- Comparing Waste Gypsum with CaCO3 as Filler Towards Developing Low-cost PBAT Composites
Tae Woong Kong*, **,# , In Tae Kim*,# , Junho Moon*, Tridib Kumar Sinha*, Dong Ho Kim**, Inseon Kim***, Kwangyong Na***, Kyum Woo Choi****, and Jeong Seok Oh*,†

*Department of Materials Engineering and Convergence Technology, ERI, Gyeongsang National University, 501 Jinju-daero, Jinju 52828, Korea
**Space-Aeronautics & Advanced Non-Metal Material Center, Jeonnam Technopark, 1448-345 Goheungman-ro, Goheung 59532, Korea
***Namhae chemical corp., 1384 Yeosusandan-ro, Yeosu-si 59618, Korea
****JS TECH Co., Ltd, Sacheon Industrial Complex, 158 Oegukgieop-ro, Sanam-Myun, Sacheon 52530, Korea- 저비용 PBAT 복합체 제조를 위한 폐석고와 탄산칼슘 충전제 비교 연구
공태웅*, **,# · 김인태*,# · 문준호* · Tridib Kumar Sinha* · 김동호** · 김인선*** · 나광용*** · 최겸우**** · 오정석*,†

*경상대학교 나노신소재융합공학과, **전남테크노파크 우주항공 첨단소재센터, ***남해화학, ****제이에스테크
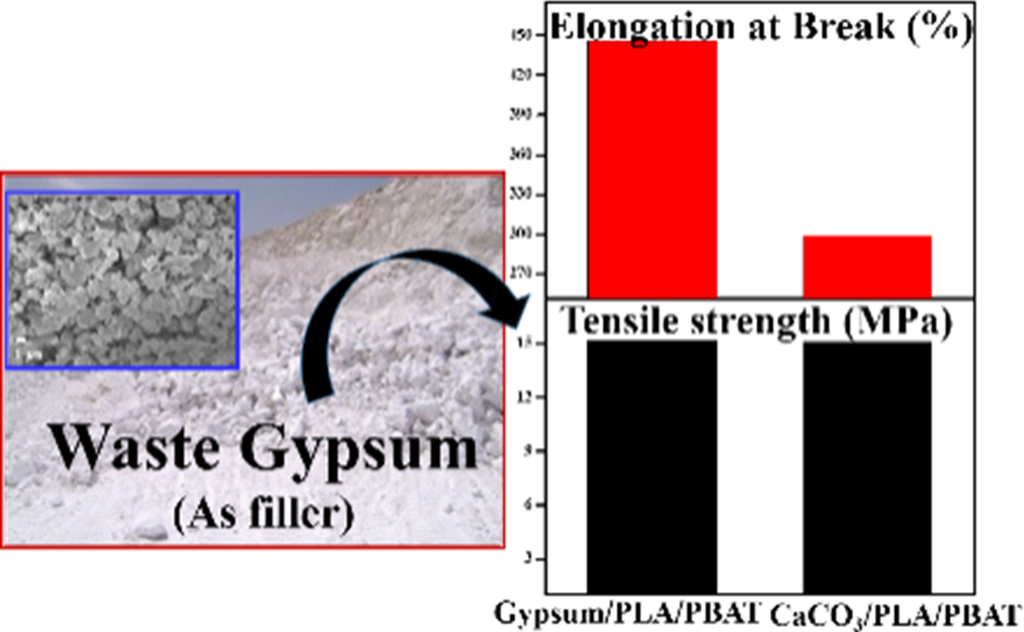
Melt-mixed composites of poly(butylene adipate-co-terephthalate) (PBAT) with the waste gypsum and calcium carbonate were prepared and their properties were compared. Calcium carbonate and gypsum contain the same cation (Ca2+) but different anions i.e., smaller CO32- and larger SO42- respectively. According to the Fajans’ rule, CaCO3 is more ionic whereas gypsum is more polarizable. Because of the abundant polarizability, gypsum was well dispersed in the PBAT matrix through the filler-matrix electrostatic interaction. The gypsum/PBAT showed lower tensile strength but higher elongation at break at a filler to matrix ratio of 20: 80 than those of CaCO3/PBAT. Further, the addition of polylactic acid (PLA) enhanced both the tensile strength and elongation at break of the gypsum/PLA/PBAT while no such effective changes were observed for CaCO3/PLA/PBAT maybe due to the possibility of depolymerization of polymers in the presence CaCO3. Thermal, morphological, and crystallography studies well corroborate with the mechanical behavior of the composites.
폐석고(CaSO4) 및 탄산칼슘(CaCO3)을 이용하여 poly(butylene adipate-co-terephthalate)(PBAT) 복합체를 용융 혼합으로 제조한 뒤 비교하였다. 석고와 탄산칼슘은 Ca2+ 양이온을 동일하게 포함하지만 다른 크기의 음이온 즉, 각각 SO42-, CO32-을 함유하고 있다. Fajans’ rule에 따르면 CaCO3는 이온성이 더 큰 반면, CaSO4는 편극성이 더 크다. 석고는 탄산칼슘에 비해 편극성이 크기 때문에 filler-matrix간 정전기적 상호작용에 의해 PBAT 내에 분산이 더 잘 되었다. 석고/PBAT 복합체는 충전제-고분자 함량 20:80의 비율에서 탄산칼슘/PBAT 복합체 대비 낮은 인장강도와 높은 연신율 값을 나타냈다. Polylactic acid(PLA)를 첨가함에 따라 석고/PLA/PBAT 복합체는 인장강도와 연신율이 향상했지만 탄산칼슘/PLA/PBAT 복합체의 경우에는 PLA의 첨가에 따른 효과가 관찰되지 않았다. 이는 탄산칼슘에 의한 PLA의 depolymerization에 기인하는 것으로 사료된다. 이러한 복합체의 기계적 거동은 열적, 형태학적, 결정학적 분석을 통해 잘 설명되었다.
Melt-mixed composite of PBAT with waste gypsum was found to exhibit better mechanical performance than that of the calcium carbonate (CaCO3), which may be due to the abundant polarizability of gypsum and its better dispersion within the PBAT matrix. Further, addition of PLA enhanced both the tensile strength and elongation at break of gypsum/PLA/PBAT while no such effective changes were observed for CaCO3/PLA/PBAT.
Keywords: poly(butylene adipate-co-terephthalate), polylactic acid, gypsum, recycling, melt-mixing
This work is results of a study on the Economic Cooperation and Development Project (P0002141), supported by the Ministry of Trade, Industry and Energy and the BK21 Plus Program (Future-oriented innovative brain raising type, 21A20151713274) funded by the Ministry of Education (MOE, Korea) and National Research Foundation of Korea (NRF).
Fast-spaced population growth significantly increases the use of petro-polymer derived plastic products in our society. But in consideration with the current environmental concerns e.g. environmental pollution, greenhouse gas emissions, and the depletion of fossil resources, different bio-polymers (e.g., starch, polylactic acid (PLA), polyhydroxyalkanoates, poly (butylene adipate-co-terephthalate) (PBAT), and poly(butylene succinate), etc.) are being considered as promising alternatives to the traditional petro-polymers.1-5 In this regard, attempts have been taken to develop different bio-degradable and bio-compatible polymers based disposal products such as bags, toys, cables, automotive parts, textile products (tees), packaging for food, medicine, cosmetic, hygiene products (nappies), foils for agriculture and cutlery, and etc. Among the numerous kinds of bio-polymers, PLA, a thermoplastic aliphatic polyester obtained from renewable resources e.g. corn starch and sugarcane, is currently considering as the most promising and popular material for developing ‘green’ eco-friendly material.4-10 However, the maximum of the bio-polymers is cost-intensive and mechanically poor.1,11 Particularly, the PLA has associated with different demerits e.g. brittleness, low heat distortion temperature, poor processability, etc., although possessing high modulus and strength comparable to that of many petro-polymers.4,7,8,12-14 Generally, plasticizers are required to improve the property of PLA,12,13 but there are some limitations regarding the processing of PLA with plasticizer (especially during melt mixing), and the safety of the food products when the PLA is used for packaging purpose. Instead of plasticizers, attempts are being taken to blend the PLA with other bio-polymers to improve its mechanical property. In this regard, immense attention has been paid to PBAT for blending with PLA.13-17 Basically, the PBAT is a commercially available aliphatic-aromatic co-polyester and exhibits high flexibility and toughness but low modulus. One more important property of PBAT is that it can fully degrade within a few weeks in the presence of an enzyme.17 Compared to the other polymers, PLA is still an expensive polymer.11,18 So, otherwise blending the PBAT with PLA matrix, it is supposed to be better to blend PLA with PBAT matrix to meet a bio-degradable PLA/PBAT composite of improved mechanical properties. Although both the PLA and PBAT are polyester in nature, they are incompatible. As a consequence, various attempts have been taken to make the blend using different compatibilizers.11-16 Other approaches improving the compatibility of the two components have been reported: Ultrasound irradiation, cross-linkers (e.g., dicummyl peroxide (DCP)), etc. However there is always a chance of chain scission (or depolymerization) which may reduce the intrinsic properties of the polymers.15 In spite of all these aforementioned pros and cons, the production cost of these polymers limits their large scale industrial upscaling for different applications e.g. in the area of agriculture and cutlery, where the bio-degradability of the polymer products are typically advantageous.4 Although the petro-polymers based agricultural plastics (e.g., low-density polyethylene (LDPE)) used for greenhouses, mulching, and silage become a popular and economically beneficial technique, longer lifetime of these waste plastics causes pollution in the environment and damages the soil quality. Also, their yearly removal and disposal cause the whole agricultural process more cost-intensive. In this regard, the production of low-cost bio-degradable polymer composite is being attempted through low-cost reinforcements by different fillers e.g. natural fibers, common minerals, etc.4,18 For mineral reinforced polymer composite based agricultural plastic, one additional achievement may be the enrichment of minerals in the soil after bio-degradation. Among different reinforcing minerals, calcium carbonate (CaCO3) became popular because of its low-cost and abundant availability.4 Because of its ionic characteristic, CaCO3 is expected to well mix with both the PLA and PBAT through polar-polar interaction and act as a compatibilizer between the incompatible PLA and PBAT. But, its catalytic efficacy during melt mixing of the polymers results in depolymerization, causing deterioration of the polymer’s intrinsic property.4 Another expected disadvantage is that during biodegradation, the pH of the soil can be altered due to presence of the CaCO3.19,20 In contrast to CaCO3, another mineral with the same cation (i.e., Ca2+) is gypsum (CaSO4). The gypsum is produced in a huge amount as a by-product from the phosphoric acid production industry.21 Being a waste material, it may cause serious ecological problems, and its conservation can be very much unpleasant to the society. Therefore, it is necessary to use this waste material for developing some value-added products. In contrast to CaCO3, its anion size is bigger. According to Fajans’ rule, thus it will be less ionic and more polarizable.22,23 Also, it has no such catalytic efficacy on the depolymerization process, so that the intrinsic property of the polymers seems to be unaltered. Another additional advantage is that the gypsum may upgrade the soil quality.24 Considering these advantages, herein we have investigated the effect of gypsum on formulating the composites of PBAT with or without the PLA towards the production of low-cost biodegradable polymer products with improved mechanical properties, which shows promising for the industrial upgrading of gypsum-based polymer composites.
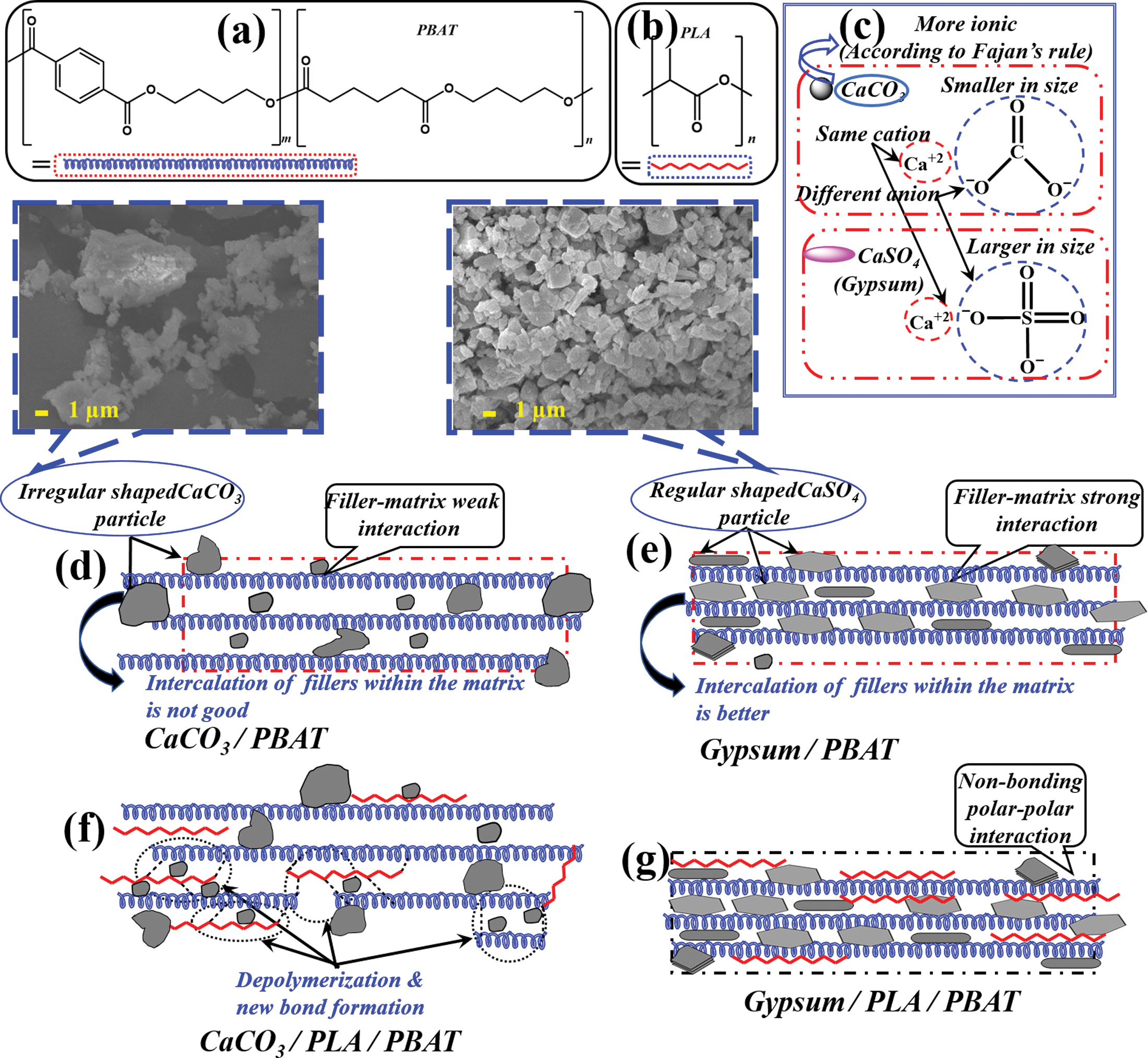
Materials. The polybutylene adipate terephthalate (PBAT) (Solpol 1000) was purchased from GIOSOL Ltd. (Korea). The polylactic acid (PLA; 2003D) was purchased from Nature Works LLC. (Korea). The by-product gypsum was first treated with inorganic acid, adjusted the neutral pH by washing with water and dried to remove the impurities and water content, and then supplied by Namhae Chemical Co.Ltd. (Korea). The CaCO3 was purchased from Omya Co.Ltd. (Korea). Gypsum was found to be regular in shape and size, whereas CaCO3 was irregular in shape and size, as observed by the field emission scanning electron microscopy (shown in Figure 1).
Preparation of the Composites. At first, the dried polymers were premixed with the dried CaCO3 and gypsum powder of various quantity (20, 30, and 40 wt% of total quantity) by tumbling. Then, the mixtures were compounded by a co-rotating twin screw extruder (BA-19, BauTek). The screw rotation velocity was 80 rpm, and the temperature of zones from hopper to die were 130, 140, 150, 160, 170, 180, 190, and 190°C during preparation of the composites made of only PBAT and the PLA mixed PBAT. The extruded blends were cooled in water bath and pelletized. The pelletized blends were then injection-molded by an injection-molding machine (WL-HV-80, Wonil Hydraulic). The injecting temperature of zones from hopper to nozzle were 130, 140, 150, and 150°C, and 160, 165, 170, and 170°C for the PBAT and PLA/PBAT composites, respectively. The filler contents were kept constant i.e., 20 wt% for PLA/PBAT composite whereas the PLA content was varied as 5, 7.5, and 10wt% of the total quantity.
Characterizations. The universal tensile tester (Tensometer 2000, Myung Ji tech, Korea) was used to investigate the mechanical properties of the composites. The tensile strength and elongation at break were measured according to the ASTM D638 standard. All tests were carried out under crosshead speed of 50mm/min.
The thermal properties of composites were measured by using thermogravimetric analysis (TGA) and diffential scanning calorimetry (DSC). For TGA, the samples were heated to 600℃ with a heating rate of 20℃/min in nitrogen atmosphere. For DSC, the samples were heated to 200℃ with a heating rate of 10℃/min and held for 5 min to remove the thermal history. Then, the samples were cooled down with a cooling rate of 10℃/min to 30℃ and finally heated with a heating rate of 10℃ to 200℃. The degree of crystallinity of composites was calculated as follows (eq. (1)).
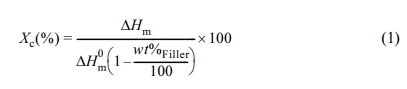
For each component in the composites, Xc is the degree of crystallinity of PBAT or PLA, respectively. The heat of fusion of 100% crystalline PBAT (ΔH0m)s 114, respectively. The heat required for melting the crystalline phase of polymers (ΔHm) was measured by DSC.
The field emission scanning electron microscopy (FE-SEM) (JSM-7610F, JEOL, Japan) was used to investigate the morphologies of the fillers and the composites. Further, to realize the dispersibility of the fillers in polymer matrix, X-ray micro-computed tomography (Skyscan 2214, Bruker Co., USA) was used. The X-ray source was applied at a voltage of 100 kV and a current of 60μA under normal pressure for an exposure time of 700 ms. The X-ray projections were recorded with the rotation increment of 0.15° within 360°.
|
Figure 1 Molecular structure of PBAT (a) and PLA (b); (c) size effect of anion (according to Fajan’s rule) regarding to the stronger ionic character of CaCO3 with respect to gypsum; (d) weak intercalation of CaCO3 due to the irregular shape, and weak filler-matrix interaction (because of more ionic character of the CaCO3); (e) better intercalation of gypsum due to its regular shape, and strong filler-matrix interaction (because of less ionic or more polarizable character of gypsum); (f) plausible depolymerization followed by new bond formation in CaCO3/ PLA/PBAT composite; (g) non-bonding polar-polar interaction among the fillers and matrix in gypsum/PLA/PBAT composite. |
In this work, we have considered the flexible PBAT as the base polymer instead of the brittle PLA.16 Although, both the polymers are polyester in nature, the existence of a long aliphatic chain makes the PBAT more flexible (as shown in Figure 1(a) and 1(b)). Because of abundant polarity, the ester group is potentially enough to electrostatically interact with the Ca-salts of CaCO3 and gypsum (CaSO4). The interaction efficacy is expected to be high for PBAT containing a higher number of ester groups. Among these two Ca-salts, because of the larger size of SO4-2 than that of CO3-2, CaCO3 is expected to execute more ionic characteristics whereas the gypsum is more polarizable according to the Fajans’ rule (as depicted in Figure 1(c)). Inset of Figure 1(d) and 1(e) show the morphology of CaCO3 and gypsum used in this work. The CaCO3 exhibits irregular shape and size whereas gypsum exhibits regular morphology (flakes and needle shape). As a consequence of more polarizability and regular morphology, there is a possibility of better intercalation of gypsum in the polymer matrix through the non-covalent polar-polar interaction. The non-covalent interaction being reversible, the dissipation of energy is highly possible for gypsum/PBAT composite during the application of mechanical force (e.g., uniaxial stretching).25 On the other hand, due to the irregular shape and size, and weak filler-matrix interaction, the improper intercalation of CaCO3 is expected to produce an inhomogeneous dispersion. Because of the more ionic character, the bonding between the cation and anion is stronger for CaCO3. As a result, the non-covalent interaction with the polymer is expected to be less for CaCO3, in comparison to that of the gypsum. But, due to the stronger bonding, the intercalated CaCO3 is expected to provide more strength to the composite. Because of weak filler-matrix interaction and the possibility to exhibit more strength, the composites containing CaCO3 are expected to be more fragile and less flexible. It is noteworthy to mention here that because of the abundant intermolecular interaction, the gypsum/PBAT composite is supposed to exhibit better thermal stability and higher crystallinity than that of CaCO3/PBAT composite. When the PLA has been added, the property of gypsum/PLA/PBAT and CaCO3/PLA/PBAT were expected to show different properties because it has been reported that there is a possibility of depolymerization followed by intermolecular crosslinking of polymers (especially PLA) during melt processing in presence of CaCO3.4 In the following section corresponding mechanical properties are briefly described.
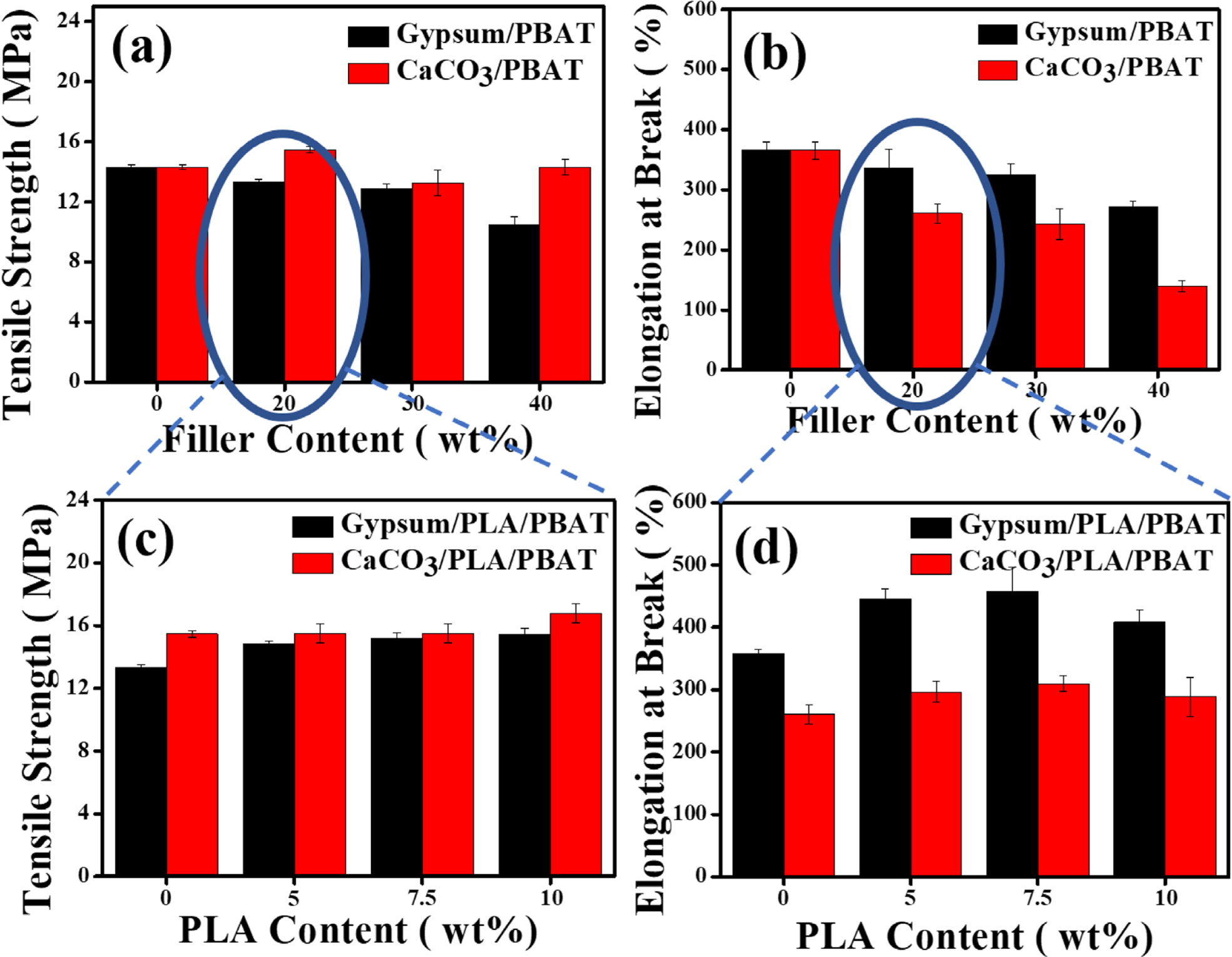
Mechanical Property. The tensile tests of the samples were performed under uniaxial disposition (strain) according to the ASTM D638 standard. The results of the comparative tensile strength and the elongation at break are shown in Figure 2(a) and 2(b) (for gypsum/PBAT and CaCO3/PBAT composites), Figure 2(c) and 2(d) (for gypsum/PLA/PBAT and CaCO3/PLA/PBAT composites), respectively. It can be observed that in comparison to the gypsum/PBAT, although the tensile strength of CaCO3/PBAT composite is increased, the elongation at break is decreased for 20 wt% of the filler content. The higher tensile strength for 20wt% CaCO3/PBAT is a consequence of the high bonding (or ionic) strength of the CaCO3. While the tensile strength increases with the addition of CaCO3, the elongation of break decreases due to the rigidity of the composite. In comparison to the CaCO3/PBAT, the gypsum/PBAT composite showed higher value of elongation at break while with slight decrease of tensile strength. CaCO3 is well known for its catalytic efficacy towards the “depolymerization” of polymer matrix.4 Signori et al. mentioned that generally the melt processing lead to chain scission of PLA and PBAT, with reduction of molecular weight, above 150℃.26 As a consequence, the formation of stable chain-ends with carbonyl and hydroxyl groups or reactive end groups is highly expected during melt-processing of PLA/PBAT in presence of CaCO3. So, in the case of CaCO3, there is a possibility towards formation of new bonds in CaCO3/PLA/PBAT composite. Whereas, in the case of gypsum, there is no such possibility, only the polar-polar interaction among the molecules of gypsum, PLA and PBAT is supposed to happen. As a consequence of the plausible depolymerization followed by new bond formation, the tensile property of the CaCO3/PLA/PBAT composite remained nearly unaltered (as shown in Figure 2(c) and 2(d), red column) with increasing the PLA content. In contrast, the gypsum/PLA/PBAT composite shows increased tensile strength (because of addition of PLA) as well as increased elongation at break (because of non-covalent interaction among the filers and matrix) (as shown in Figure 2(c) and 2(d), black column), resulting in mechanically better performances of gypsum/PLA/PBAT composite. The mechanical properties of both the composite are promising for the PLA content of 7.5 wt%. Thus, herein we prioritize the composites for both the gypsum and CaCO3 having 7.5 wt% of PLA content.
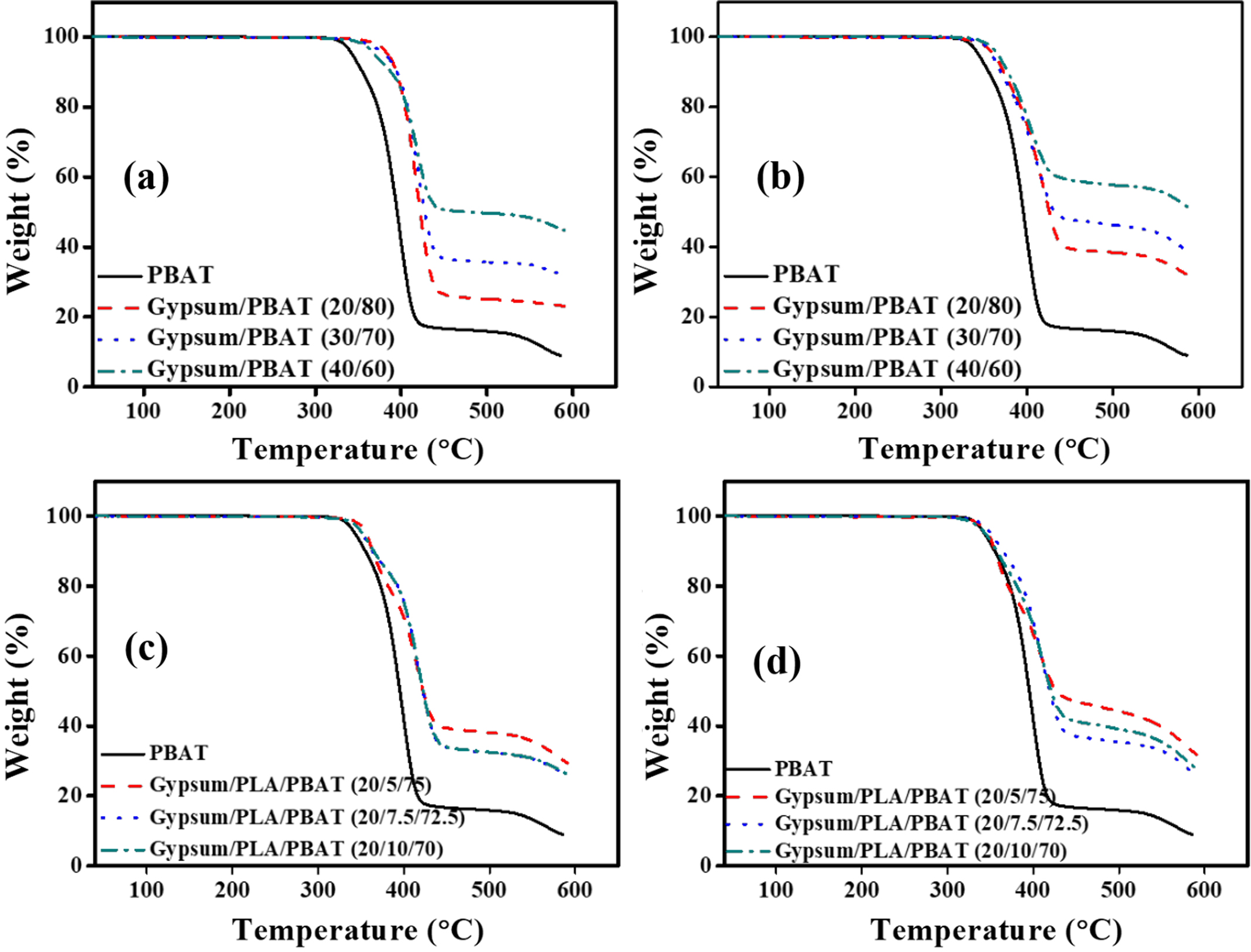
Thermal Property. The TGA of the samples was investigated to check their thermal stability, as shown in Figure 3. For both the composites of PBAT with gypsum and CaCO3 (Figure 3(a) & 3(b)), the thermal stability increases from that of the neat PBAT, which is due to the electrostatic interaction of the dispersed fillers with the matrix. Because of the high polarizability of CaSO4, the filler-matrix interaction is more favorable for gypsum/PBAT composite, leading to more thermal stability than that of CaCO3/PBAT composite. In contrast, although the gypsum/PLA/PBAT (Figure 3(c)) shows better thermal stability than that of neat PBAT, the CaCO3/PLA/PBAT (Figure 3(d)) shows lower thermal stability for 5 and 10wt% of the PLA content due to the plausible depolymerization in presence of CaCO3. Only the composite having 7.5wt% of PLA content shows better thermal stability which can be considered as a proper ratio for the composite. It is noteworthy to mention that the degradation temperatures of the composites are above the processing and application temperatures, which allows them to be applicable to produce different agricultural (e.g., mulching films) commodity products.
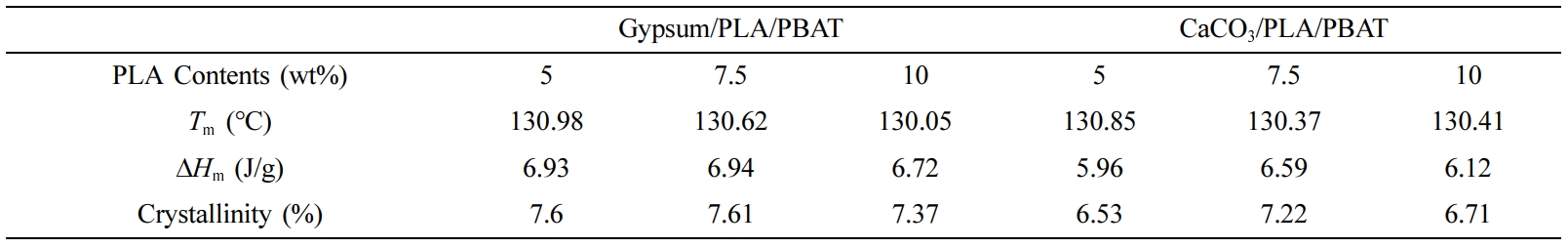
Table 1 represents the results obtained from the DSC curves of the gypsum/PLA/PBAT and CaCO3/PLA/PBAT samples. It can be observed that both the composites of gypsum and CaCO3 containing 7.5 wt% of PLA exhibits a higher degree of crystallinity which resembles that the proper ratio for the composite meets at the 7.5 wt% of PLA content. Also, it can be said that the composites containing 7.5 wt% of PLA content are expected to exhibit better mechanical property. The slight increase of crystallinity for gypsum/PLA/PBAT is due to the better intermolecular interaction among the fillers and matrix. Thus, the results obtained from thermal analyses well corroborates with our presumption and the mechanical properties.
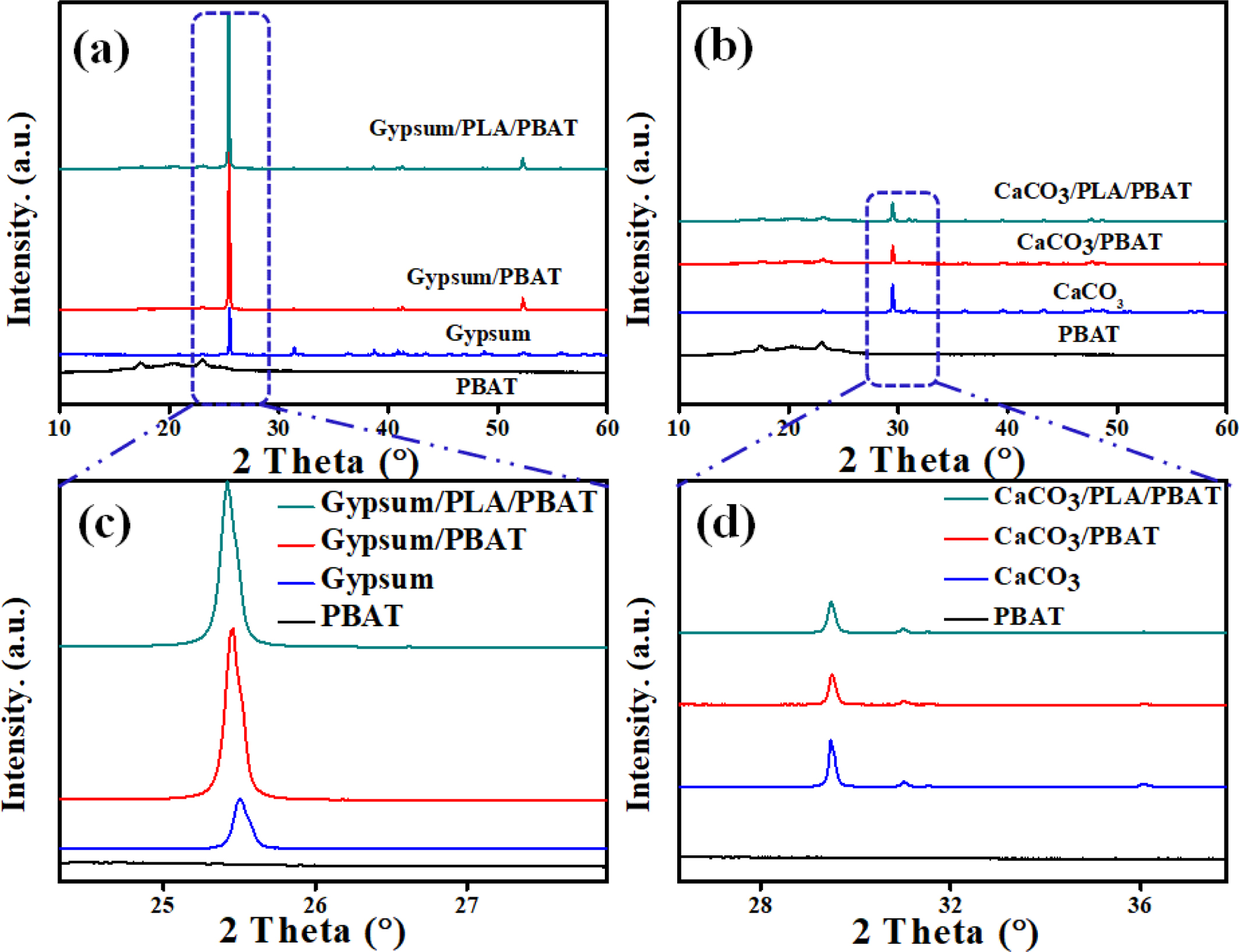
XRD. Further to realize the interaction mechanism, the XRD of the samples were analyzed. Figure 4 represents the comparative XRD patterns of the composite samples containing 20wt% of each of the fillers (gypsum or CaCO3). In the case of gypsum based composites (Figure 4(a)), it can be observed that compared to the virgin gypsum there is a peak shift (as shown in an enlarged form) in its composites, due to the better filler-matrix interaction.27,28 However, such a shift is absent for the CaCO3 (Figure 4(b)) which may be due to the absence or presence of very negligible filler-matrix interaction.
Morphology. Figures 5(a), 5(b), and 5(c) represent the SEM micrographs of only PBAT, gypsum/PBAT, and CaCO3/PBAT. For both the composites of PBAT, an immiscible behavior between the components are observed. Although PBAT containing polyester groups interact both the calcium salts because of polar-polar interactions, it is not enough for homogeneous coating of polymer over the salt surfaces, resulting in segregation of the calcium salts over the PBAT matrix. More segregation can be observed for the CaCO3/PBAT composite (as marked by the blue circle in Figure 5(c)), in comparison to that of the gypsum/PBAT composite, which infers the better interaction of PBAT with the gypsum. In contrast, the PBAT-composites of gypsum and CaCO3 show better morphology when the PLA is added (Figure 5(d) & 5(e)) which resemble some positive effects of PLA on the morphologies of the composites. Because of the possibility of depolymerization in presence of CaCO3, the fillers (i.e., CaCO3) can be dispersed well through the more available interstitial spaces of depolymerized polymer matrixes during the formulation of CaCO3/PLA/PBAT composite, resulting in a better morphology of the composite when compared with that of the gypsum/PLA/PBAT. More segregation can be noticed for the gypsum/PLA/PBAT (as marked by the white circle in Figure 5(d)), due to the incorporation of PLA, which occupies the spaces within the PBAT entanglements by replacing the gypsum.
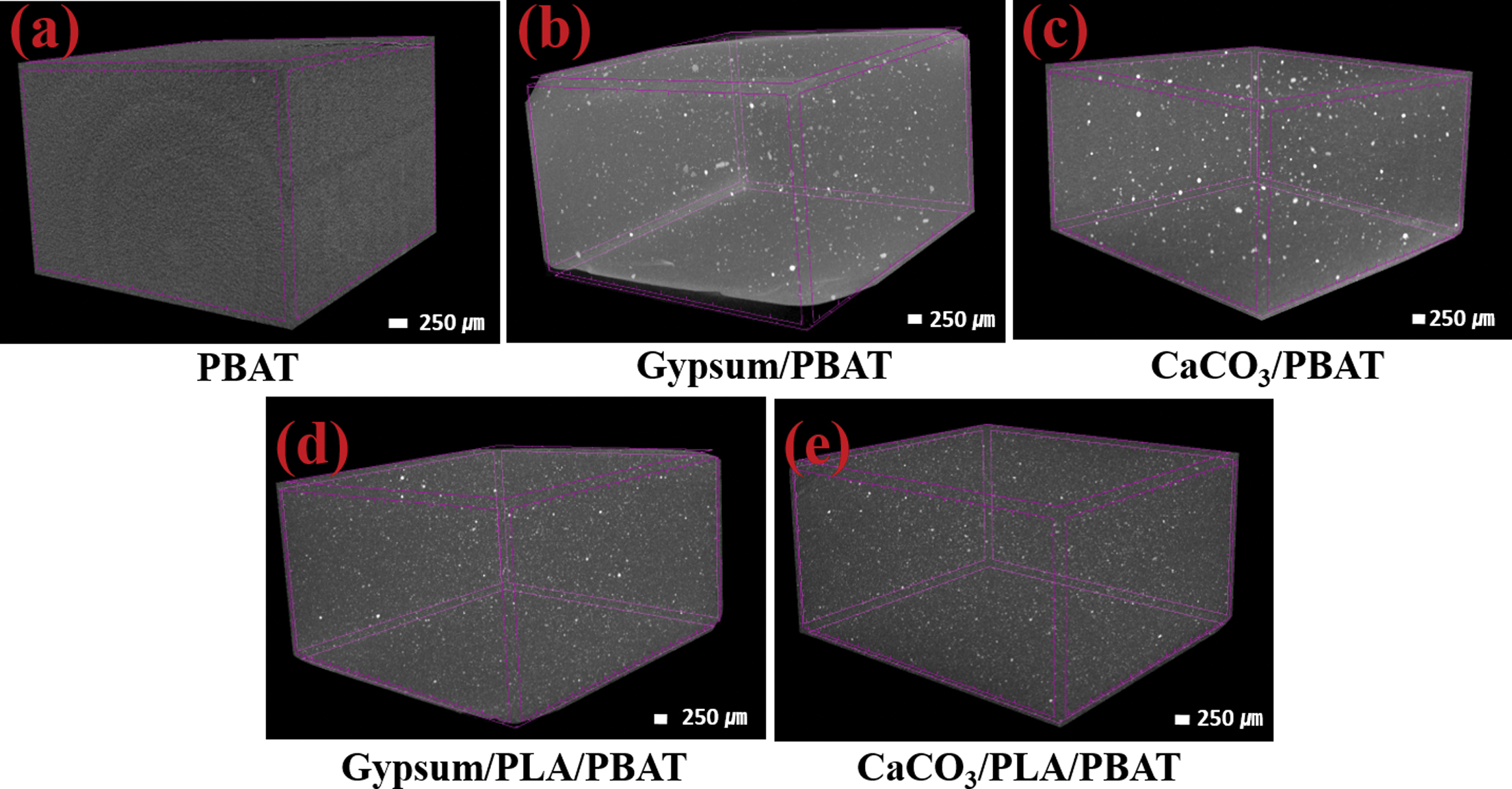
To check the dispersion as well as the distribution of the fillers in the polymer matrix, further, the micro-CT images of the composites have been taken and shown in Figure 6. In case of only PBAT composites (i.e., without PLA), it can be observed that the matrix of gypsum composites for (Figure 6(b)) is slightly visible in comparison to that of CaCO3 composites (Figure 6(c)), which infers that the gypsum because of its regular shape, and presence of abundant filler-matrix interaction, dispersed well in the matrix.29 However, it can be observed that both the fillers are well distributed within the matrix. Compared to the CaCO3/PBAT, the matrix area of CaCO3/PLA/PBAT (Figure 6(e)) is slightly visible because of enhancement in distribution as well as dispersion of the CaCO3 within the PLA/PBAT matrix due to increase in availability of interstitial spaces within the matrix after the plausible depolymerization, as discussed in earlier sections. On the other hand, when compared to gypsum/PBAT, the agglomeration of fillers in gypsum/PLA/PBAT is observed, may be due to the interference of PLA. Overall, the degree of gypsum dispersion is not significantly different from that of calcium carbonate.
|
Figure 2 Comparative tensile strength and elongation at break of only PBAT based composites ((a) & (b) respectively), and PLA/PBAT based composites ((c) & (d) respectively). |
|
Figure 3 Comparative TGA curve of PBAT based composites ((a) & (b) respectively); PLA/PBAT based composites ((c) & (d) respectively). |
|
Figure 4 Comparative XRD patterns of the composites of (a) gypsum; (b) CaCO3. |

|
Figure 5 FESEM images of (a) PBAT; (b) Gypsum/PBAT; (c) CaCO3/PBAT; (d) gypsum/PLA/PBAT; (e) CaCO3/PLA/PBAT. |
|
Figure 6 Micro-CT images of (a) PBAT; (b) gypsum/PBAT; (c) CaCO3/PBAT; (d) gypsum/PLA/PBAT; (e) CaCO3/PLA/PBAT. |
|
Table 1 Comparative Thermal Properties (Derived from the DSC Curves) of PL A/PBAT Composites with Gypsum and CaCO3. |
Herein, we have investigated the usability of waste gypsum towards the production of low-cost biodegradable polymer composites to be used for developing different agricultural (e.g., mulching, green hose, etc.) or commodity products. Because of regular morphology and abundant polarizability, gypsum well intercalates in the flexible PBAT matrix through the filler-matrix electrostatic interaction. The addition of PLA, because of its better thermal and mechanical properties, enhances the mechanical performance and thermal stability of the composite. Compared to the composites of CaCO3, the gypsum composite exhibited superior mechanical properties, which can be considered potential for the industrial upscaling to develop different bio-degradable products in the future. In addition, this work may open a new strategy to formulate the polymer composites using different inorganic fillers and to manipulate their property depending on the polarizability (chemical formula) of the filler materials.
- 1. Schrijvers, D. L.; Leroux, F.; Verney, V.; Patel, M. K. Ex-ante Life Cycle Assessment of Polymer Nanocomposites Using Organo-modified Layered Double Hydroxides for Potential Application in Agricultural Films. Green Chem. 2014, 16, 4969-4984.
-

- 2. Gnanasekaran, D. Green Biopolymers and Their Nanocomposites; Springer: Singapore, 2019; pp 1-27.
-

- 3. Hu, H.; Zhang, R.; Wang, J.; Ying, W. B.; Shi, L.; Yao, C.; Kong, J.; Wang, K.; Zhu, J. A. Mild Method to Prepare High Molecular Weight Poly(Butylene Furandicarboxylate-co-glycolate) Copoly-esters: Effects of the Glycolate Content on Thermal, Mechanical, and Barrier Properties and Biodegradability. Green Chem. 2019, 21, 3013-3022.
-

- 4. Rocha, D. B.; Souza de Carvalho, J.; de Oliveira, S. A.; dos Santos Rosa, D. A New Approach for Flexible PBAT/PLA/CaCO3 Films Into Agriculture. J. Appl. Polym. Sci. 2018, 135, 46660.
-

- 5. Spierling, S.; Knüpffer, E.; Behnsen, H.; Mudersbach, M.; Krieg, H.; Springer, S.; Springer, S.; Albrecht, S.; Herrmann, C.; Endres, H.-J. Bio-based Plastics - A Review of Environmental, Social and Economic Impact Assessments. J. Clean. Prod. 2018, 185, 476-491.
-

- 6. Aung, S. P. S.; Shein, H. H. H.; Aye, K. N.; Nwe, N. Environment-Friendly Biopolymers for Food Packaging: Starch, Protein, and Poly-lactic Acid (PLA). In Bio-based Materials for Food Packaging; Ahmed, S. Ed.; Springer: Singapore, 2018; pp 173-195.
-

- 7. Bajpai, P. K.; Singh, I.; Madaan, J. Development and Characterization of PLA-based Green Composites. Compos. Mater. 2014, 27, 52-81.
-

- 8. Koronis, G.; Silva, A.; Fontul, M. Green Composites: A Review of Adequate Materials for Automotive Applications. Compos. Part B 2013, 44, 120-127.
-

- 9. Zhou, H.; Lawrence, J. G.; Bhaduri, S. B. Fabrication Aspects of PLA-CaP/PLGA-CaP Composites for Orthopedic Applications: A Review. Acta Biomater. 2012, 8, 1999-2016.
-

- 10. Garlotta, D. A Literature Review of Poly(Lactic Acid). J. Polym. Environ. 2001, 9, 63-84.
-

- 11. Hamad, K.; Kaseem, M.; Ko, Y. G.; Deri, F. Biodegradable Polymer Blends and Composites: An Overview. Polymer Science Series A 2014, 56, 812-829.
-

- 12. Burgos, N.; Martino, V. P.; Jiménez, A. Characterization and Ageing Study of Poly(Lactic Acid) Films Plasticized with Oligomeric Lactic Acid. Polym. Degrad. Stab. 2013, 98, 651-658.
-

- 13. Osman, M. J.; Ibrahim, N. A.; Yunus, W. M. Z. W. Effect of Modified Clay on the Morphological and Thermal Properties of PLA/PBAT Nanocomposites. Orient. J. Chem. 2017, 33, 3015-3023.
-

- 14. Nofar, M.; Heuzey, M. C.; Carreau, P. J.; Kamal, M. R.; Randall, J. Coalescence in PLA-PBAT Blends under Shear Flow: Effects of Blend Preparation and PLA Molecular Weight. J. Rheol. 2016, 60, 637-648.
-

- 15. Sirisinha, K.; Somboon, W. Melt Characteristics, Mechanical, and Thermal Properties of Blown Film from Modified Blends of Poly(butylene adipate‐co‐terephthalate) and Poly(lactide). J. Appl. Polym. Sci. 2012, 124, 4986-4992.
-

- 16. Kim, T.-J.; Kim, T.-H.; Kim, S.-G.; Seo, K.-H. Structural, Thermal, and Mechanical Properties of PLA/PBAT/MEA Blend. Polym. Korea 2016, 40, 371-379.
-

- 17. Xie, J.; Wang, Z.; Zhao, Q.; Yang, Y.; Xu, J.; Waterhouse, G. I. N.; Zhang, K.; Li, S.; Jin, P.; Jin, G. Scale-Up Fabrication of Biodegradable Poly(butylene adipate-co-terephthalate)/Organophilic-Clay Nanocomposite Films for Potential Packaging Applications. ACS Omega 2018, 3, 1187-1196.
-

- 18. Jiang, L.; Wolcott, M. P.; Zhang, J. Study of Biodegradable Polylactide/Poly(butylene adipate-co-terephthalate) Blends. Bio-macromolecules 2006, 7, 199-207.
-

- 19. França, D. C.; Almeida, T. G.; Abels, G.; Canedo, E. L.; Carvalho, L. H.; Wellen, R. M. R.; Haag, K.; Koschek, K. Tailoring PBAT/PLA/Babassu Films for Suitability of Agriculture Mulch Application. J. Nat. Fibers 2019, 16, 933-943.
-

- 20. Van Breemen, N.; Mulder, J.; Driscoll, C. T. Acidification and Alkalinization of Soils. Plant Soil. 1983, 75, 283-308.
-

- 21. Kara, Ö.; Bolat, İ. Impact of Alkaline Dust Pollution on Soil Microbial Biomass Carbon. Turk. J. Agric. For. 2007, 31, 181-187.
- 22. Denev, Y. G.; Denev, G. D.; Popov, A. N. Surface Modification of Phosphogypsum Used as Reinforcing Material in Polyethylene Composites. J. Elastomers Plast. 2009, 41, 119-132.
-

- 23. Gopikrishnan, C. R.; Jose, D.; Datta, A. Electronic Structure, Lattice Energies and Born Exponents for Alkali Halides from First Principles. AIP Adv. 2012, 2, 012131.
-

- 24. Fajans, K. Degrees of Polarity and Mutual Polarization of Ions in the Molecules of Alkali Fluorides, SrO, and BaO. In Structure and Bonding; Jørgensen, C. K., Neilands, J. B., Nyholm, S. R. S., Reinen, D., Williams, R. J. P. Eds.; Springer: Berlin, Germany, 1967; vol 3, pp 88-105.
-

- 25. Shainberg, I.; Sumner, M. E.; Miller, W. P.; Farina, M. P. W.; Pavan, M. A.; Fey, M. V. Use of Gypsum on Soils: A Review. In Advances in Soil Science; Stewart, B. A. Ed.; Springer: NewYork, USA, 1989; pp 1-111.
-

- 26. Neal, J. A.; Mozhdehi, D.; Guan, Z. Enhancing Mechanical Performance of a Covalent Self-Healing Material by Sacrificial Noncovalent Bonds. J. Am. Chem. Soc. 2015, 137, 4846-4850.
-

- 27. Signori, F.; Coltelli, M.-B.; Bronco, S. Thermal Degradation of Poly(lactic acid) (PLA) and Poly(butylene adipate-co-terephthalate) (PBAT) and Their Blends upon Melt Processing. Polym. Degrad. Stab. 2009, 94, 74-82.
-

- 28. Dhole, S. G.; Dake, S. A.; Prajapati, T. A.; Helambe, S. N. Effect of ZnO Filler on Structural and Optical Properties of Polyaniline-ZnO Nanocomposites. Procedia Manuf. 2018, 20, 127-134.
-

- 29. Cho, J.; Jeon, I.; Kim, S. Y.; Lim, S.; Jho, J. Y. Improving Dispersion and Barrier Properties of Polyketone/Graphene Nanoplatelet Composites via Noncovalent Functionalization Using Aminopyrene. ACS Appl. Mater. Interfaces 2017, 9, 27984-27994.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2021; 45(1): 119-128
Published online Jan 25, 2021
- 10.7317/pk.2021.45.1.119
- Received on Aug 31, 2020
- Revised on Oct 13, 2020
- Accepted on Oct 13, 2020
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Jeong Seok Oh
-
Department of Materials Engineering and Convergence Technology, ERI, Gyeongsang National University, 501 Jinju-daero, Jinju 52828, Korea
- E-mail: ohjs@gnu.ac.kr
- ORCID:
0000-0003-3163-4826









 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.