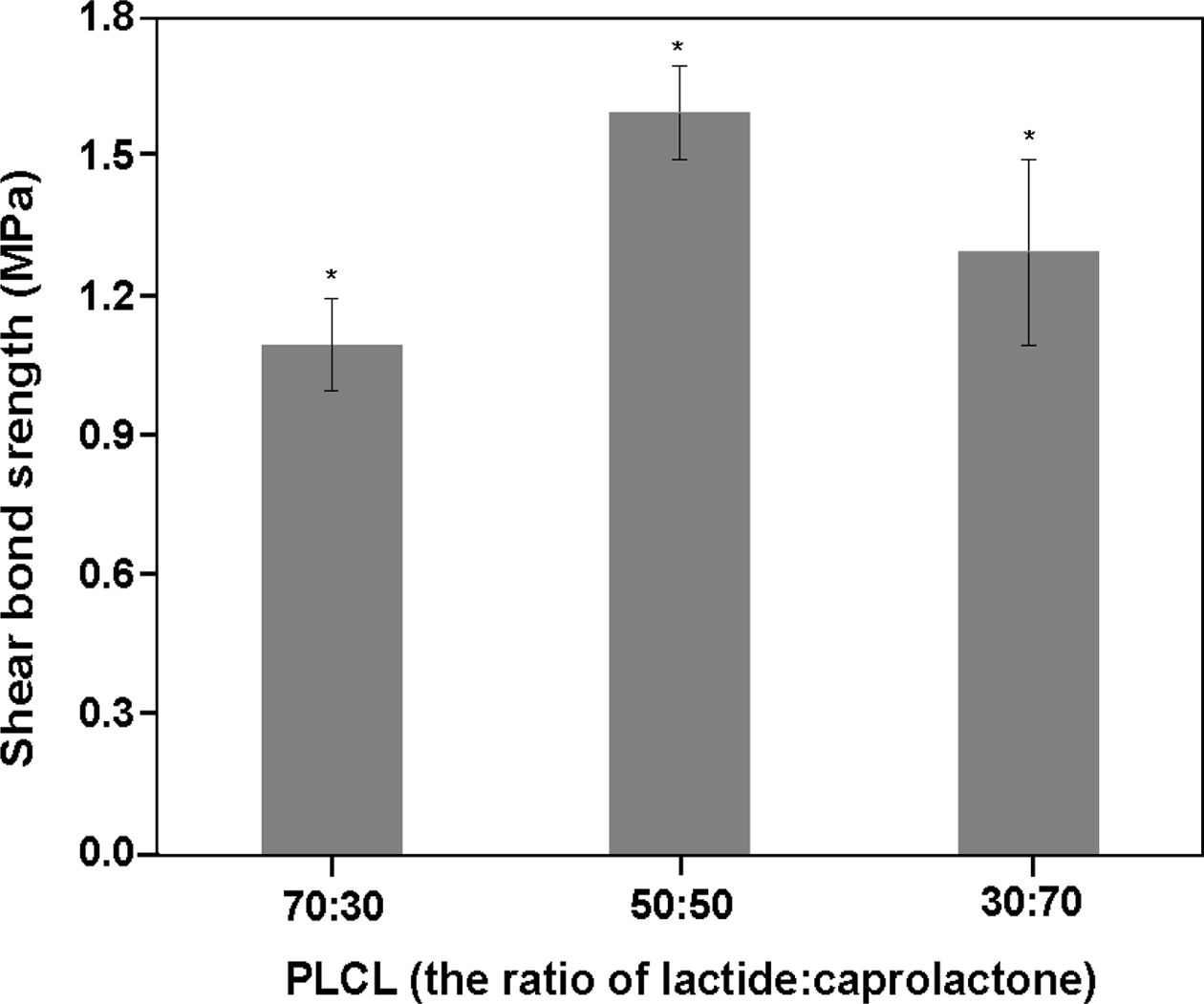
Result of Shear Bond Strength.The shear bond strength values of the PLCLs at molar ratios of 70:30, 50:50, and 30:70 were measured. The PLCLs at a molar ratio of 50:50 showed more than 1.5-fold increase in the bond strength compared with the PLCLs at molar ratios of 30:70 and 70:30, as shown in Figure 1. The high bond strength of the specimens of the PLCLs at a molar ratio of 50:50 was because of the amorphous crystal structure of PLCL and the inherent stickiness and toughness on dentin. Meanwhile, lower bond strength was observed in the PLCLs at molar ratios of 70:30 and 30:70. This lower strength might have been because of the decreased impact strength due to the increased rigidity caused by the variation in the crystallization with changes in the molar ratio. Therefore, as shown in Table 1 and Figure 1, the 50:50 molar ratio is suitable for enhancing adhesive properties on the tooth; hence, it was prepared for further study.
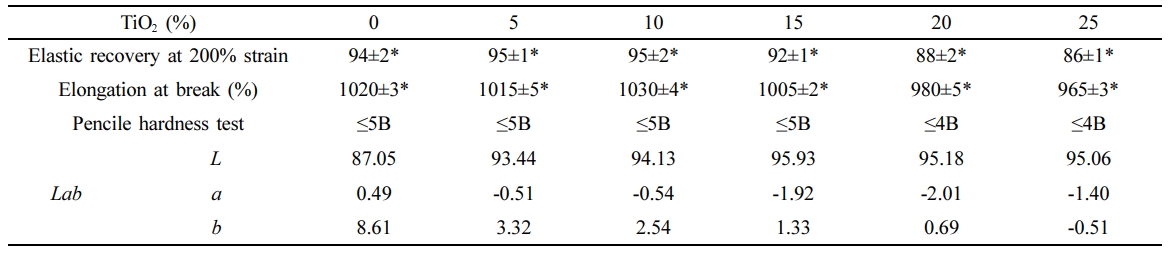
Mechanical Properties of the TiO2/PLCL Composites. The mechanical properties of the TiO2/PLCL (50:50) composites with TiO2 concentrations of 0, 5, 10, 15, 20, and 25% are shown in Table 2. Superior elastic recovery and elongation properties were observed in the TiO2/PLCL composite at 10% and 15%; however, these were lower in the rest of the samples. In addition, a low hardness of 4 B was observed from the surface hardness test of the TiO2/PLCL composites at 20% and 25%. Meanwhile, composites with a TiO2 concentration of less than 20% had a relatively high hardness of 5 B. The hardness of 5 B is sufficient for removing the coated tooth manicure by brushing teeth. Therefore, we suppose that the lower mechanical properties at 20% and 25% were because of the weakened interconnectivity and higher surface roughness due to excess TiO2 particles in the composite. Enhanced whitening effect and good mechanical properties were observed with increasing TiO2 concentration at a TiO2 concentration of less than 20%. However, there was no clearly observed difference in the whitening effect in the composite at 10% and 15%. Therefore, a TiO2 concentration of 10% was considered optimal because it resulted in good mechanical properties and an enhanced whitening effect.
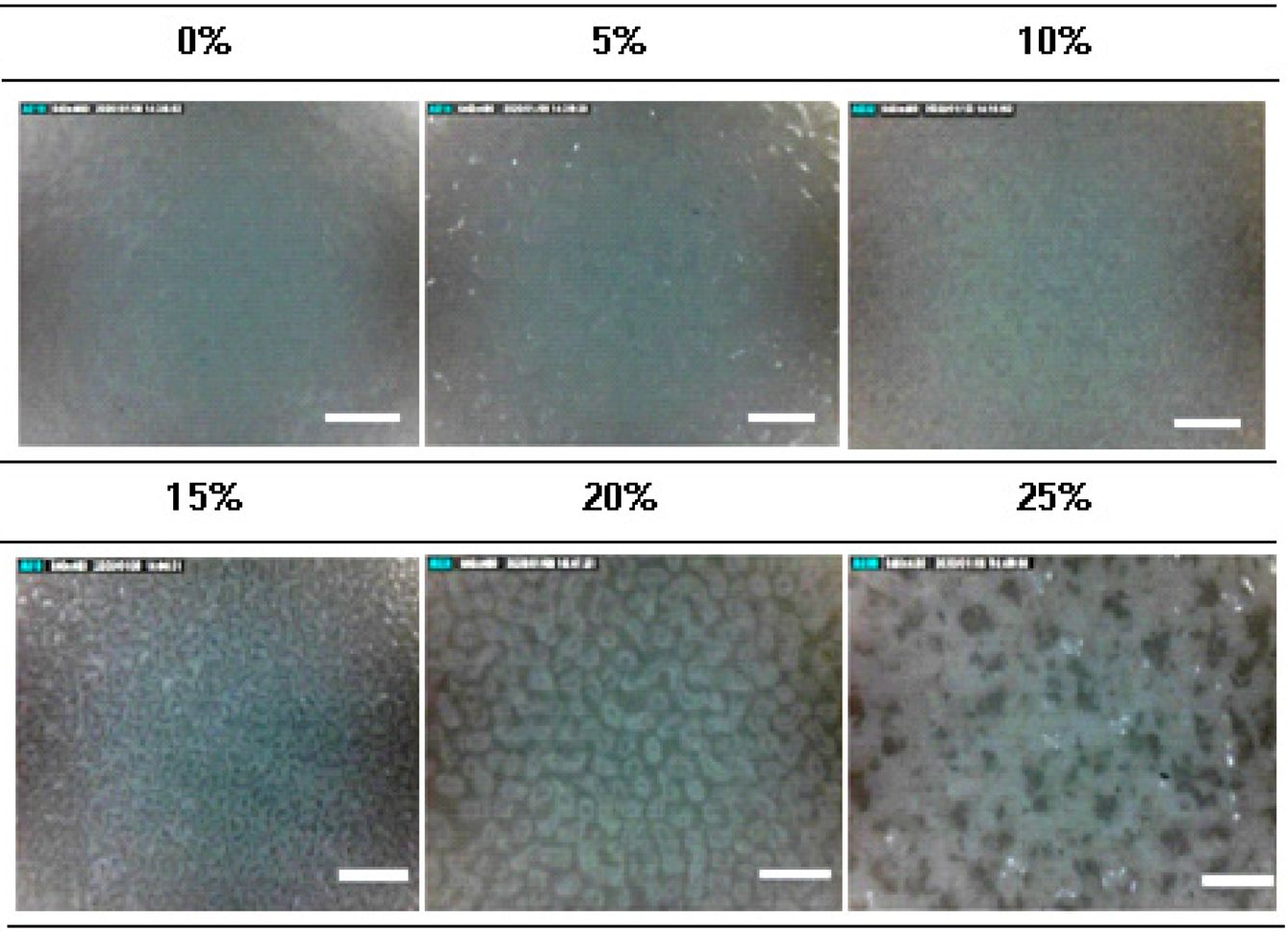
Measurement of Dispersion State of TiO2 Particles.The dispersion state of TiO2 particles and the surface morphology of the films were studied using a digital microscope. As shown in Figure 2, the dispersion states of TiO2 particles on the TiO2/PLCL composites at 0, 5, and 10% were similar in a homogeneous state. Meanwhile, composites with more than 10% concentration showed an increase in clumps and surface roughness because of the lower dispersion efficiency of the white TiO2 particles as pigments. The increased clumps and surface roughness were because of the higher surface area of TiO2 particles.
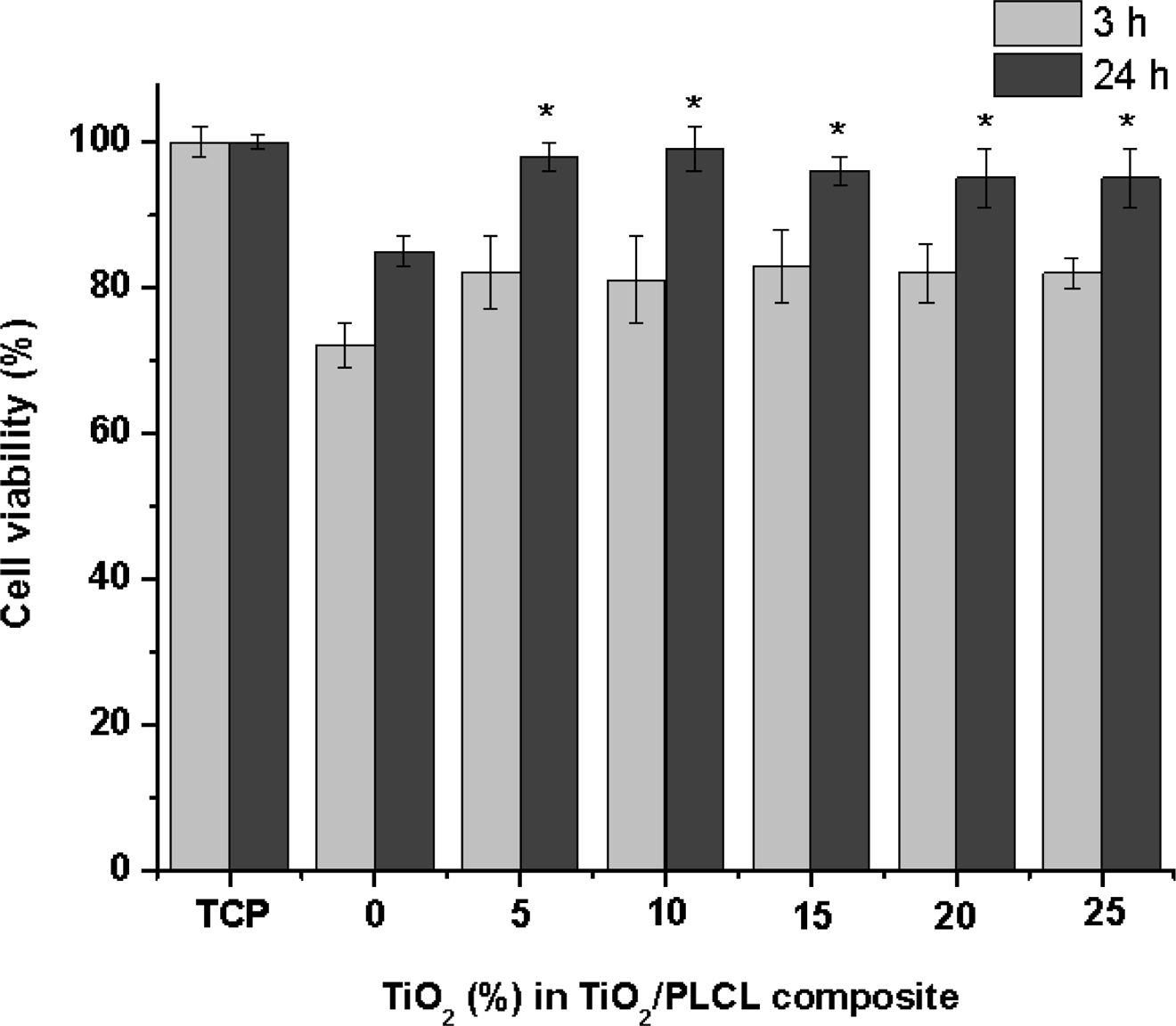
Cytotoxicity of TiO2/PLCL Composite Films.For cell toxicity evaluation, NIH3T3 cells were cultured on TiO2/PLCL (50:50) composite films at TiO2 concentrations of 0, 5, 10, 15, 20, and 25%. The initial cell adhesion and proliferation were affected by the TiO2 concentration, except in case of the PLCL film prepared without TiO2. However, low cell adhesion and proliferation were observed in the pure PLCL film prepared without TiO2 (Figure 3). The cell initial adhesion efficiency is affected by the hydrophobic ratio on the surface. The contact angles of the TiO2/PLCL (50:50) composite films with TiO2 concentrations of 0, 5, 10, 15, 20, and 25% were 68, 63, 62, 62, 61, and 61o, respectively. However, the overall TiO2/PLCL composites appeared to be more hydrophilic than pure PLCL. Furthermore, no significant difference was observed between the films at TiO2 concentration of 5, 10, 15, 20, and 25% and tissue culture plate after culturing for 24 h. Therefore, there was no toxicity.
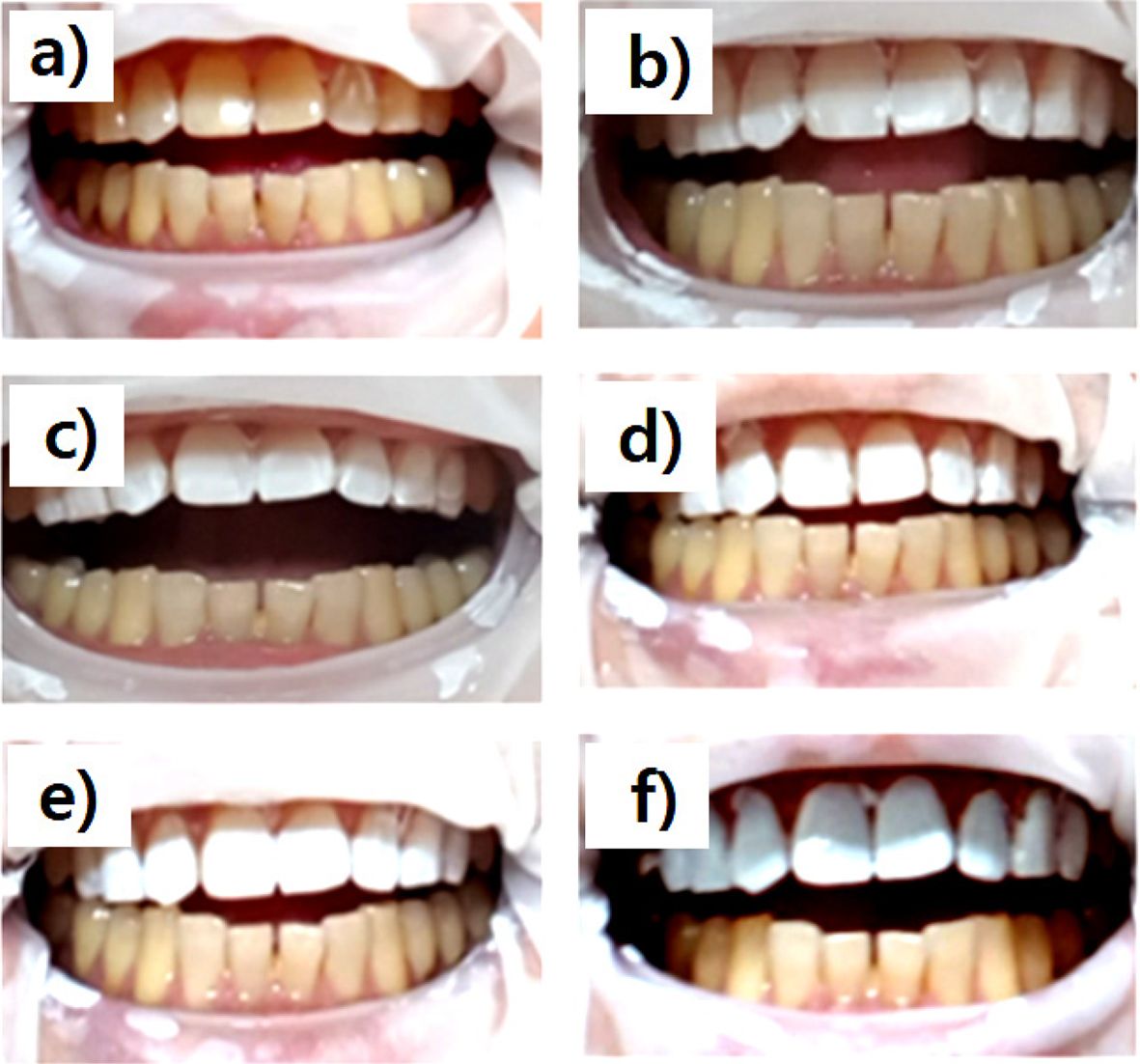
Applies to Actual Tooth.Figure 4 shows photographs of the TiO2/PLCL (50:50) composite at various TiO2 concentrations applied to the teeth. As seen in Figure 4(a), pure PLCL applied to the tooth surface exhibited a colorless transparent coating film on yellow teeth. In contrast, Figure 4(b)-(f) show an increased whitening effect with increasing TiO2 content. However, in the case of the PLCL composite with >15% TiO2 concentration, an unnaturally painted strong white tooth was observed [(Figure 4(d)-(f))]. In contrast, uniform and natural coloring was observed in PLCL composites at 5% and 10%. This may have been because proper pigment content and good dispersion degree were used. Therefore, a PLCL composite with a TiO2 concentration of 10% was considered optimal for dental manicure applications.
Fabrication of Yellow-colored Composites.As the amount of gardenia yellow increased, the yellow color became stronger and clearer (Figure 5). From the quantitative analysis of colors, the colorimeter showed that the L value, as a white indicator, decreased while the b value, as a yellow indicator, increased with increasing gardenia yellow (Table 3).
The study also confirmed that it could be applied as a tooth manicure and has a variety of colors and whitening coating.
|
Figure 1 Shear bond strength of poly(L-lactide-co-ε-caprolactone) (PLCL) according to molar ratio (n=5, *P<0.05). |
|
Figure 2 Surface morphological study and dispersion state of TiO2 using a digital microscope (TiO2 = 0, 5, 10, 15, 20, 25%, ×250, scale bar: 400 µm). |
|
Figure 3 Cell viability of TiO2/PLCL (50:50) according to TiO2 concentration (n=5, *P>0.05). |
|
Figure 4 Photographs of the TiO2/PLCL (50:50) composite at various TiO2 concentrations applied to the teeth. (a) 0%; (b) 5%; (c) 10%; (d) 15%; (e) 20%; (f) 25% |

|
Figure 5 Various colors producing using gardenia yellow (sample number in Table 3). |
|
Table 1 Properties of Poly(L-lactide-co-ε-caprolactone) (PLCL) according to Molar Ratio (n=5, *P<0.05) |
|
Table 2 Properties of Poly(L-lactide-co-ε-caprolactone) (PLCL 50:50) according to TiO2 Concentration (n=5, *P<0.05) |
Effective dental manicure material was prepared by a simple method using a biodegradable elastic poly(L-lactide-co-ε-caprolactone) (PLCL) copolymer and TiO2 particles in ethyl acetate solution. The tensile strength, elastic recovery, analysis of color, and cytotoxicity of the composites were studied to determine the optimal composition for tooth manicure applications. We found via mechanical property tests that PLCL at a molar ratio of 50:50 was the most effective composition to increase the elastic recovery and adhesive properties of the tooth coating. The TiO2/PLCL mixture with a TiO2 weight of 10% exhibited the highest dispersion of pigments and mechanical properties. Meanwhile, cell viability was improved in TiO2/PLCL composites compared with pure PLCL. Further studies on the biological evaluation and biodegradable properties are required; however, the TiO2/PLCL composite shows promise for use in implantable materials, for tooth whitening, and as protecting agent in the field of dentistry.
- 1. Yu, H.; Zhang, C.-Y.; Cheng, S.-L.; Cheng, H. Effects of Bleaching Agents on Dental Restorative Materials: A Review of the Literature and Recommendation to Dental Practitioners and Researchers. J. Dent. Sci. 2015, 10, 345-351.
-

- 2. Kwon, S. R. Whitening the Single Discolored Tooth. Dent. Clin. N. Am. 2011, 55, 229-239.
-

- 3. Gerlach, R. W.; Barker, M. L.; Karpinia, K.; Magnusson, I. Single Site Meta-analysis of 6% Hydrogen Peroxide Whitening Strip Effectiveness and Safety Over 2 Weeks. J. Dent. 2009, 37, 360-365.
-

- 4. Farawati, F. A. L.; Hsu, S.-M.; O’Neill, E.; Neal, D.; Clark, A.; Josephine, E.-U. Effect of Carbamide Peroxide Bleaching on Enamel Characteristics and Susceptibility to Further Discoloration.J. Prosthet. Dent. 2019, 121, 340-346.
-

- 5. Junyuan, Q.; LiZeng, W.; Licheng, T.; Ruizhi, L.; Yiwang, C. A bio-safety tooth-whitening composite gels with novel phthalimide peroxy caproic acid. Compos. Commun. 2019, 13, 107-111.
-

- 6. Fatemeh, A.; Kiana, B.; Mohammad, M. I.; Amin, G.; Roya, S.-F. Effect of Bleaching with Carbamide Peroxide on Shear Bond Strength of Orthodontic Brackets: A Meta-analysis of in Vitro Studies. Int. Orthod. 2020, 18, 214-224.
-

- 7. Mohan, N.; Westland, S.; Brunton, P.; Ellwood, R.; Pretty, I. A.; Luo, W. A Clinical Study to Evaluate the Efficacy of a Novel Tray Based Tooth Whitening System. J. Dent. 2008, 36,21-26.
-

- 8. Choi, J. W.; Yang, S. Y.; Kwon, J. S.; Lee, S. B. Study on Testing Methods for Efficacy of Tooth Manicure Products. Dent. Mater. 2019, 35, E10-e11.
-

- 9. Hashimura, T.; Yamada, A.; Iwamoto, T.; Arakaki, M.; Saito, K.; Fukumoto, S. Application of a Tooth-surface Coating Material to Teeth with Discolored Crowns. Pediatr. Dent. J. 2013, 23, 44-50.
-

- 10. Eric, J. T. Recent Coating Developments for Combination Devices in Orthopedic and Dental Applications: A Literature Review. Adv. Drug Deliv. Rev. 2017, 112, 88-100.
-

- 11. Fabián, M.-G.; Mario, F. D. G. Bonding Effectiveness of Tooth-colored Materials to Resin Cement Provided by Self-etching Silane Primer after Short- and Long-term Storage.J. Prosthet. Dent. 2019, 212, 713.e1-713.e8.
-

- 12. Pitacas, H. M. G.; Cavalheiro, A.; Coito, C.; Silva, A.; Eira, R.; Lopes, M. Effect of External Tooth Bleaching on the Surface of Resin Composites – An In Vitro Studyefeito Do Branqueamento Dentário Externo Na Superfície Das Resinas Compostas – Estudo In Vitro. Revista Portuguesa de Estomatologia, Medicina Dentária e Cirurgia Maxilofacial. 2015,56,149-155.
-

- 13. Yue, S.; Wu, J.; Zhang, Q.; Zhang, K.; Weir, M. D.; Imazato, S.; Bai, Y.; Xu, H. H. K. Novel Dental Adhesive Resin with Crack Self-healing, Antimicrobial and Remineralization Properties. J. Dent. 2018, 75, 48-57.
-

- 14. Krifka, S.; Spagnuolo, G.; Schmalz, G.; Schweikl, H. A Review of Adaptive Mechanisms in Cell Responses Towards Oxidative Stress Caused by Dental Resin Monomers. Biomaterials 2013, 34, 4555-4563.
-

- 15. Ma, P. X. Biomimetic Materials for Tissue Engineering. Adv. Drug Deliv. Rev. 2008, 60, 184-198.
-

- 16. Nur, R. E. P.; Xiuhui, W.; Ying, C.; Xiaomeng, L.; Naoki, K.; Guoping, C. Preparation of PLGA-collagen hybrid scaffolds with controlled pore structures for cartilage tissue engineering. Prog. Nat. Sci.-Mater. 2020, 30, 642-650.
-

- 17. Frydrych, M.; Román, S.; MacNeil, S.; Chen, B. Biomimetic poly(glycerol sebacate)/poly(l-lactic acid) Blend Scaffolds for Adipose Tissue Engineering. Acta Biomater. 2015, 18, 40-49.
-

- 18. Chung, S.; Ingle, N. P.; Montero, G. A.; Kim, S. H.; King, M. W. Bioresorbable Elastomeric Vascular Tissue Engineering Scaffolds via Melt Spinning and Electrospinning. Acta Biomater. 2010, 6, 1958-1967.
-

- 19. Kijeńska, E.; Prabhakaran, M. P.; Swieszkowski, W.; Kurzydlowski, K. J.; Ramakrishna, S. Interaction of Schwann Cells with Laminin Encapsulated PLCL Core–shell Nanofibers for Nerve Tissue Engineering. Eur. Polym. J. 2014, 50, 30-38.
-

- 20. Chen, Z.; Han, S.; Zhou, S.; Feng, H.; Liu, Y.; Jia, G. Review of Health Safety Aspects of Titanium Dioxide Nanoparticles in Food Application. NanoImpact. 2020, 18, 100224.
-

- 21. Padmanabhan, N. T.; John, H. Titanium Dioxide Based Self-cleaning Smart Surfaces: A Short Review. J. Environ. Chem. Eng. 2020, 8, 104211.
-

- 22. Fernández, J.; Meaurio, E.; Chaos, A.; Etxeberria, A.; Alonso-Varona, A.; Sarasua, J. R. Synthesis and Characterization of Poly (L-lactide/ε-caprolactone) Statistical Copolymers with Well Resolved Chain Microstructures. Polymer 2013, 54, 2621-2631.
-

- 23. Lim, J. I.; Lim, K. J.; Lim, H. N.; Lee, Y. K. Bond Strength of Experimental Cyanoacrylate-modified Dental Glass Ionomer Cement. J. Mater. Sci. 2010, 45, 5211-5217.
-

- 24. Wang, C.; Zeng, J.; Wang, S.; Yang, Z.; Huang, Q.; Chen, P.; Zhou, S.; Liu, X. Influence of Surface Treatments on the Shear Bond Strength of Orthodontic Brackets to Porcelain. Appl. Surf. Sci. 2008, 255, 416-418.
-

- 25. Landegren, T.; Risling, M.; Persson, J. K. E.; Sonde´n, A. Cyanoacrylate in Nerve Repair: Transient Cytotoxic Effect. Int. J. Oral Maxillofac. 2010, 39, 705-712.
-

- 26. SilvaNunes-Halldorson, V. D.; Steiner, R. L.; Smith, G. B. Residual Toxicity after Biodegradation: Interactions Among Benzene, Toluene, and Chloroform. Ecotoxicol. Environ. Saf. 2004, 57, 162-167.
-

- 27. Kim, H. A Case of Acute Toxic Hepatitis after Suicidal Chloroform and Dichloromethane Ingestion. Am. J. Emerg. Med. 2008, 26, 1073.e3-1073.e6.
-

- 28. Khan, M. A.; Ahmad, R.; Srivastava, A. N. Effect of Ethyl Acetate Aroma on Viability of Human Breast Cancer and Normal Kidney Epithelial Cells. In Vitro Integr. Med. Res. 2017, 6, 47-59.
-

- 29. Dudic, V. B.; Lang, N. P.; Mombelli, A. Microbiological and Clinical Effects of an Antiseptic Dental Varnish after Mechanical Periodontal Therapy. J. Clin. Periodontol. 1999, 26, 341-346.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2021; 45(3): 421-427
Published online May 25, 2021
- 10.7317/pk.2021.45.3.421
- Received on Dec 10, 2020
- Revised on Jan 30, 2021
- Accepted on Feb 8, 2021
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Jin Ik Lim
-
Department of Chemical Engineering, College of Engineering, Dankook University, Jukjeon-dong, Yongin-si, Gyeonggi-do 16890, Korea
- E-mail: limjinik@dankook.ac.kr
- ORCID:
0000-0003-4803-0455










 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.