- Fabrication of Starch-Lauric Acid Nanoparticles for Potential Tumor Therapy
Guk-Young Ahn, Inseong Choi, Tae Hoon Yun, and Sung-Wook Choi†

Department of Biomedical Chemical Engineering, The Catholic University of Korea, 43 Jibong-ro, Wonmi-gu, Bucheon-si, Gyeonggi-do, Korea
- 잠재적 종양 치료를 위한 전분-라우르산 나노 입자의 제조
가톨릭대학교 바이오메디컬화학공학과
This manuscript reports on the fabrication of starch-lauric acid (St-LA) nanoparticles having potential cancer therapy. St-LA nanoparticles were fabricated by varying the amount of LA, and the optimized St-LA nanoparticles were found. In addition, it was confirmed that the St-LA nanoparticles were successfully fabricated through scanning electron microscopy (SEM) and Fourier transform infrared spectroscopy (FTIR) analysis. In vitro, the St-LA nanoparticles showed cytotoxicity in A549 and Caco-2 cells but not NIH/3T3 cells. These results were confirmed by LIVE/DEAD staining and fluorescence imaging. Our next goal is to evaluate the St-LA nanoparticles containing various anticancer drugs through in vitro and in vivo studies. St-LA nanoparticles can potentially be applied to cancer chemotherapy.
라우르산의 양을 변화시킴으로써 잠재적인 암 요법을 갖는 전분-라우르산(starch-lauric acid; St-LA) 나노 입자를 제조하고 최적화된 St-LA 나노 입자를 발견하였다. 또한 주사 전자 현미경(SEM) 및 푸리에 변환 적외선 분광법(FTIR) 분석을 통해 St-LA 나노 입자가 성공적으로 제조되었음을 확인하였다. 세포 실험에서, St-LA 나노 입자는 A549 및 Caco-2 세포에서 세포 독성을 나타내지만 NIH/3T3 세포는 나타내지 않았음이 LIVE/DEAD 염색 및 형광 이미징에 의해 확인되었다. 따라서 St-LA 나노 입자는 잠재적으로 암 화학 요법에 적용될 수 있을 것으로 판단된다.
Lauric acid is a saturated fatty acid with 12-carbon atoms. It potentially induces apoptosis in various cancer cells. Starch-conjugated lauric acid (St-LA) nanoparticles were synthesized by DMTMM via esterification between carboxyl group of LA and hydroxyl group of starch. Based on this property, St-LA nanoparticles were fabricated using dialysis. St-LA nanoparticles specifically target cancer cells due to their EGFR overexpression resulting in increased apoptotic signals compared with normal cells. Therefore, St-LA nanoparticles can potentially be used as drug carriers for tumor therapy.
Keywords: lauric acid, nanoparticles, antitumor effect.
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2015R1A4A1042350 and NRF-2017R1A2B4008093), a grant awarded by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) and funded by the Ministry Health & Welfare, Republic of Korea (HI17C0886), and the Research Fund, 2019 of The Catholic University of Korea.
Nanotherapy is attracting attention as a promising technology for the treatment, diagnosis, and prevention of disease using nanoscale materials. Nanoparticles are nano-sized particles measuring less than 1000 nm and are used primarily in nanotherapy of cancer, and in bioimaging and drug delivery systems.1-4 Various nanoparticles include polymer nanoparticles [poly(lactide-co-glycolide), polylactic acid, gelatin, and chitosan],5-7 liposomes,8 micelles,9 dendrimers,10 and inorganic nanoparticles (gold, carbon nanotube, SiO2, and Fe3O4).11 These nanoparticles circulate in blood vessels and are able to penetrate cancer tissues most effectively with enhanced permeability and retention (EPR) effect (passive targeting).12 Grafting cancer target molecules onto nanoparticles increases intracellular drug concentration in cancer cells while preventing damage to normal cells (active targeting).13 Nanoparticle-based cancer therapy has been actively studied based on these characteristics.14
Lauric acid (LA) is a saturated fatty acid with a 12-carbon atom chain and properties of medium-chain fatty acids.15 LA is also present in fruits, vegetable oils, and breast milk.16 LA is reported to exhibit antimicrobial, antifungal,17 and anti-inflammatory activities,18 in addition to preventing prostatic hyperplasia,19 and triggering apoptosis of colon cancer cells.20 In particular, LA induces apoptosis in various cancer cells and therefore, used in cancer chemotherapy.21 Cancer chemotherapy via induction of cellular apoptosis is mediated via increased oxidative stress.22 The oxidative stress occurs when the glutathione (GSH) level is lowered or the reactive oxygen species (ROS) level is increased, leading to an imbalance in cell proliferation and apoptosis.23 LA produces a substantial amount of ROS at the beta-oxidation stage, one of the metabolic degradation mechanisms, leading to cancer cell death.24 The increased ROS also trigger the epidermal growth factor receptor/extracellular signal-regulated kinase (EGFR/ERK) transduction pathway referred to as the cell proliferation regulatory pathways and altered gene expression, leading to apoptosis.21
In this study, we fabricated starch-conjugated lauric acid (St-LA) nanoparticles for cancer chemotherapy. Starch encapsulating LA is biodegradable cost-effective, and nontoxic.25 We believe that the St-LA nanoparticles have significant potential in enhancing the anticancer effect.
Materials. Starch, LA, capric acid (CA), and dimethyl sulfoxide (DMSO) were purchased from Sigma-Aldrich (St Louis, MO, USA). 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (DMTMM) was ordered from Tokyo Chemical Industry Co., Ltd (TCI, Tokyo, Japan). Dialysis membranes (MWCO 6–8 kDa) were supplied by CelluSep (Sequin, TX, USA). The culture medium consisted of Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% antibiotics (containing penicillin and streptomycin). Cell Counting Kit-8 (CCK-8, Dojindo Co. Ltd., Tokyo, Japan) was used to quantitatively measure the number of cells.
Synthesis and Characterization of Starch-Lauric Acid Nanoparticles. Starch (100mg) was mixed with 20mL of DMSO and stirred for 3h. LA (10, 25, 50, and 70mg). DMTMM (0.2g) was added into the starch dispersion and stirred for another 24 h to obtain St-LA, which was dialyzed in DMSO for 1 day to remove DMTMM. Subsequently, the St-LA solution was dialyzed in deionized water (DW) for 2 days to obtain St-LA nanoparticles, which were lyophilized for 2 days for further use. Starch-conjugated capric acid (St-CA) nanoparticles were also synthesized according to the same protocol. The size and zeta potential were measured via a dynamic light scattering method (Zetasizer nano ZS, Malvern Instruments, Malvern, UK). The morphology was observed by scanning electron microscopy (SEM, S-4800, Hitachi, Tokyo, Japan). Fourier transform infrared spectroscopy (FTIR, IRAffinity-1, Shimadzu, Tokyo, Japan) was used to characterize the St-LA nanoparticles.
Cell Viability. Mouse embryonic fibroblast (NIH/3T3), adenocarcinomic human alveolar basal epithelial cells (A549), and human epithelial colorectal adenocarcinoma cells (Caco-2) purchased from the Korea Cell Line Bank (Seoul, Korea) were selected as model cells to evaluate the cell viability of St-LA and St-CA nanoparticles. Aqueous dispersions of St-LA and St-CA nanoparticles at concentrations of 50, 75, 100, 200, and 500 μg/mL were added to each well containing 5×103 cells in a 96-well plate and incubated in a humidified atmosphere containing 5% CO2 at 37°C for 48h. The cells were counted using the CCK-8 assay. Cell viability was calculated as the number of live cells divided by the number of total cells. The cells were stained with a LIVE/DEAD Viability/Cytotoxicity Kit and observed with fluorescence microscopy (FM, IX71, Olympus Co. Ltd., Tokyo, Japan).
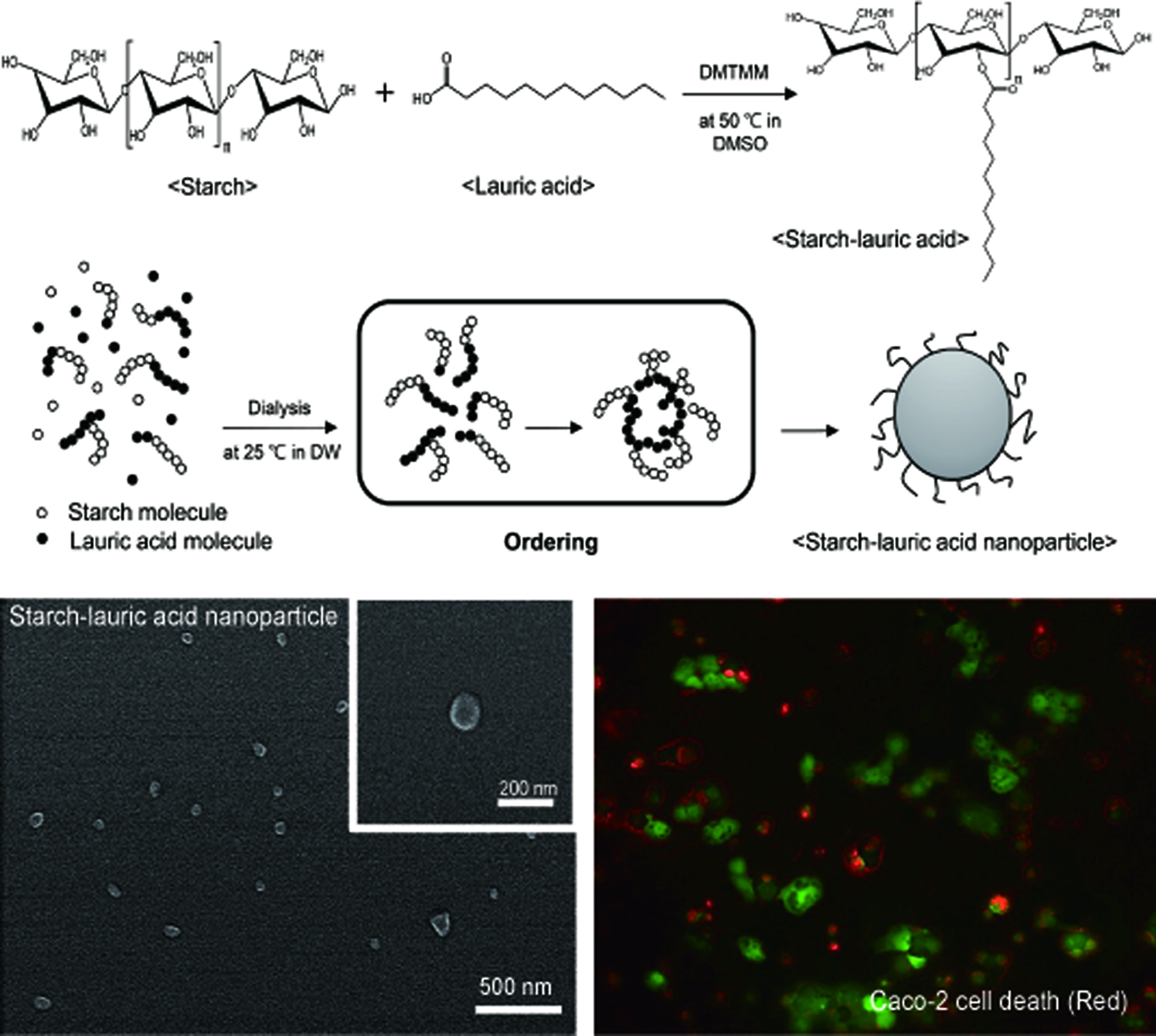
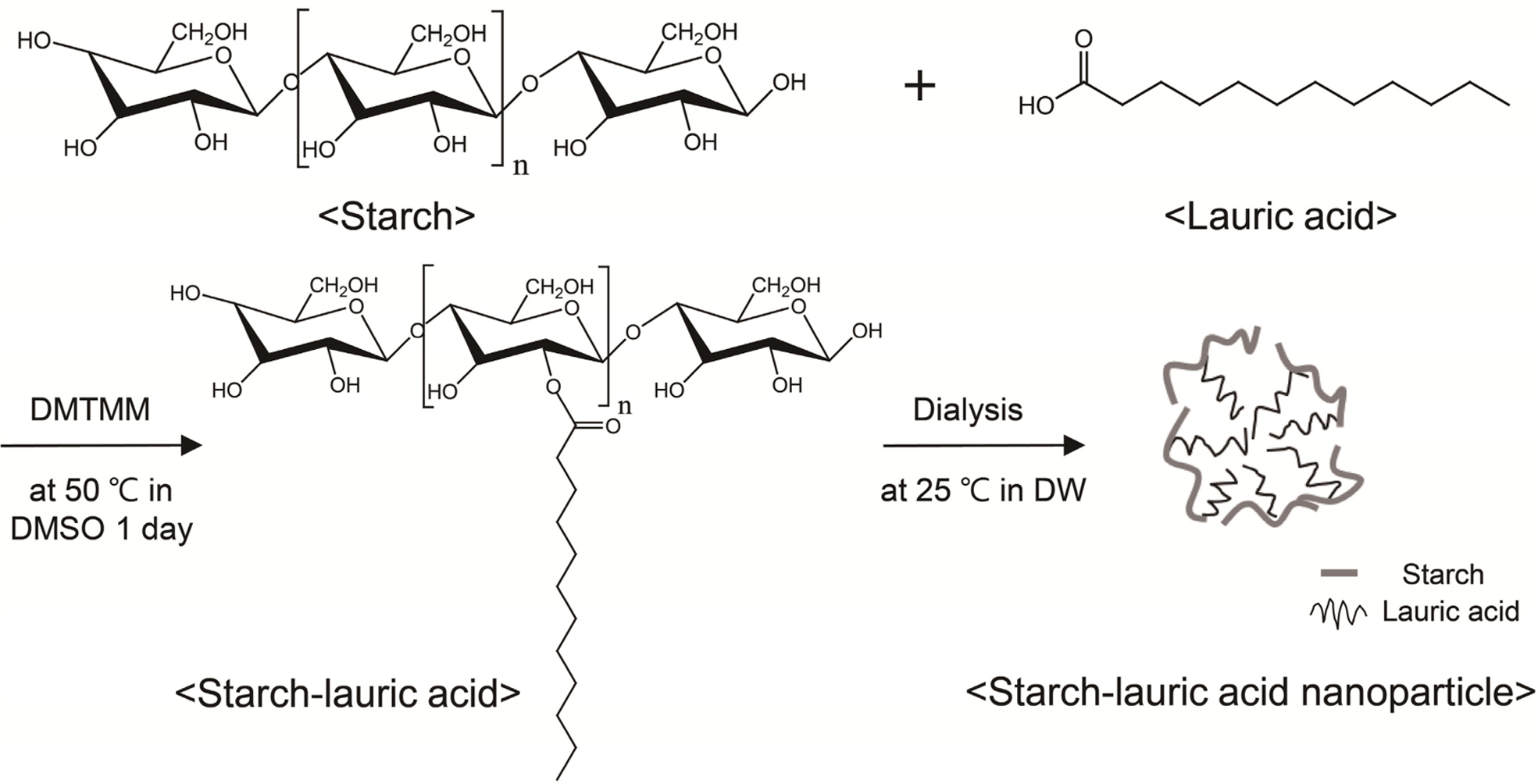
Preparation and Characterization of Starch-Lauric Acid Nanoparticles. Figure 1 shows the synthesis of St-LA nanoparticle. St-LA was synthesized between the LA and starch by DMTMM.26 DMTMM activates carboxyl groups of LA and induces esterification reaction with the hydroxyl groups of starch. DMTMM was used as a conjugation agent due to its non-toxicity and high conjugation efficiency.27 The synthesized St-LA solution with the DMSO phase was dialyzed in the DMSO phase to remove DMTMM. The St-LA solution loaded on the dialysis membrane was transferred into the aqueous phase of DW to obtain nanoparticles. In the aqueous phase, hydrophobic aggregation of LA particles occurred with starch as the hydrophilic component surrounding LA to form spherical nanoparticles.28
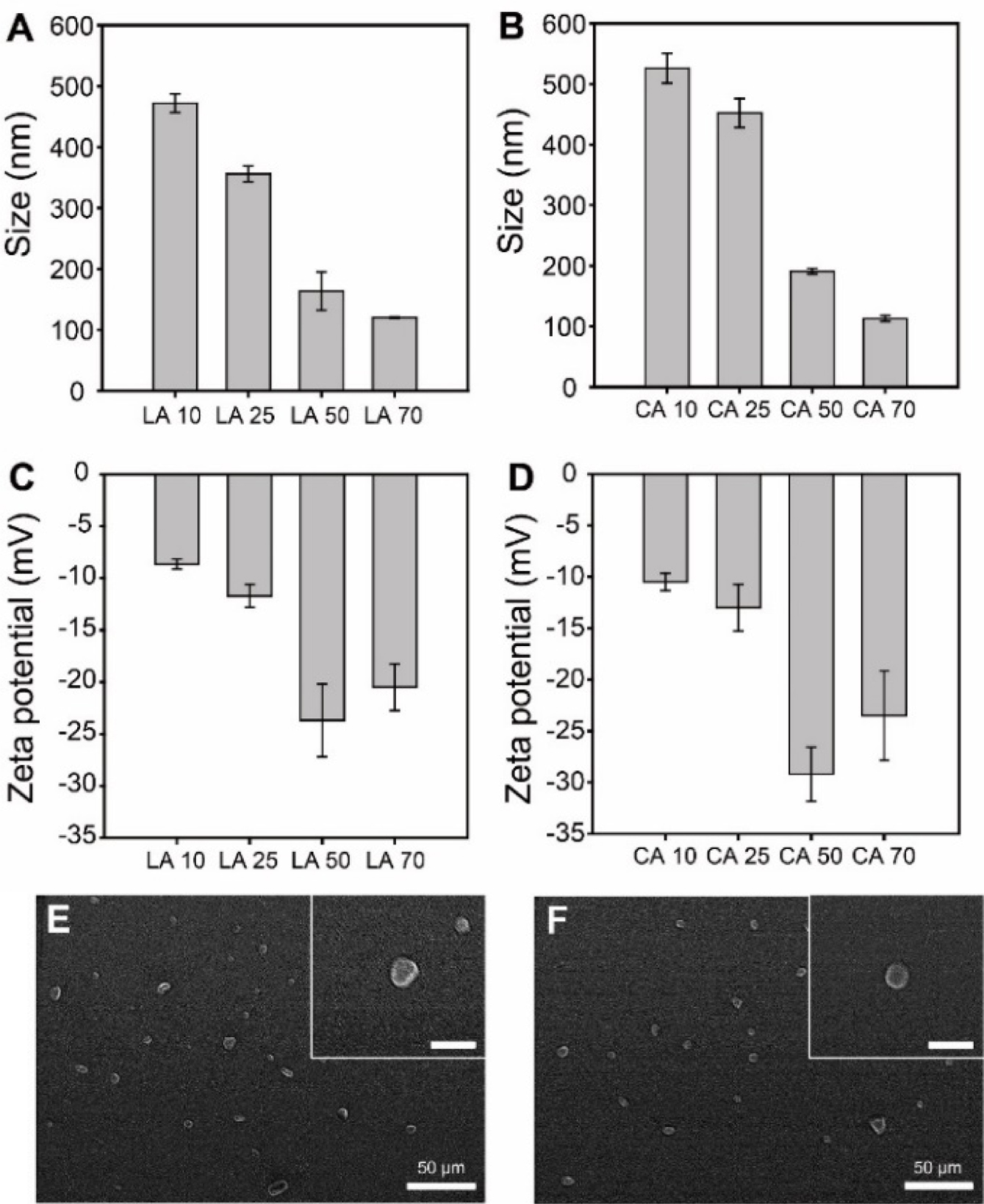
St-LA nanoparticles were prepared by varying the amount of LA (10, 25, 50, and 70mg) to determine the optimized St-LA nanoparticles. Figure 2(A) and 2(B) show the size of St-LA and St-CA nanoparticles. The St-LA nanoparticles containing 10 and 25 mg of LA measured 472.0±15.0 and 356.0±13.1nm, respectively. However, the sizes of St-LA nanoparticles carrying 50 and 70mg of LA were 163.7±31.2 and 120.2±1.2nm, suggesting that the size of St-LA nanoparticles decreased with an increased amount of LA. The reduction in size is due to the stronger hydrophobic interaction between nanoparticle cores as the amount of LA increases.29 The size of St-CA nanoparticles also showed a similar tendency as St-LA.
As shown in Figure 2(C), the zeta potentials of St-LA nanoparticles with 50 and 70 mg of LA were -23.7±3.5 and -20.5±2.2mV, higher than the zeta potential of starch. In the synthesis, the hydroxyl group of starch was decreased and the zeta potential was increased.30 The zeta potentials of St-LA nanoparticles with 10 and 25mg of LA were -8.6±0.5 and -11.7±1.1mV similar to the zeta value of LA, indicating the incomplete formation of the nanoparticles.31
The zeta potentials of St-CA nanoparticles also showed a similar tendency as St-LA. Therefore, the St-LA nanoparticles with 70mg of LA were selected for the subsequent study because of their high LA content and appropriate size for cellular uptake. He et al. demonstrated that the smaller size of nanoparticles facilitated efficient cellular uptake because larger nanoparticles require stronger driving force for cellular internalization.32 Studies in vitro showed that nanoparticles measuring 100nm in size showed optimal uptake.33,34 Figure 2(E) and 2(F) represent the SEM images of St-LA and St-CA nanoparticles with 70mg LA and CA. Both nanoparticles exhibit spherical morphology.
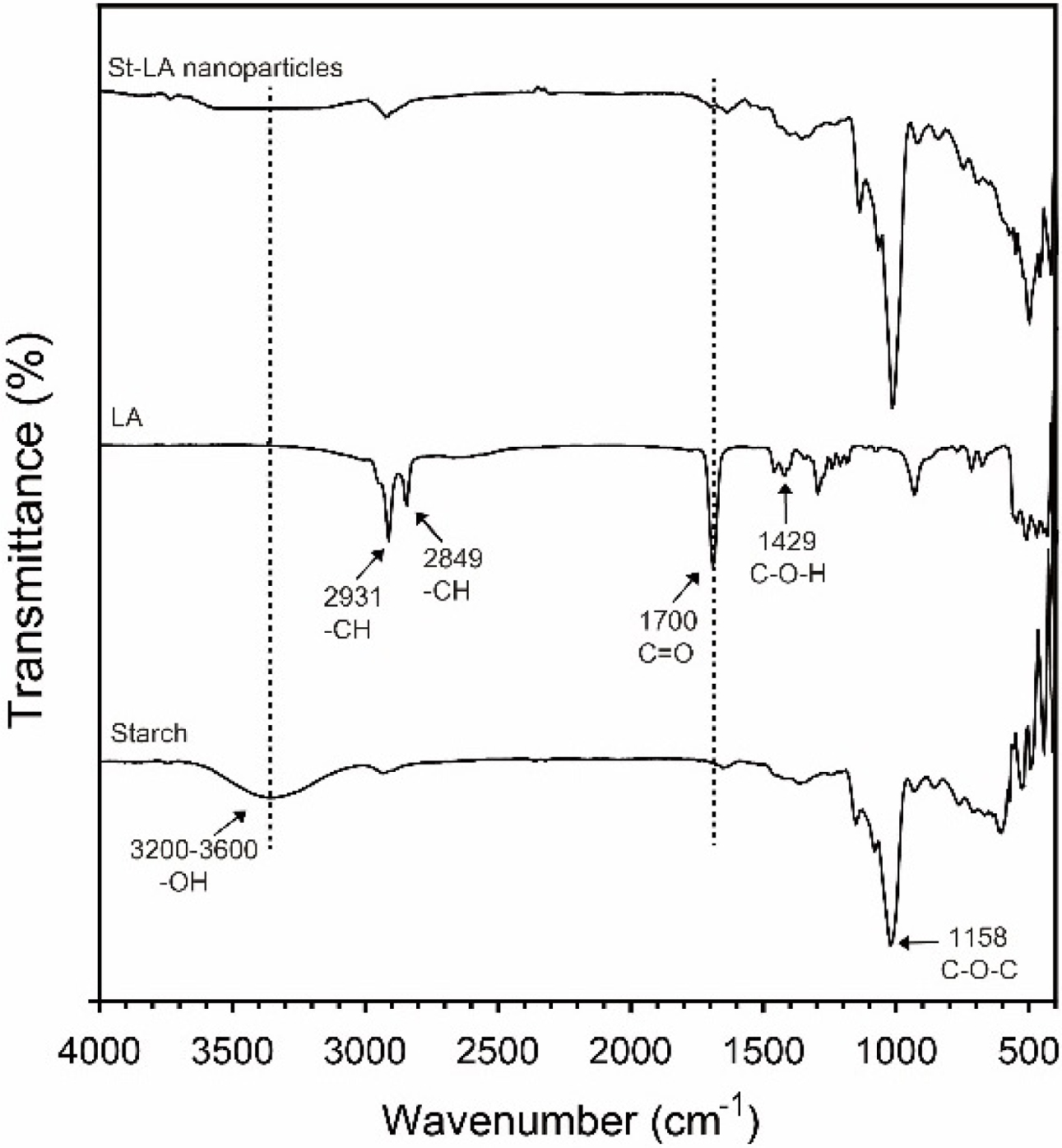
Figure 3 presents the FTIR spectrum of starch, LA, and St-LA nanoparticles highlighting the esterification between hydroxyl groups in starch and carboxyl groups in LA. The region 3200 to 3600cm-1 corresponds to the peak of hydroxyl groups (-OH stretch) in starch.35 However, the hydroxyl groups in St-LA nanoparticles disappeared due to the reduction of hydroxyl groups in the esterification reaction. The decreased peaks of carboxylic groups in C=O (1700cm-1) and -OH (3200-3600 cm-1) indicate that the carboxylic groups were reduced in the esterification reaction.36 These results demonstrate the successful synthesis of St-LA nanoparticles.
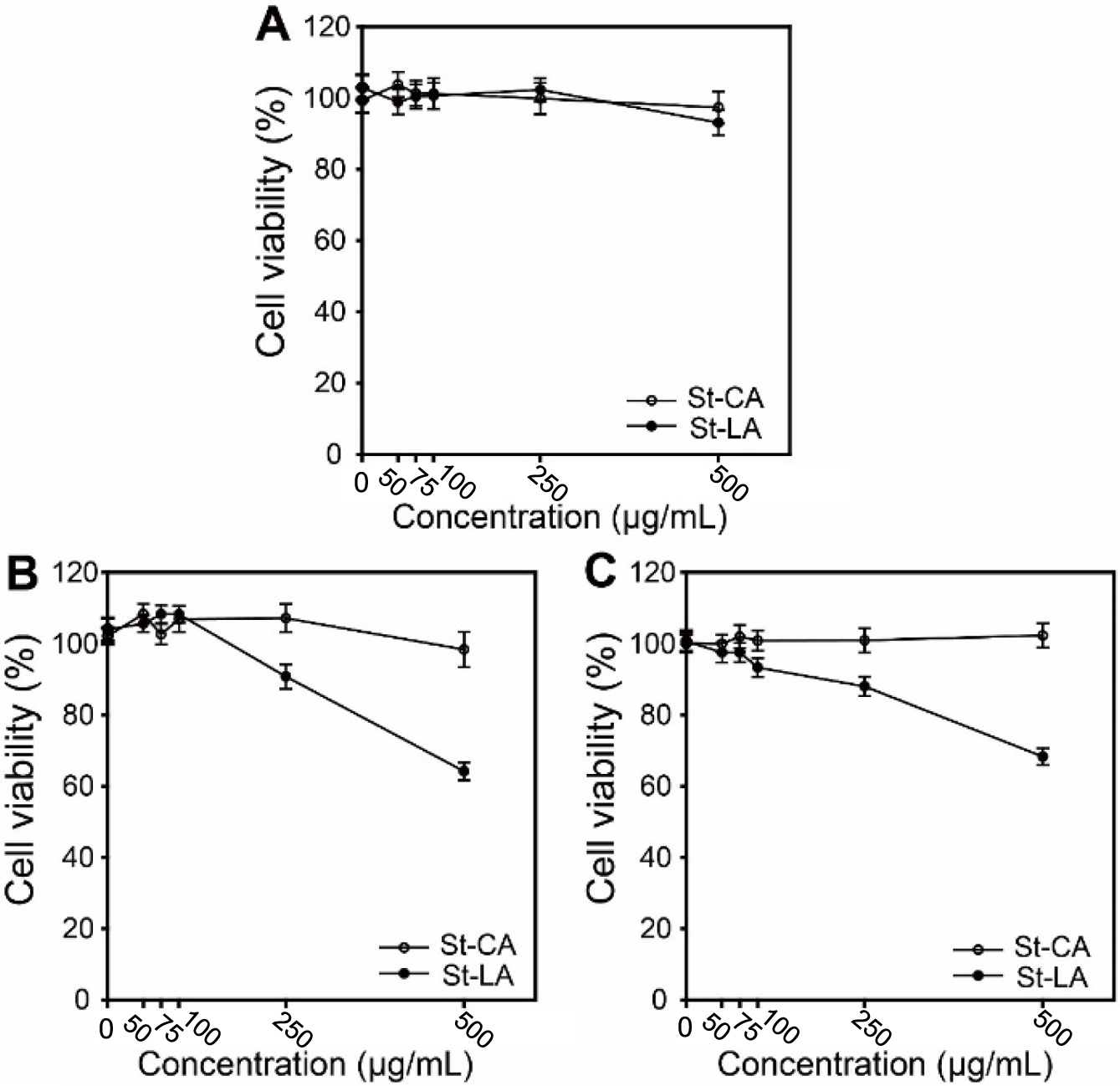
In Vitro Experiment of Starch-Lauric Acid Nanoparticles. Figure 4 shows the viabilities of NIH/3T3, A549, and Caco-2 cells treated with St-LA, and St-CA nanoparticles during 48 h at different nanoparticles concentrations. In the case of NIH/3T3 cells (Figure 4(A)), a minor variation in cell viability was observed depending on the concentration of nanoparticles, indicating that the St-LA nanoparticles were non-toxic in normal cells. EGF-induced apoptosis in the EGFR/ERK transduction pathway also occurred in normal cells as a ‘normal’ physiological event, and the apoptosis of St-LA nanoparticles was insignificant to affect normal cell viability.37 As shown in Figures 4(B) and 4(C), the viabilities of A549 and Caco-2 cells treated with St-LA nanoparticles at 500μg/mL were reduced to 64.1% and 66.9%, respectively. However, St-CA nanoparticles did not affect either cell viability because of the different metabolism of LA in CA oxidation in mitochondria.38 St-LA nanoparticles specifically target cancer cells due to their EGFR overexpression resulting in increased apoptotic signals compared with normal cells. EGFR mainly plays a role in regulating cell proliferation, migration, and differentiation, but it has been demonstrated that EGFR may ‘paradoxically’ mediate apoptosis signals in some cell types. EGFR overexpression increases the apoptotic effect by mechanism impairing Akt activation by blocking Ras signaling. In EGFR-overexpressing cells, the apoptotic effect can reach a threshold, and the balance beyond that is tipped from proliferation to apoptosis due to misregulation of the signaling circuitry.37
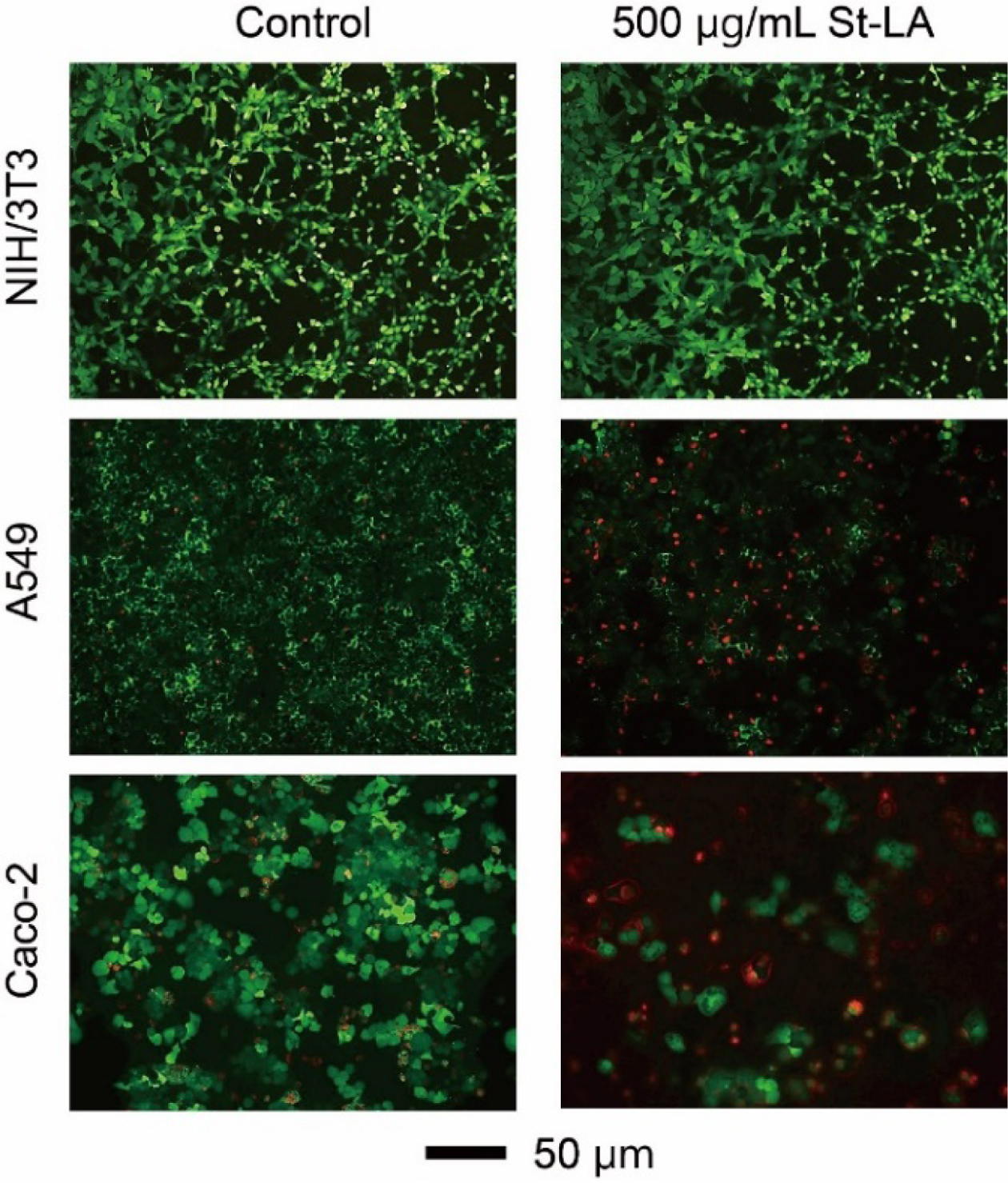
Figure 5 shows a visual representation of cell death following treatment with 500μg/mL St-LA nanoparticles. The cells were stained with the live and dead assay kit, with green and red colors indicating live and dead cells. The fluorescence images matched the cell viability data closely.
|
Figure 1 Schematic illustration of the synthesis of St-LA nanoparticle. |
|
Figure 2 Size (A, B) and zeta potentials (C, D) of St-LA (A, C); St-CA nanoparticles (B, D). SEM images of (E) St-LA (LA 70); (F) St-CA (CA 70) nanoparticles. The inserts are magnified SEM images and scale bars represent 200 nm. |
|
Figure 3 FTIR spectra of starch, LA, and St-LA nanoparticles. |
|
Figure 4 Viability of (A) NIH/3T3; (B) A549; (C) Caco-2 cells after treatment with St-CA and St-LA nanoparticles during 48 h of incubation at different concentrations. |
|
Figure 5 Fluorescence microscopy images of NIH/3T3, A549 and Caco-2 cells treated with St-LA nanoparticles for 48 h, followed by LIVE/DEAD staining (Green: live cells, Red: dead cells). |
St-LA nanoparticles were fabricated for potential application in cancer chemotherapy. Since St-LA nanoparticles were fabricated based on their hydrophobic interaction in dialysis, hydrophobic anticancer drugs such as doxorubicin can be encapsulated together. Although the anticancer effect of St-LA nanoparticles alone is about 65% in vitro, the effect is increased when the nanoparticles are used as carriers of various anticancer drugs. Our next goal is to combine St-LA nanoparticles with various anticancer agents and cancer target moieties to generate highly efficient anticancer nanoparticles. We believe that the St-LA nanoparticles carry great potential for anticancer therapy.
- 1. Hu, C.-M. J.; Aryal, S.; Zhang, L. Nanoparticle-assisted Combination Therapies for Effective Cancer Treatment. Ther. Deliv. 2010, 1, 323-334.
-

- 2. Kim, Y. M.; Lim, S. K.; Yoon, M. S. Paclitaxel-loaded Nanoparticles of Cholanic Acid-Modified Hyaluronan Oligosaccharide for Tumor-site Specific Delivery. Polym. Korea 2015, 39, 967-975.
-

- 3. Bhunia, S. K.; Saha, A.; Maity, A. R.; Ray, S. C.; Jana, N. R. Carbon Nanoparticle-based Fluorescent Bioimaging Probes. Sci. Rep. 2013, 3, 1473.
-

- 4. Singh, R.; Lillard, J. W. Nanoparticle-based Targeted Drug Delivery. Exp. Mol. Pathol. 2009, 86, 215-223.
-

- 5. Park, J. K.; Kim, D. G.; Choi, C.; Jeong, Y. I.; Kim, M. Y.; Jang, M. K.; Nah, J. W. Preparation and Characterization of Lithocholic Acid Conjugated Chitosan Oligosaccharide Nanoparticles for Hydrophobic Anticancer Agent Carriers. Polym. Korea 2008, 32, 263-269.
- 6. Cong, D. V.; Trang, N. T. T.; Giang, N. V.; Trung, T. H.; Chinh, N. T.; Huynh, M. D.; Hoang, T.; Park, J.-S. Preparation and Characterization of Nanocomposites Based on Poly(ethylene-co-vinyl acetate), Polylactic Acid, and TiO2 Nanoparticles. Polym. Korea 2016, 40, 355-364.
-

- 7. Kumari, A.; Yadav, S. K.; Yadav, S. C. Biodegradable Polymeric Nanoparticles Based Drug Delivery Systems. Colloid. Surface B 2010, 75, 1-18.
-

- 8. Yan, W.; Huang, L. Recent Advances in Liposome-Based Nanoparticles for Antigen Delivery. Polym. Rev. 2007, 47, 329-344.
-

- 9. Khanal, A.; Inoue, Y.; Yada, M.; Nakashima, K. Synthesis of Silica Hollow Nanoparticles Templated by Polymeric Micelle with Core−Shell−Corona Structure. J. Am. Chem. Soc. 2007, 129, 1534-1535.
-

- 10. Scott, R. W. J.; Wilson, O. M.; Crooks, R. M. Synthesis, Characterization, and Applications of Dendrimer-Encapsulated Nanoparticles. J. Phys. Chem. B 2005, 109, 692-704.
-

- 11. Xu, Z. P.; Zeng, Q. H.; Lu, G. Q.; Yu, A. B. Inorganic Nanoparticles as Carriers for Efficient Cellular Delivery. Chem. Eng. Sci. 2006, 61, 1027-1040.
-

- 12. Torchilin, V. Tumor Delivery of Macromolecular Drugs Based on the EPR Effect. Adv. Drug Deliver. Rev. 2011, 63, 131-135.
-

- 13. Wang, M.; Thanou, M. Targeting Nanoparticles to Cancer. Pharmacol. Res. 2010, 62, 90-99.
-

- 14. Haley, B.; Frenkel, E. Nanoparticles for Drug Delivery in Cancer Treatment. Urol. Oncol.-Semin. O. I. 2008, 26, 57-64.
-

- 15. Dayrit, F. M. The Properties of Lauric Acid and Their Significance in Coconut Oil. J. Am. Oil Chem. Soc. 2015, 92, 1-15.
-

- 16. Odenigbo, U. M.; Otisi, C. A. O. Fatty Acids and Phytochemical Contents of Different Coconut Seed Flesh in Nigeria. Int. J. Plant Physiol. Biochem. 2011, 3, 176-182.
-

- 17. Agoramoorthy, G.; Chandrasekaran, M.; Venkatesalu, V.; Hsu, M. J. Antibacterial and Antifungal Activities of Fatty Acid Methyl Esters of the Blind-your-eye Mangrove from India. Braz. J. Microbiol. 2007, 38, 739-742.
-

- 18. Calder, P. C. Long-chain Fatty Acids and Inflammation. P. Nurt. Soc. 2012, 71, 284-289.
-

- 19. Veeresh Babu, S. V.; Veeresh, B.; Patil, A. A.; Warke, Y. B. Lauric acid and Myristic Acid Prevent Testosterone Induced Prostatic Hyperplasia in Rats. Eur. J. Pharmacol. 2010, 626, 262-265.
-

- 20. Weng, W.-H.; Leung, W.-H.; Pang, Y.-J.; Hsu, H.-H. Lauric Acid Can Improve The Sensitization of Cetuximab in KRAS/BRAF Mutated Colorectal Cancer Cells by Retrievable microRNA-378 Expression. Oncol. Rep. 2016, 35, 107-116.
-

- 21. Lappano, R.; Sebastiani, A.; Cirillo, F.; Rigiracciolo, D. C.; Galli, G. R.; Curcio, R.; Malaguarnera, R.; elfiore, A.; Cappello, A. R.; Maggiolini, M. The Lauric Acid-activated Signaling Prompts Apoptosis in Cancer Cells. Cell Death Discov. 2017, 3, 17063.
-

- 22. Ozben, T. Oxidative Stress and Apoptosis: Impact on Cancer Therapy. J. Pharm. Sci. 2007, 96, 2181-2196.
-

- 23. Matés, J. M.; Segura, J. A.; Alonso, F. J.; Márquez, J. Oxidative Stress in Apoptosis and Cancer: An Update. Arch. Toxicol. 2012, 86, 1649-1665.
-

- 24. Tracey, T. J.; Steyn, F. J.; Wolvetang, E. J.; Ngo, S. T. Neuronal Lipid Metabolism: Multiple Pathways Driving Functional Outcomes in Health and Disease. Front. Mol. Neurosci. 2018, 11, 1-25.
-

- 25. Lu, D. R.; Xiao, C. M.; Xu, S. J. Starch-based Completely Biodegradable Polymer Materials. Express Polym. Lett. 2009, 3, 366–375.
-

- 26. Kunishima, M.; Morita, J.; Kawachi, C.; Iwasaki, F.; Terao, K.; Tani, S. Esterification of Carboxylic Acids with Alcohols by 4-(4,6-Dimethoxy-1,3,5-triazin-2-yl)-4-methyl-morpholinium Chloride (DMTMM). Synlett. 1999, 8, 1255-1256.
-

- 27. D’Este, M.; Eglin, D.; Alini, M. A Systematic Analysis of DMTMM vs EDC/NHS for Ligation of Amines to Hyaluronan in Water. Carbohydr. Polym. 2014, 108, 239-246.
-

- 28. Lee, J.-H.; Jung, S.-W.; Kim, I.-S.; Jeong, Y.-I.; Kim, Y.-H.; Kim, S.-H. Polymeric Nanoparticle Composed of Fatty Acids and Poly(ethylene glycol) as a Drug Carrier. Int. J. Pharm. 2003, 251, 23-32.
-

- 29. Na, K.; Park, K.-H.; Kim, S. W.; Bae, Y. H. Self-assembled Hydrogel Nanoparticles from Curdlan Derivatives: Characterization, Anti-cancer Drug Release and Interaction with a Hepatoma Cell Line (HepG2). J. Control. Release 2000, 69, 225-236.
-

- 30. Lim, S. A.; Park, H.; Lee, J. M.; Lee, E. S. Chlorin E6-embedded Starch Nanogels for Improved Photodynamic Tumor Ablation. Polym. Adv. Technol. 2018, 29, 2766-2773.
-

- 31. Yang, D.; Pornpattananangkul, D.; Nakatsuji, T.; Chan, M.; Carson, D.; Huang, C.-M.; Zhang, L. The Antimicrobial Activity of Liposomal Lauric Acids Against Propionibacterium Acnes. Biomaterials 2009, 30, 6035-6040.
-

- 32. He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of Particle Size and Surface Charge on Cellular Uptake and Biodistribution of Polymeric Nanoparticles. Biomaterials 2010, 31, 3657-3666.
-

- 33. Kulkarni, S. A.; Feng, S.-S. Effects of Particle Size and Surface Modification on Cellular Uptake and Biodistribution of Polymeric Nanoparticles for Drug Delivery. Pharm. Res. 2013, 30, 2512-2522.
-

- 34. Win, K. Y.; Feng, S.-S. Effects of Particle Size and Surface Coating on Cellular Uptake of Polymeric Nanoparticles for Oral Delivery of Anticancer Drugs. Biomaterials 2005, 26, 2713-2722.
-

- 35. Zhang, H. Y.; Firempong, C. K.; Wang, Y. W.; Xu, W. Q.; Wang, M. M.; Cao, X.; Zhu, Y.; Tong, S. S.; Yu, J. N.; Xu, X. M. Ergosterol-loaded Poly(lactide-co-glycolide) Nanoparticles with Enhanced In Vitro Antitumor Activity and Oral Bioavailability. Acta Pharmacol. Sin. 2016, 37, 834-844.
-

- 36. Song, S.; Zhang, Y. Carbon Nanotube/reduced Graphene Oxide Hybrid for Simultaneously Enhancing the Thermal Conductivity and Mechanical Properties of Styrene -butadiene Rubber. Carbon 2017, 123, 158-167.
-

- 37. Lehto, V.-P. EGF Receptor: Which Way to Go? FEBS Lett. 2001, 491, 1-3.
-

- 38. Kong, J. Y.; Rabkin, S. W. Lovastatin Does Not Accentuate but is Rather Additive to Palmitate-induced Apoptosis in Cardiomyocytes. Prostag. Leukotr. Ess. 2002, 67, 293-302.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2021; 45(1): 62-67
Published online Jan 25, 2021
- 10.7317/pk.2021.45.1.62
- Received on Jul 15, 2020
- Revised on Aug 26, 2020
- Accepted on Sep 9, 2020
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Sung-Wook Choi
-
Department of Biomedical Chemical Engineering, The Catholic University of Korea, 43 Jibong-ro, Wonmi-gu, Bucheon-si, Gyeonggi-do, Korea
- E-mail: choisw@catholic.ac.kr
- ORCID:
0000-5075-8798









 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.