Hydrogels have potential in many biomedical applications, such as tissue engineering and drug delivery system, mainly because they have similar physical properties and high water content to soft tissues. In this study, we developed novel PEGylated hybrid hydrogels by a combination of methacrylated hyaluronic acid (MeHA) or n-isopropylacrylamide (NIPAM) with PEG-based hydrogels. We synthesized the hybrid hydrogels by free radical polymerization via UV irradiation or mild chemical crosslinking and their physicochemical properties such as swelling ratios, degradation profiles were evaluated. The data indicate that their physicochemical properties can be tunable depending on the types of PEG-based macromers and the amount of PEG macromers. In addition, their biocompatibility was also examined using zebra-fish, which was one of the widely investigated model of animals. The initial behavioral data suggest that the PEGylated hydrogels are biocompatible with tunable physicochemical properties, thus these novel PEGylated hybrid hydrogels may be useful as tissue engineering scaffold or drug delivery system.
하이드로젤은 연조직과 유사한 물리적 특성 및 높은 수분함량을 가지고 있기 때문에 조직 공학 및 약물 전달 시스템과 같은 의료용 분야에서 잠재력을 가지고 있다. 본 연구에서는 methacrylated 히알루론산(MeHA) 또는 n-isopropylacrylamide(NIPAM)와 PEG 기반 하이드로젤을 조합하여 새로운 페길레이티드 하이브리드 하이드로젤을 개발하였다. UV 경화 또는 약한 화학적 가교를 통해 자유 라디칼 중합에 의해 하이브리드 하이드로젤을 합성하고 팽윤율, 분해속도와 같은 물리 화학적 특성을 평가하였다. 데이터는 PEG 기반 고분자의 유형과 PEG 고분자 양에 따라 하이브리드 하이드로젤의 물리 화학적 특성을 조절할 수 있음을 나타내었다. 또한 널리 알려진 모델 동물 중 하나인 zebrafish를 사용하여 합성한 하이드로젤의 생체 적합성도 평가하였다. 초기 독성 데이터는 페길레이티드 하이브리드 하이드로젤이 실험동물의 생체 적합성을 나타내는 것을 알 수 있었고 결과적으로 본 연구에서 합성한 하이브리드 하이드로젤은 조직 공학 지지체 또는 약물 전달 시스템으로 유용하게 사용될 수 있으리라고 사료된다.
PEGylated hybrid hydrogels by a combination of methacrylated hyaluronic acid (MeHA) or n-isopropylacrylamide (NIPAM) with PEG-based hydrogels. The resulting hybrid hydrogels can have tunable physical properties depending on the types of PEG-based macromers and the amount of PEG macromers in the hybrid hydrogel networks. These hydrogels may be useful for tissue engineering and drug delivery applications.
Keywords: PEGylation, hybrid hydrogels, hyaluronic acid, biocompatibility, biodegradability
This work was supported by 2018 Hongik university research fund. The authors acknowledge Dr. Sang Kyu Jung’s laboratory for providing technical help for zebrafish testing. This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2016R1D1A3B01008280).
Despite extensive research and development, tissue engineering strategies has not been successful to precisely restore and repair structure and function of complex tissues, mainly due to lack of appropriate cell sources, biomaterials and/or biomolecules.1,2 Hydrogels have been utilized for many years as favorable biomaterials for tissue engineering applications because of their similarity to soft tissues such as cartilage in terms of high water content and mechanical properties.3,4 Among natural hydrogels, hyaluronic acid (HA) has been extensively explored as tissue engineering scaffolds and drug delivery systems due to its specific chemical properties; wide range of molecular weight, remodeling capability by enzymes or cells, and functionality with reactive groups such as hydroxyl (-OH) and carboxylic acid (-COOH) groups.5,6 However, its relatively low mechanical properties have limited its hard tissue applications including cartilage tissue engineering.5,7 To improve its properties, HA-based composites have been suggested by incorporating other polymers such as collagen,8 poly(lactic-glycolic acid) (PLGA)9 and poly(propylene fumarate).10
On the other hand, among synthetic hydrogels, poly(n-isopropylacrylamide) (PNIPAM) is also widely studied for tissue engineering application due to their excellent thermal sensitivity.11,12 However, it is often difficult to precisely control its mechanical properties and biodegradability.13,14 Therefore, there have been great attention devoted to improve its properties by incorporating other polymers or using different polymerization methods such as controlled radical polymerization (CRP) methods.13,15,16
Meanwhile, polyethylene glycol (PEG)-based biodegradable polymers have been developed as tissue engineering scaffolds and/or drug delivery systems for many years.17,18 However, most PEG-based hydrogels have not been properly used for desired biomedical applications due to extremely high swelling properties and non-biodegradability.19 In our laboratory, we have developed novel biodegradable PEG-based hydrogels by addition of dicarboxylic acids into the backbone to improve their properties such as swelling ratio and cell adhesion.20,21 There have been extensive efforts to improve mechanical properties of HA or NIPAM by copolymerizing with PEG-based polymers such as PEG diacrylate (PEGDA).22,23
Here, we hypothesized that the hybridization of HA or NIPAM with PEG-based biodegradable polymers (e.g., PEG sebacic acid diacrylate or PEGSDA) may improve their properties without compromising their biocompatibility. To test our hypothesis, we developed novel biodegradable hydrogels by combining HA or NIPAM with PEG-based macromers such as PEG sebacic acid diacrylates (PEGSDA) via free radical polymerization and their physicochemical properties (e.g., swelling ratios and degradation profiles) were evaluated. In addition, we also explored behavioral responses of aquatic organisms (e.g., zebrafish) as a method for evaluating the toxicity to biomaterials.
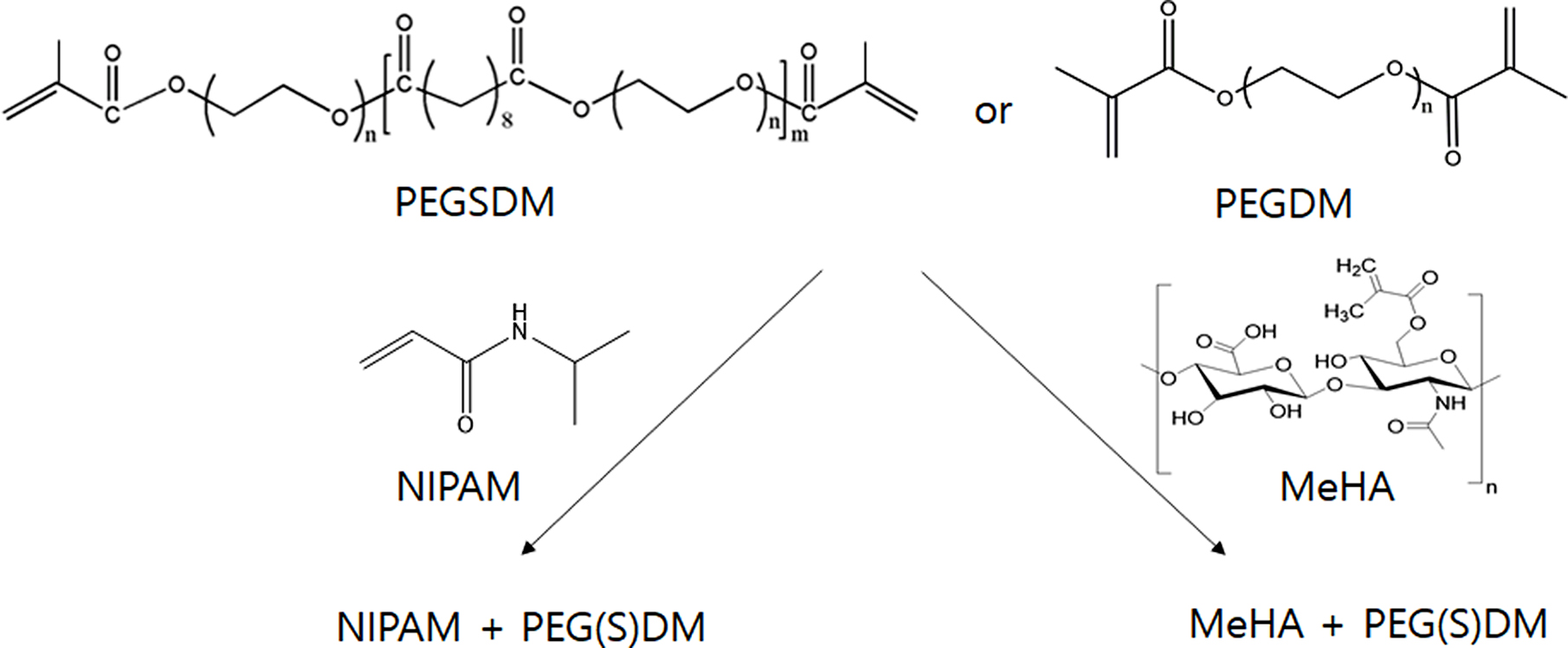
Synthesis and Characterization of PEGylated Hybrid Hydrogels. Hyaluronic acid (HA, MW: ~500kDa) was provided from SK Bioland (Korea) and all chemicals were purchased from Sigma-Aldrich (MO, USA) and used as received. PEGSD(M)A (PEG, MW: 1000kDa) and methacrylated HA (MeHA) was prepared as described previously.20,24 Briefly, for PEG sebacate (PEGS) synthesis, poly(ethylene glycol) (PEG, Mn: 1000Da) was dried by azeotropic distillation and removed residual water under reduced pressure. PEG (1.0 mol) was then dissolved in anhydrous methylene chloride. Dried sebacoyl chloride (0.9 mol) and triethylamine (1.5 mol) were subsequently added into the solution with vigorous stirring. The reaction was run for 24 h at room temperature, and the product (PEGS) was obtained after precipitation in petroleum ether. Similarly, PEGSDM was synthesized by adding 1.5 mols of methacryloyl chloride and triethylamine (TEA) into the PEGS (1.0 mol) solution, the reaction was run for another 24 h, and the resulting polymer (PEGSDM) was obtained after precipitation in petroleum ether and drying in vacuum overnight. PEGSDA was synthesized similarly to PEGSDM, except for using acryloyl chloride instead of using methacryloyl chloride. For MeHA synthesis, glycidyl methacrylate was added to an aqueous solution of 1% (w/v) HA (pH 9.0) with 1.1 molar ratio of glycidyl methacrylate to the primary amine groups in HA then proceed to react via gentle shaking at room temperature for 36 h. The reaction mixture was then neutralized, dialyzed against water for 15h using membrane with cutoff molecular weight of 10 kDa and lyophilized for further studies. The degree of methacrylation to PEGSDM and MeHA was determined via proton nuclear magnetic resonance (1H NMR, 300MHz, Jeol, Japan) spectrometry.
PEGylated HA were prepared via free radical polymerization under mild ultraviolet light (UV) in the presence of a photoinitiator (Irgacure 2959) after mixing of 0.03 g PEGSD(M)A with the same amount of MeHA in 1mL of deionized water (Figure 1). PEGylated NIPAM were also prepared via free radical irradiation with ascorbic acid (AA) and ammonium persulfate (APS). To incorporate different amount of PEGSDM into the hybrid hydrogels, up to 67% of PEGSDM was into the NIPAM solution. The surface morphology of the hydrogels was observed using a scanning electron microscopy (SEM, Teskan, Czech Republic). The hydrogels were lyophilized, and surface morphology was imaged in low vacuum mode.
Physicochemical Properties of Hybrid Hydrogels. After crosslinking, the samples were incubated in phosphate-buffered saline (PBS, pH 7.4) at designated temperatures. Swelling ratios of PEGylated hybrid hydrogels were determined by measuring the initial weight of the samples (Wi) and the swollen weight of the samples (Ws) at different time points and different temperatures. In the separate experiments, degradation profiles of PEGylated hybrid hydrogels were determined by measuring the initial dry weight (Wi) and the final dry weight (Wd) of the samples at each time period in PBS at different temperatures. Finally, zebrafish larvae were used as a model organism to examine the biocompatibility of the hybrid hydrogels. The hybrid hydrogel solutions (1% w/v) were injected into each tissue culture well containing zebrafish and the movement of zebrafish was captured by taking images by a camera in real-time.

|
Figure 1 Schematic diagram of PEGylated hybrid hydrogels. First, PEGSDM was prepared by adding PEG and sebacoyl chloride and then PEGSDM were subsequently synthesized by adding methacryloyl chloride. PEGDM was also synthesized by adding PEG and methacryloyl chloride.20 Subsequently, PEGylated hybrid hydrogels were formed by UV irradiation (MeHA+PEGSDM) or chemical redox initiators (NIPAM+PEGSDM). |
In this study, novel PEGylated hybrid hydrogels were synthesized by combining HA or NIPAM with biodegradable PEG based polymers (PEGSD(M)A) via free radical polymerization and their physicochemical properties were evaluated. Various PEGylated hybrid hydrogels were prepared by using nondegradable PEG-based hydrogels (PEGDA or PEGDM) or degradable PEG-based hydrogels (PEGSDA or PEGSDM) (Figure 1). After crosslinking, the microscopic appearance of the hydrogels revealed that the surface of PEGylated HA hydrogels became smoother and had denser and more compact surface morphology as compared with MeHA hydrogel alone, especially in the case of adding biodegradable PEGSDA(or PEGSDM) (Figure 2). The difference in the surface morphology between MeHA and PEGylated MeHA may be due to greater crosslinking density of PEGylated hybrid hydrogels than that of MeHA alone, thus the hybrid hydrogels have more compact network structure.25
Swelling Ratio. Swelling ratio data indicate that PEGylated HA hydrogel significantly reduced swelling of the hydrogels, especially when added PEGSDA hydrogels into HA hydrogel network (Figure 3). These results well corresponded to the surface morphology data mentioned above since when added PEG-based hydrogels, then the hybrid hydrogels increased surface network density, thus less absorb water into the network.26 In addition, all MeHA containing hydrogels displayed a typical temperature-dependent swelling behavior, which was demonstrated by the decrease in swelling ratios as temperature increases (Figure 3).27
For NIPAM hydrogels, the addition of PEGDM decreased swelling ratio as well and the swelling ratio of the PEGylated NIPAM hydrogels also decreased with the increase of temperature as compared with PEGylated MeHA hybrid hydrogel results (Figure 4). However, at temperatures above low critical solution temperature (LCST) of ~32℃, swelling ratios of PEGylated NIPAM with PEGSDM were very small and not much different among tested hybrid hydrogels, possibly because the hydrogels shrunk above LCST and the structure became similar as reported by others.23,26,28 When added PEGDM into NIPAM hydrogels, similar temperature-dependent behavior in swelling ratio was found, however the extent of swelling ratio was much greater, compared to the hydrogels with PEGSDM mainly due to the hydrophobic segments in PEGSDM, which significantly reduced water absorption into the hydrogel network.20
Degradation Profiles. Regarding degradation profiles of the hybrid hydrogels, PEGylation degreased the degradation rate of the hybrid HA hydrogels as compared with HA hydrogel alone. The addition of PEGDM into HA hydrogels further decreased the degradation rate of the hydrogels, compared with PEGSDM addition (Figure 5), possibly due to the increase of crosslinking density of the hybrid hydrogels as seen in the SEM data. There appeared to have no significant effects of temperature on degradation rate of the hybrid HA hydrogels.
Degradation profiles of PEGylated NIPAM hybrid hydrogels were also measured up to 63 days (9 weeks) and the data showed a much slower degradation rate, compared with PEGylated HA hydrogels as expected, mainly due to the difference in hydrophilicity between HA and NIPAM (Figure 6). It is important to note that the degradation data revealed that PEGylated NIPAM hybrid hydrogels can be biodegradable by the incorporation of biodegradable PEGSDM hydrogels into PNIPAM network. Biodegradable hybrid NIPAM hydrogels may be useful as tissue engineering scaffold and/or drug delivery system, because PNIPAM itself is not biodegradable, which may induce chronic foreign body reaction after serving its function for biomedical applications.16,29
Toxicity Test Using Zebrafish. Zebrafish has been used for decades as a method for aquatic toxicity monitoring.30 In this study, zebrafish was used as model organism to test toxicity of PEGylated hybrid hydrogels. The behavior of the fish was monitored by a camera. All zebrafishes in the wells containing hybrid hydrogel macromers (1% w/v), survived and there was no abnormal appearance after 16h of time periods (Figure 7). While this early time period may not be enough to assess the toxicity of the hydrogels and more systematic toxicity studies need to be done,31 these preliminary in vivo data appeared to show that the hybrid hydrogels showed minimal toxicity to the organism. Currently, quantitative behavioral analysis is underway by measuring moving activities using an image analysis. Overall, the data demonstrate that PEGylated hybrid hydrogels have tunable physicochemical properties and appeared to have no adverse effects on their biocompatibility.

|
Figure 2 SEM images of various PEGylated hybrid hdyrogels. Scale bar = 200 mm. MeHA: Methacrylated hyaluronic acid, PEGDM: Poly(ethylene glycol) (PEG) dimethacrylate, PEGSDA: PEG sebacic acid diacrylate, PEGSDM: PEG sebacic acid dimethacrylate, NIPAM67+PEGSDM33; NIPAM 67 wt% + PEGSDM 33 wt% in total macromer amounts in the hydrogels. |

|
Figure 3 Swelling ratios of PEGylated MeHA hybrid hydrogels at different temperature. Swelling ratio was determined as (Ws – Wi)/ Wi, where Ws was swollen weight of hydrogels and Wi was initial weight. Error bars represents means±standard deviation between the measurements (n=3). |

|
Figure 4 Swelling ratios of PEGylated NIPAM hybrid hydrogels at different temperatures. Swelling ratio was determined as (Ws – Wi)/Wi, where Ws was swollen weight of hydrogels and Wi was initial weight. Error bars represents means±standard deviation between the measurements (n=3). (A) PEGylated NIPAM hydrogels with PEGSDM, NIPAM67+PEGSDM33 stands for NIPAM 67 wt%+PEGSDM 33 wt% in total macromer amounts in the hydrogels; (B) PEGylated NIPAM hydrogels with PEGDM. |

|
Figure 5 Degradation profiles of PEGylated MeHA hybrid hydrogels at different temperature in PBS at 37 ℃. Mass remaining (%) was determined as (Wi – Wd)/Wd×100, where Wi was initial dry weight of hydrogels and Wd was dry weight of hydrogels at various time periods. Error bars represents means±standard deviation between the measurements (n=5). |

|
Figure 6 Degradation profiles of PEGylated NIPAM hybrid hydrogels at room temperature. Mass remaining (%) was determined as (Wi – Wd)/Wd×100, where Wi was initial dry weight of hydrogels and Wd was dry weight of hydrogels at various time periods. Error bars represents means±standard deviation between the measurements (n=3). |

|
Figure 7 Zebrafish appearance in the wells containing various PEGylated hybrid hydrogels. |
Novel PEGylated hybrid hydrogels with tunable properties were synthesized and their physical properties such as swelling ratios and degradation rate were characterized. For MeHA hydrogels, the PEGylation increased surface density and decreased swelling ratio and degradation rate. For NIPAM hydrogels, PEG incorporation decreased swelling ratio as the amount of PEG incorporation increased and PEGylation also made NIPAM hydrogels biodegradable over time. These data demonstrated that novel hybrid hydrogels containing PEG-based macromers can have tunable physical properties depending on the types of PEG-based macromers and the amount of PEG macromers. In addition, initial zebrafish toxicity data appeared to show minimal toxicity of the hybrid hydrogels. Overall, the data indicate that novel biodegradable hybrid hydrogels may be useful as tissue engineering scaffolds and/or drug delivery system.
- 1. Hubbell, J. A. Biomaterials in Tissue Engineering. Bio-Technology 1995, 13, 565-576.
-

- 2. Ratner, B. D.; Bryant, S. J. Biomaterials: Where We have been and Where We are Going. Annu. Rev. Biomed. Eng. 2004, 6, 41-75.
- 3. Hoffman, A. S. Hydrogels for Biomedical Applications. Adv. Drug Deliv. Rev. 2002, 54, 3-12.
- 4. Choi, B.; Kim, S.; Lin, B.; Li, K.; Bezouglaia, O.; Kim, J.; Evseenko, D.; Aghaloo, T.; Lee, M. Visible-light-initiated Hydrogels Preserving Cartilage Extracellular Signaling for Inducing Chondrogenesis of Mesenchymal Stem Cells. Acta Biomater. 2015, 12, 30-41.
-

- 5. Kim, I. L.; Mauck, R. L.; Burdick, J. A. Hydrogel Design for Cartilage Tissue Engineering: A Case Study with Hyaluronic Acid. Biomaterials 2011, 32, 8771-82.
-

- 6. Schante, C.; Zuber, G.; Herlin, C.; Vandamme, T. Chemical Modifications of Hyaluronic Acid for the Synthesis of Derivatives for a Broad Range of Biomedical Applications. Carbohydr. Polym. 2011, 85, 469-489.
-

- 7. Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-Based Hydrogels as Scaffolds for Tissue Engineering Applications: A Review. Biomacromolecules 2011, 12, 1387-1408.
-

- 8. Tang, S.; Vickers, S.; Hsu, H.; Spector, M. Fabrication and Characterization of Porous Hyaluronic Acid-collagen Composite Scaffolds. J. Biomed. Mater. Res. Part A 2007, 82A, 323-335.
-

- 9. Chang, N.; Jhung, Y.; Yao, C.; Yeh, M. Hydrophilic Gelatin and Hyaluronic Acid-treated PLGA Scaffolds for Cartilage Tissue Engineering. J. Appl. Biomater. Funct. Mater. 2013, 11, 45-52.
-

- 10. Liao, E.; Yaszemski, M.; Krebsbach, P.; Hollister, S. Tissue-Engineered Cartilage Constructs Using Composite Hyaluronic Acid/Collagen I Hydrogels and Designed Poly(Propylene Fumarate) Scaffolds. Tissue Eng. 2007, 13, 537-550.
-

- 11. Francis, R.; Baby, D.; Kumar, D. Poly(N-isopropylacrylamide) Hydrogel: Effect of Hydrophilicity on Controlled Release of Ibuprofen at Different pH. J. Appl. Polym. Sci. 2012, 124, 5079-5088.
-

- 12. Kang, M.; Hong, S.; Kim, J. Release Property of Microgels Formed by Electrostatic Interaction between Poly(N-isopropyl-acrylamide-co-methacrylic acid) and Poly(N-isopropylacrylamide-co-dimethylaminoethylmethacrylate). J. Appl. Polym. Sci. 2012, 125, 1993-1999.
-

- 13. Galperin, A.; Long, T.; Ratner, B. Degradable, Thermo-Sensitive Poly(N-isopropyl acrylamide)-Based Scaffolds with Controlled Porosity for Tissue Engineering Applications. Biomacromolecules 2010, 11, 2583-2592.
-

- 14. Rennerfeldt, D.; Renth, A.; Talata, Z.; Gehrke, S.; Detamore, M. Tuning Mechanical Performance of Poly(ethylene glycol) and Agarose Interpenetrating Network Hydrogels for Cartilage Tissue Engineering. Biomaterials 2013, 34, 8241-8257.
-

- 15. Kim, S.; Healy, K. Synthesis and Characterization of Injectable Poly(N-isopropylacrylamide-co-acrylic acid) Hydrogels with Proteolytically Degradable Cross-links. Biomacromolecules 2003, 4, 1214-1223.
-

- 16. Zhang, X.; Sun, G.; Wu, D.; Chu, C. Synthesis and Characterization of Partially Biodegradable and Thermosensitive Hydrogel. J. Mater. Sci.-Mater. Med. 2004, 15, 865-875.
-

- 17. Hern, D. L.; Hubbell, J. A. Incorporation of Adhesion Peptides into Nonadhesive Hydrogels Useful for Tissue Resurfacing. J. Biomed. Mater. Res. 1998, 39, 266-276.
-

- 18. Drury, J. L.; Mooney, D. J. Hydrogels for Tissue Engineering: Scaffold Design Variables and Applications. Biomaterials 2003, 24, 4337-4351.
-

- 19. Lee, K.; Rowley, J.; Eiselt, P.; Moy, E.; Bouhadir, K.; Mooney, D. Controlling Mechanical and Swelling Properties of Alginate Hydrogels Independently by Cross-linker Type and Cross-linking Density. Macromolecules 2000, 33, 4291-4294.
-

- 20. Kim, J.; Lee, K. W.; Hefferan, T. E.; Currier, B. L.; Yaszemski, M. J.; Lu, L. Synthesis and Evaluation of Novel Biodegradable Hydrogels Based on Poly(ethylene glycol) and Sebacic Acid as Tissue Engineering Scaffolds. Biomacromolecules 2008, 9, 149-157.
-

- 21. Kim, J.; Yaszemski, M. J.; Lu, L. Development of Biodegradable and Injectable Macromers Based on Poly(ethylene glycol) and Diacid Monomers. J. Biomed. Mater. Res. 2009, 90, 1010-1020.
-

- 22. Park, Y.; Tirelli, N.; Hubbell, J. Photopolymerized Hyaluronic Acid-based Hydrogels and Interpenetrating Networks. Biomaterials 2003, 24, 893-900.
-

- 23. Son, K.; Lee, J. Synthesis and Characterization of Poly(Ethylene Glycol) Based Thermo-Responsive Hydrogels for Cell Sheet Engineering. Materials 2016, 9, 854.
-

- 24. Seidlits, S.; Khaing, Z.; Petersen, R.; Nickels, J.; Vanscoy, J.; Shear, J.; Schmidt, C. The effects of Hyaluronic Acid Hydrogels with Tunable Mechanical Properties on Neural Progenitor Cell Differentiation. Biomaterials 2010, 31, 3930-3940.
-

- 25. Hou, X.; Yang, W.; Li, A.; Hou, J.; Zhang, C. Effects of Incorporating Acrylolsobutyl Polyhedral Oligomeric Silsesquioxane on the Properties of P(N-isopropylacrylamide-co-poly(ethylene glycol) diacrylate) Hybrid Hydrogels. Polym. Bull. 2017, 74, 1831-1847.
-

- 26. Li, P.; Hou, X.; Qu, L.; Dai, X.; Zhang, C. PNIPAM-MAPOSS Hybrid Hydrogels with Excellent Swelling Behavior and Enhanced Mechanical Performance: Preparation and Drug Release of 5-Fluorouracil. Polymers 2018, 10, 137.
-

- 27. Zhang, X.; Li, C.; Hu, Y.; Liu, R.; He, L.; Fang, S. A Novel Temperature and pH Dual-responsive Hybrid Hydrogel with Polyhedral Oligomeric Silsesquioxane as Crosslinker: Synthesis, Characterization and Drug Release Properties. Polym. Int. 2014, 63, 2030-2041.
-

- 28. Chen, S.; Jiang, L.; Dan, Y. Preparation and Thermal Response Behavior of Poly(N-isopropylacrylamide-co-acrylic acid) Microgels via Soap-Free Emulsion Polymerization Based on AIBN Initiator. J. Appl. Polym. Sci. 2011, 121, 3322-3331.
-

- 29. Ratner, B. D.; Hoffman, A. S.; Schoen, F. J.; Lemons, J. E. Biomaterials Science : An Introduction to Materials in Medicine, 3rd ed.; Academic Press: San Diego, CA, 2013.
- 30. Melvin, S.; Wilson, S. The Utility of Behavioral Studies for Aquatic Toxicology Testing: A Meta-analysis. Chemosphere 2013, 93, 2217-2223.
-

- 31. Fako, V.; Furgeson, D. Zebrafish as a Correlative and Predictive Model for Assessing Biomaterial Nanotoxicity. Adv. Drug Deliv. Rev. 2009, 61, 478-486.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2021; 45(1): 171-177
Published online Jan 25, 2021
- 10.7317/pk.2021.45.1.171
- Received on Nov 10, 2020
- Revised on Dec 2, 2020
- Accepted on Dec 4, 2020
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Jinku Kim
-
Department of Bio and Chemical Engineering, Hongik University, Sejong 30016, Korea
- E-mail: jinkukim@hongik.ac.kr
- ORCID:
0000-0002-8379-7208










 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.