- Synthesis and Application of Cationic Waterborne Polyurethane as Fixing Agent for Cotton Fabric
*College of Textile and Clothing Engineering, Soochow University, Suzhou 215021, China **National Engineering Laboratory for Modern Silk, Suzhou 215123, China
- 면섬유 고정제를 위한 양이온 수용성 폴리우레탄의 합성 및 응용
The traditional reactive dyeing of cotton has the disadvantage of poor fastness. In order to improve the fastness, a novel environment-friendly cationic waterborne polyurethane (CWPU) was successfully synthesized via step-by-step polymerization. The CWPU was prepared from isophorone diisocyanate (IPDI), polypropylene glycol (PPG-1000), methyl ethyl ketone oxime and hydrophilic monomer (EGDEA). The chemical structure of CWPU was characterized by Fourier transform infrared spectroscopy. Effects of n (IPDI/PPG-1000), EGDEA dosage, and the neutralizational degree on the properties of the waterborne polyurethane emulsion and the fastness of the treated dyed cotton fabrics were investigated. Particle size and zeta potential were tested to evaluate the emulsion stability. Scanning electron microscope showed that CWPU had been successfully finished on the fabric. Compared with the untreated cotton fabric, the dry and wet rubbing fastness of the treated cotton fabric dyed with reactive dyes increase 0~0.5 and 0.5~1 level, respectively, and the wash fastness increases 0.5~1 level.
The hydrophilic monomer (EGDEA) synthesized by the reaction of ethylene glycol diglycidyl ether (EGDE) and diethylamine (DEA) as a cationic chain extender is introduced into the polyurethane primary polymer to synthesize cationic waterborne polyurethane. The dry rubbing fastness, wet rubbing fastness, and soaping fastness of cotton fabrics dyed with reactive dyes can be increased by 0~0.5, 0.5~1, and 0.5~1 after treatment with a fixing agent.
Keywords: polyurethane, cation, fixing agent, reactive dyes, cotton fabric
This study was supported by the National Natural Science Foundation of China (NO. 21774085).
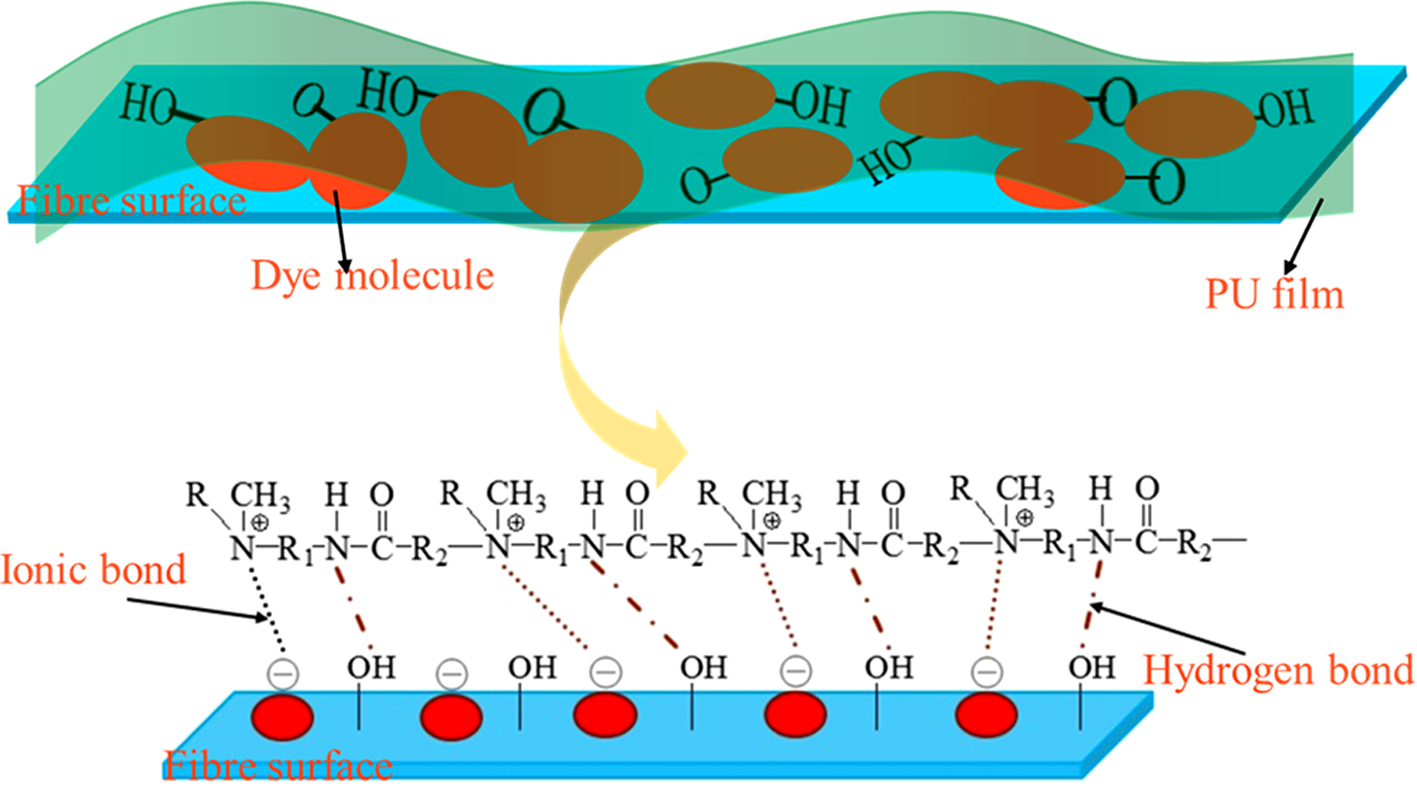
Reactive dyes are most preferred dyes for dyeing of cellulosic fibres as they can occur chemically bonded to the fibres which are being dyed,1,2 present bright and brilliant colour in various shade ranges and also inexpensive to apply.3-5 Under alkaline conditions, reactive dyes react with hydroxyl groups of cellulose, mostly by nucleophilic substitution or addition, to form the covalent bonds.6-8 However, the following problems exist with reactive dyed cotton fabrics: 1) Some reactive groups are hydrolyzed and inactivated during the dyeing process, resulting in a low dyeing rate.9,10 2) The chemical stability of the covalent bond formed between the dye and cellulose is poor.11 Under humid and hot conditions, it is easy to break the bond and fade, and the fixation rate is low. According to statistics, about half of the dye is lost with the drainage. The distribution of dye on the fibers is shown in Figure 1. As a result, there are a large number of unfixed dyes on the dyed fabric and the dyeing fastness is unqualified. So, it must be treated with a fixing agent.
Notable improvements in the fastness properties of anionic dyes can be brought about by pretreatment or after treatment of textile fibers.12-14 The use of pretreatments or after treatments to improve the fastness properties of dyeing has a long and prolific history. The traditional fixing agent Y has the problem of formaldehyde release and has been banned.15 Polyamine-based fixing agents have good color fixing effects, but this type of fixing agents contains more free amines in addition to quaternary ammonium salt groups, which can cause discoloration on fabrics.16 Polycationic fixing agents have excellent color fixing effects, but the raw material diallylamine is difficult to purchase due to environmental regulations.17 As a fixing agent, water-based polyurethane has the advantages of good film-forming property and less harm to people and the environment, and has become one of the research hotspots.18-20 However, most researchers use N-methyldiethanolamine, triethanolamine, etc. as hydrophilic monomers.21-23 Such tertiary amine-based diols are primary alcohols. They react violently during the prepolymerization stage, tend to gelled, and require more solvent, resulting in an increase in the VOC content of the emulsion.24,25 At the same time, the hydrophilic group is on the main chain of the polyurethane molecule. It not only has low degree of freedom, but also can easily wrap hydrophilic groups in the particles of polyurethane dispersions, which cannot well combine with anionic static electricity on dyes to form insoluble lakes.26,27
In this study, tertiary amino cationic hydrophilic monomer containing a secondary alcohol was synthesized with ethylene glycol diglycidyl ether and diethylamine. Hydrophilic monomers were introduced into the polyurethane as side chain to form a cationic aqueous polyurethane fixing agent. The synthesis of cationic polyurethane fixing agent was optimized by taking polyurethane emulsion performance and fixing performance as indicators. Then, the fixing agent synthesized by the optimized process was compared with the traditional commercial fixing agent.
|
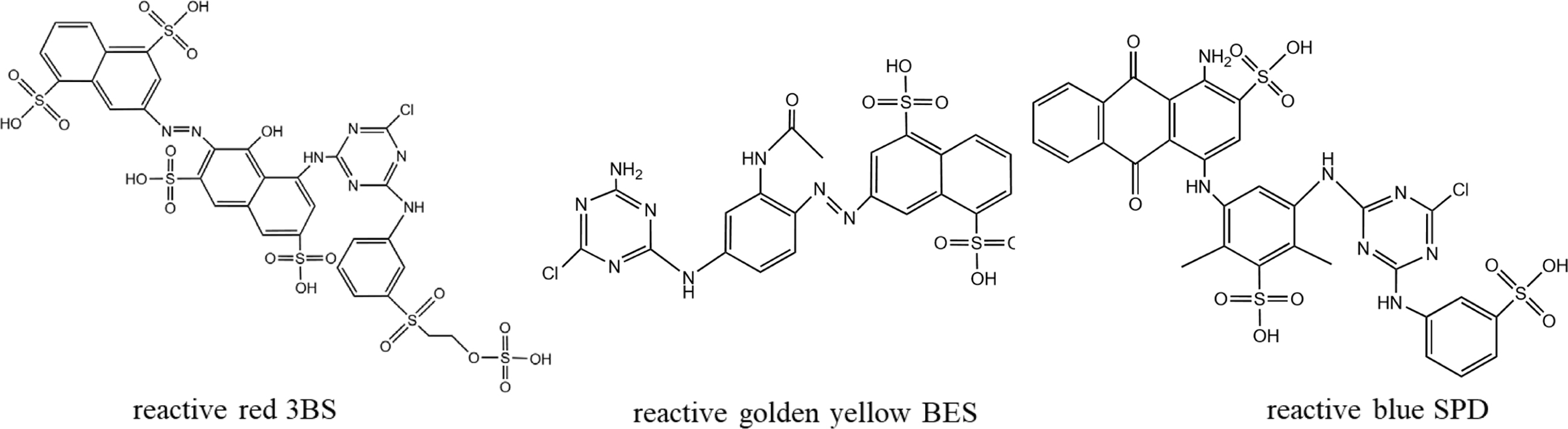
Figure 1 The distribution of dye on the fibers. |
Materials. The fabric used in this study was a 100% scoured and bleached cotton fabric (plain weave, weighing 140 g/m2). The cotton fabric was kindly supplied by Shaoxing Weifeng Textile Co., Ltd. (Zhejiang, China). Isophorone diisocyanate (IPDI), dibutyltin dilaurate (DBTDL) were obtained from Sinopharm Chemical Reagent Suzhou Co., Ltd. (China). Polypropylene glycol (PPG-1000), methyl ethyl ketone oxime (MEKO), acetone, di-n-butylamine, hydrochloric acid, acetic acid were supplied Jiangsu Yonghua Fine Chemical Co., Ltd. (China). Ethylene glycol diglycidyl ether (EGDE) and diethylamine (DEA) were purchased from Saen Chemical Technology Co., Ltd. (Shanghai) and Jiuding Chemical Technology Co., Ltd. (Shanghai), respectively. Anhydrous sodium carbonate and anhydrous sodium sulphate were obtained Yixing Chemical Reagent Factory Co., Ltd. (China). Fixing agent Y and fixing agent CS-10 were purchased Transfar Group Co., Ltd. (China). Three typical reactive dyes (reactive red 3BS, reactive golden yellow BES, reactive blue SPD; as shown in Figure 2) were purchased from Zhejiang Runtu Co., Ltd. (China). Deionized water was used throughout all the experiments.
Synthesis. Synthesis of Cationic Chain Extender: A certain amount of EGDE was added to a 250mL four-necked flask equipped with a stirrer, a thermometer and a condensing reflux device, and the temperature was raised to 60°C. The measured DEA was added dropwise with a constant pressure dropping funnel, and the reaction was kept for 4 h at 60°C. Under the condition of 60℃ and -0.09MPa, the unreacted DEA in the system was removed by a vacuum pump to obtain cationic chain extender (EGDEA). The synthesis equation of EGDEA was shown in Scheme 1.
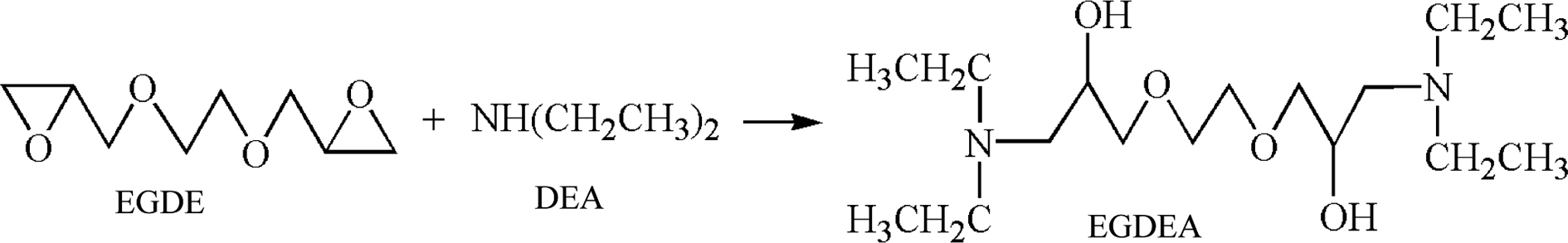
Scheme 1. Synthetic route to the cationic chain extender (EGDEA).
Synthesis of Cationic Waterborne Polyurethane Fixing Agent: First, a certain amount of PPG-1000 (vacuum dehydrated), DBTDL as catalyst and acetone as solvent were charged into a 250 mL round-bottom and four-necked flask equipped with a mechanical stirrer, a condenser, a nitrogen inlet and outlet, and a dropping funnel. Second, the stoichiometric amount of IPDI was slowly dropped into the flask and the temperature was raised to 70°C under nitrogen protection, the reaction was kept for 2 h. When the -NCO reached the theoretical value, the temperature was reduced to 45℃, certain amount of EGDEA was added dropwise, and the reaction was kept for about 1.5 h. After that, the calculated MEKO was added into the four-neck flask over a period of 0.5h. Acetic acid was used to neutralize the reaction in the after 0.5 h. Finally, the reaction was emulsified with deionized water under vigorous stirring and the CWPU emulsion was achieved after acetone was distilled under reduced pressure. The synthesis equation of CWPU was shown in Scheme 2.
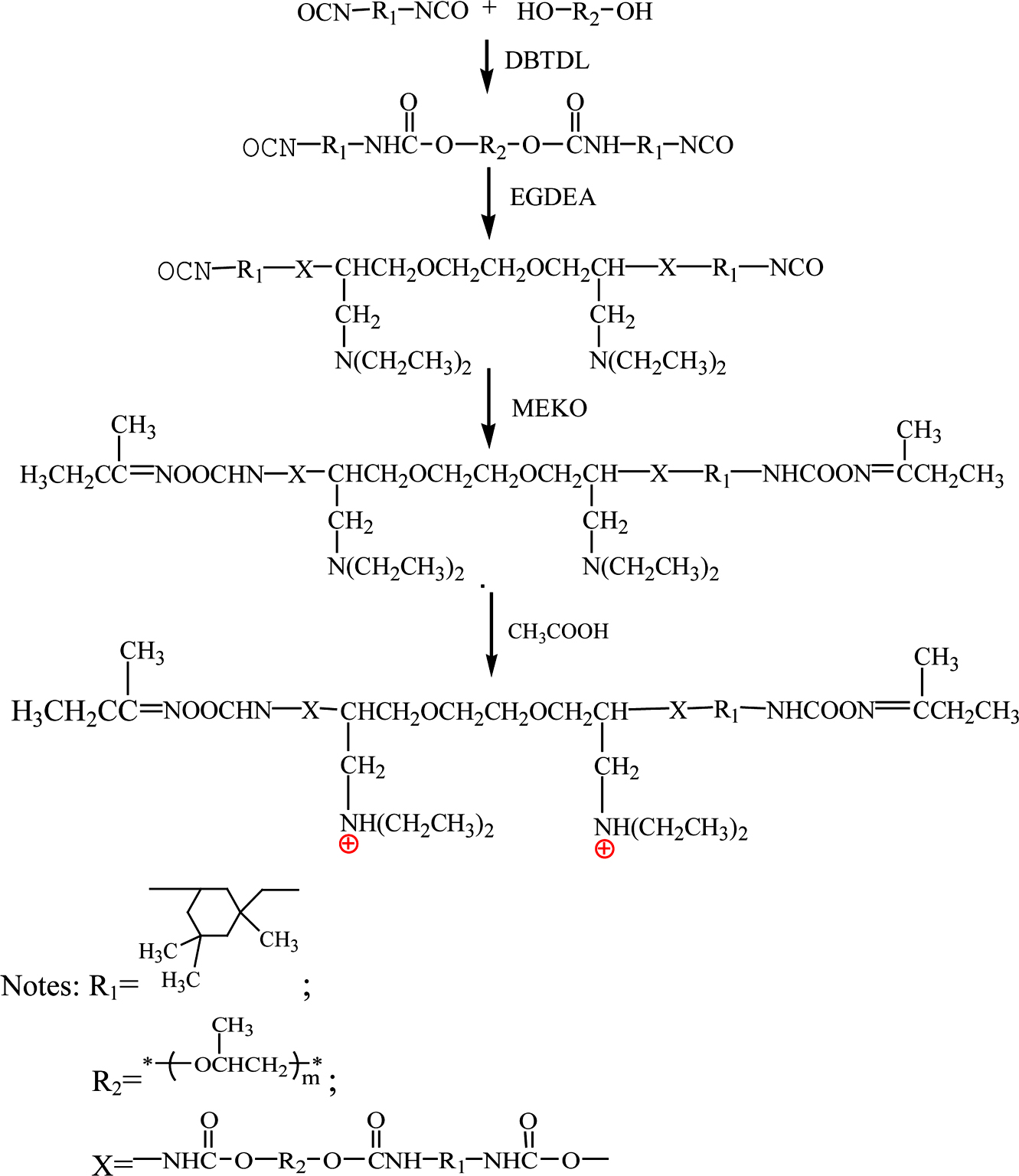
Scheme 2. Synthetic route to the cationic waterborne polyurethane fixing agent (CWPU).
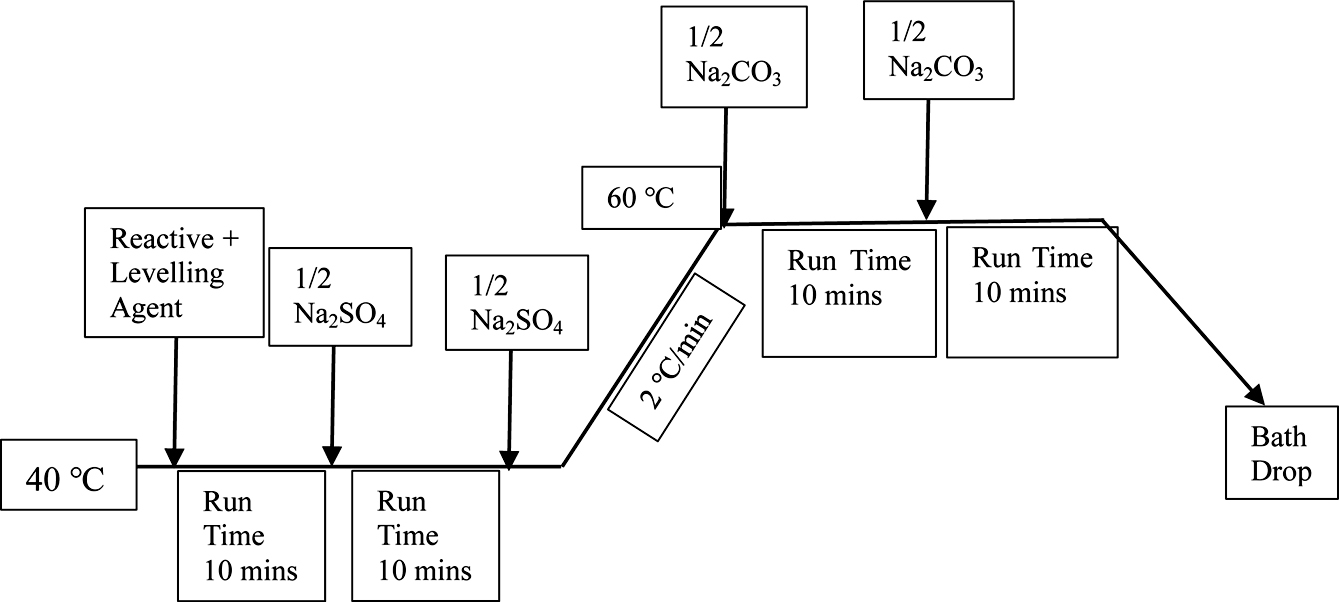
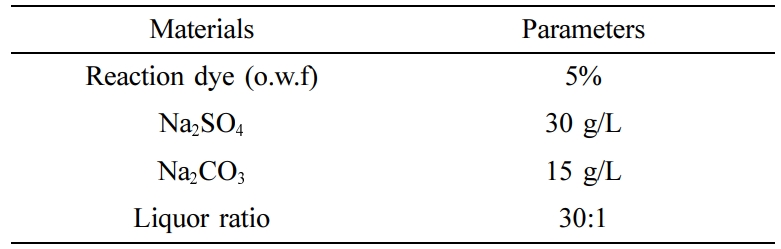
Dyeing of Cotton Fabrics. After the cotton fabric samples had been wetted in cold deionized water. Fabrics were dyed with reactive red 3BS, reactive golden yellow BES, reactive blue SPD in a laboratory dyeing machine at a liquor ratio of 30:1, respectively. The dyeing process formula was shown in Table 1. The process curve was shown in Figure 3.
Dipping Cotton Fabrics in CWPU. Dyed cotton fabric samples were dipped in finishing solution which contained fixing agents (2% o.w.f), at a liquor ratio of 1: 30 for 60 s. Wet pickup was kept about 75% after two-dips, two-nips. The fabric was dried in a heat-setting stenter at 60℃ for 150 s, and then cured at 120℃ for 180s.
Characterization. The FTIR spectra were used to detect the chemical structures of EGDE, EGDEA and CWPU. Measurements were recorded on a Nicolet 5700 FTIR spectrometer with KBr pellet method. The ATR-FTIR spectra of bleached cotton fabrics, dyed fabrics and fixed-color fabrics samples were performed between 4000 and 500 cm-1 on a Nicolet 8700 FTIR spectrometer. The resolution for all the infrared spectra was 4cm-1 and there were 32 scans for each spectrum.
Scanning electron microscope (SEM) images of the samples that had treated before and after were obtained by TM3030 table scanning electron microscope.
The sample was added in the centrifuge tube and the test condition was 3000 r/min. Centrifuge for 15 min to observe whether the CWPU is stratification or oil drift.
Particle size, particle size distribution and zeta potential of CWPU emulsions were measured by a Malvern Zetasizer Nano ZS90. The samples were prepared by diluting the emulsions with deionized water to adjust the solid content to 0.01wt%.
The water resistance of the film can be expressed by measuring the water absorption.28 The CWPU was formed into a film with a diameter of 50mm and a thickness of about 0.2mm on a glass surface with a known weight by rolling. The sample was air-dried at room temperature, baked for 2 h at 60℃ in an oven, and then dried for 1h at 100℃, until the weight had no change more. The obtained weight minus the weight of the glass piece is W1. The film-carrying glass piece was immersed in distilled water for 24 h, and the surface moisture was taken out by using a filter paper to absorb the moisture. The obtained weight minus the glass piece weight is W2. The water absorption rate was measured by eq. (1)
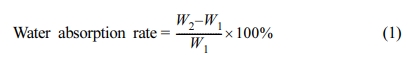
Color fastness to rubbing of cotton fabrics were measured according to GB-T 3920-2008 ‘Textiles-Tests for colour fastness-Colour fastness to rubbing’. The test was used to measure the color fastness of dry and wet rubbing before and after fixing. Then, according to GB-T 215-2008, the gray sample card for staining was used to evaluate the dyeing fastness of the fabric.
Color fastness to soaping: refer to GB-T 3921-2008 “Textile color fastness test for soaping color fastness” to test the discoloration and color fastness of dyed fabric before and after fixing. Gray sample cards for color and GB-T 250-1995 were used to evaluate the gray sample cards for fading, and the dyeing fastness of the fabric was visually rated.
The reflectance values of the dyed samples were measured using Datacolor 650 Spectraflash (USA) attached to a personal computer under illuminant D65 using a 10° standard observer.
|
Figure 2 The chemical structure of reactive red 3BS, reactive golden yellow BES, and reactive blue SPD. |
|
Figure 3 The dyeing curve. |
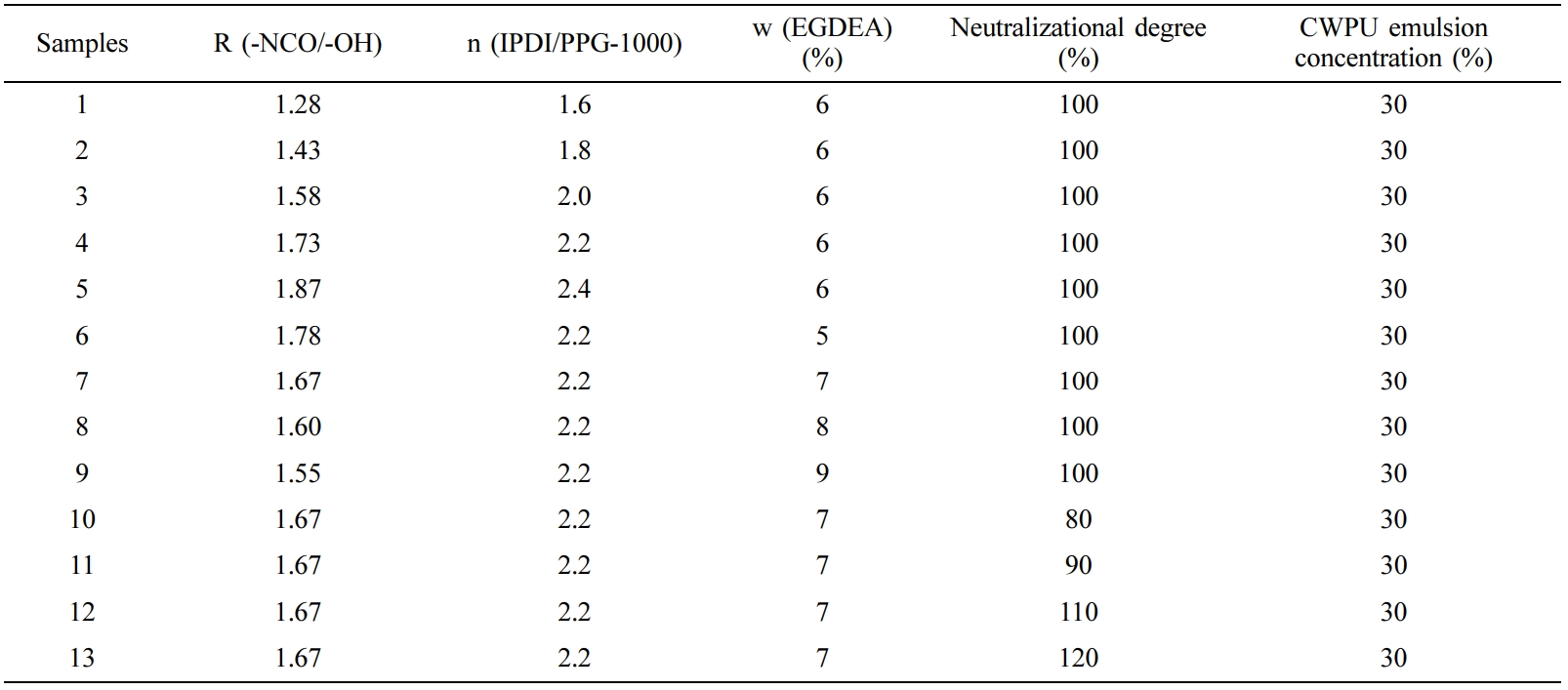
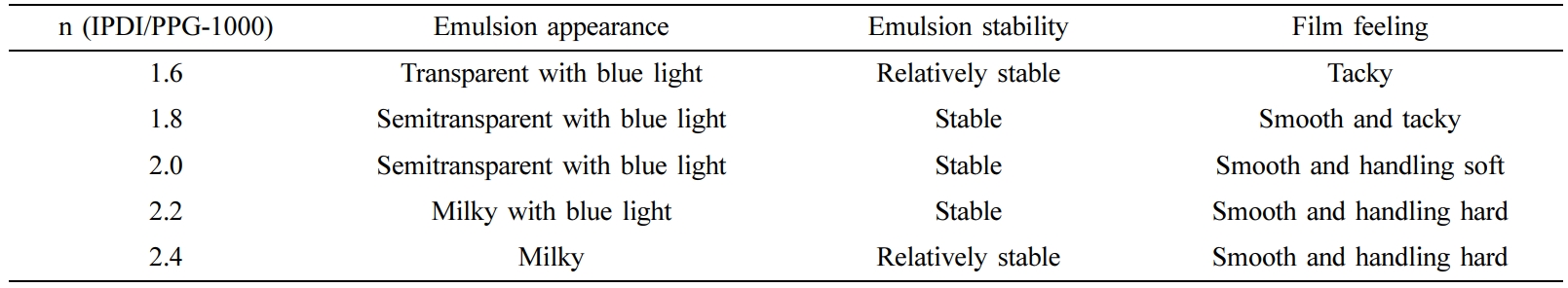
The Optimization of Synthesis Process for CWPU. The performance of CWPU emulsions was studied with different n (IPDI/PPG-1000) (molar ratio), EGDEA dosage (the percentage of the total mass of the monomers) and neutralizational degree (the neutralizational degree refers to the ratio of the amount of acid added to the amount of acid required to completely neutralize the amino group on the polymer when -NR2 on the polymer chain is neutralized with an acid). The compositions of CWPU prepared in this paper with different n (IPDI/PPG-1000), EGDEA dosage and neutralizational degree are shown in Table 2.
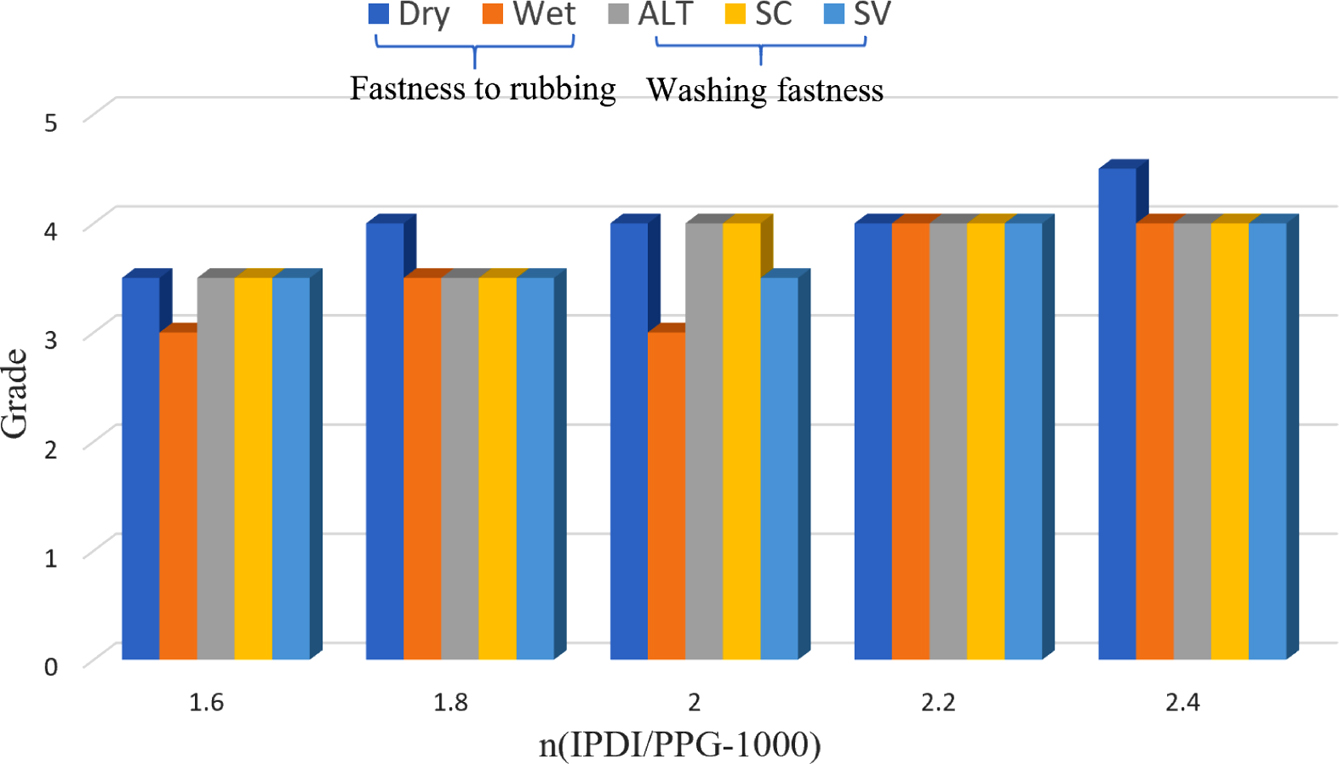
Molar Ratio of IPDI to PPG-1000: In the system of synthesizing CWPU, the amount of EGDEA was set to 6%, and the neutralizational degree was 100%. The effect of n (IPDI/PPG-1000) is as shown in the following Table 3 and Figure 4.
It can be seen from Table 3 and Figure 4 that under the same condition of others, the stability of the emulsion improves with the increases in the n (IPDI/PPG-1000). This is the molecular weight of the obtained polyurethane prepolymer is smaller by increasing the n (IPDI/PPG-1000), so it is easier to obtain a stable emulsion with a smaller particle size. However, when the ratio is large enough, more polyurea is generated by the reaction of monomer with water in the emulsification stage. Due to the strong hydrophobicity of urea, the dispersion of waterborne polyurethane is poor, and the stability of the emulsion will decrease slightly. At the same time, with the increase of n (IPDI/PPG-1000), the performance of the adhesive film becomes better, and the rubbing fastness and soaping fastness are generally on the rise. Evidently, this result was attributed to the rigid segment ratio increases and the film-forming performance becomes better when the n (IPDI/PPG-1000) increases.27 However, when the ratio of rigid segment is large enough, the hardness of the film will be enhanced. Considering the stability, film-forming property and fastness grade of the emulsion comprehensively, it is more appropriate to choose n (IPDI/PPG-1000) at 2.2.
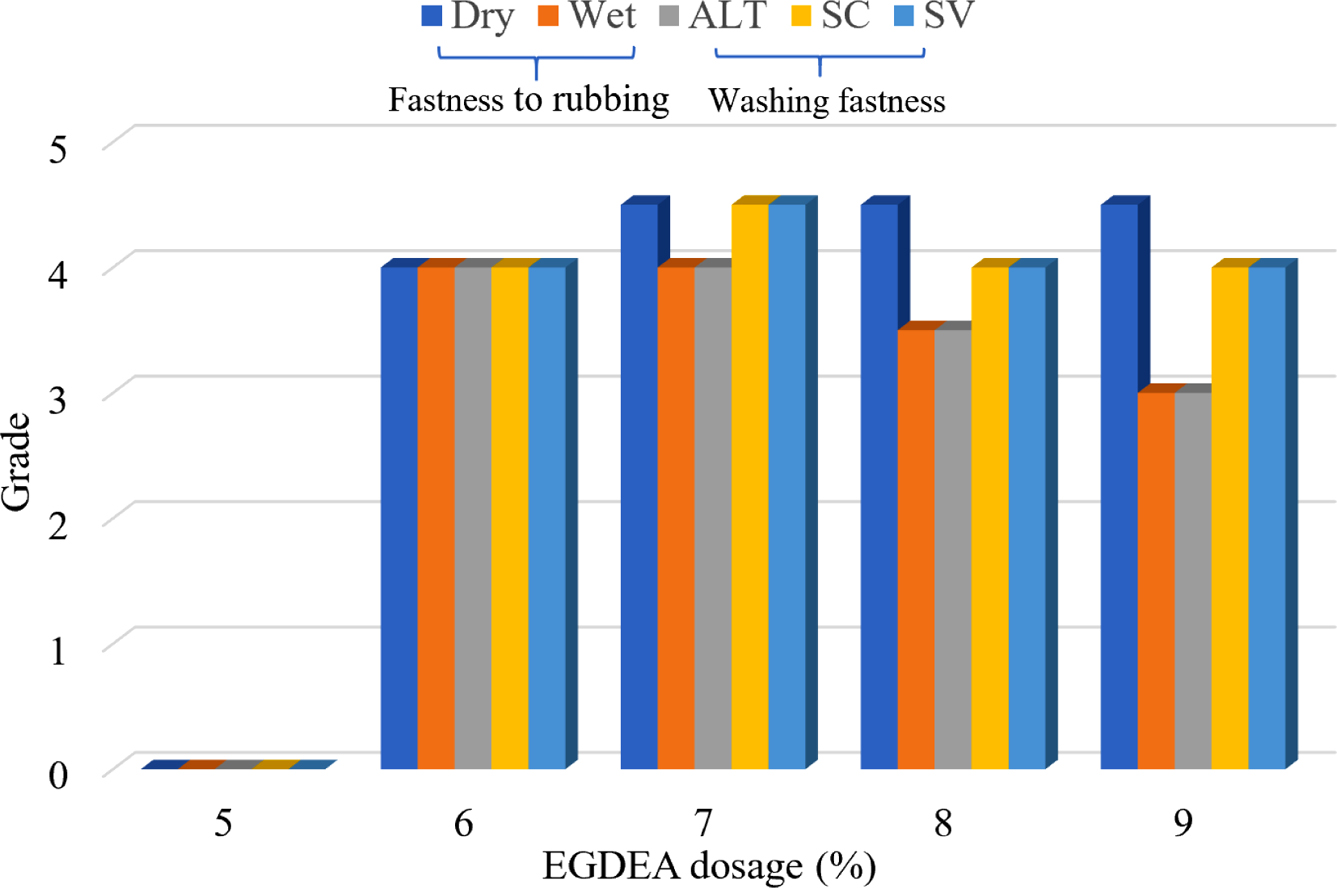
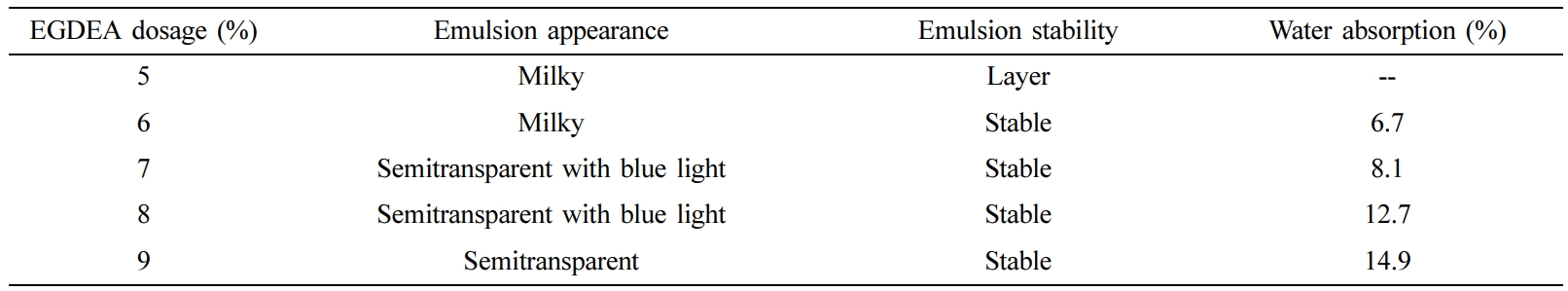
EGDEA Dosage: In the system of the synthesis of CWPU, the n (IPDI/PPG-1000) was set to 2.2, and the neutralizational degree was 100 %. The properties of CWPU emulsion and the fastness of treated cotton fabrics under different EGDEA dosage were shown in Table 4 and Figure 5.
The experimental data are shown in Table 4 and Figure 5. It was observed that under the same condition of others, as the EGDEA content increases, the appearance of the emulsion changes from milky white to transparent. This indicated that the particle size of the emulsion is gradually diminished and the stability of the emulsion is also improved. In the meantime, as the content of EGDEA increases, the water resistance of the adhesive film decreases. It may be explained by the fact that the hydrophilicity of CWPU is enhanced, the polymer molecular chains are not easily entangled with each other and the crosslink density between the molecules is reduced. This process ultimately resulted in that water molecules enter the adhesive film more easily, thus the water resistance of the film is reduced.29 But, the fastness level of the color fixing effect increased initially, then decreased as EGDEA concentration increased, meaning that the appropriate cationic group can react with the dye anionic group to improve the adhesion fastness of the reactive dye on the fabric. After that, the auxiliary molecules which has been absorbed will have a large repulsive force on the continuously diffused auxiliary molecules, and affect the color fixing effect. Based on the above analysis, considering emulsion stability, film-forming property, and fastness level comprehensively, it is more appropriate to choose EGDEA dosage at 7%.
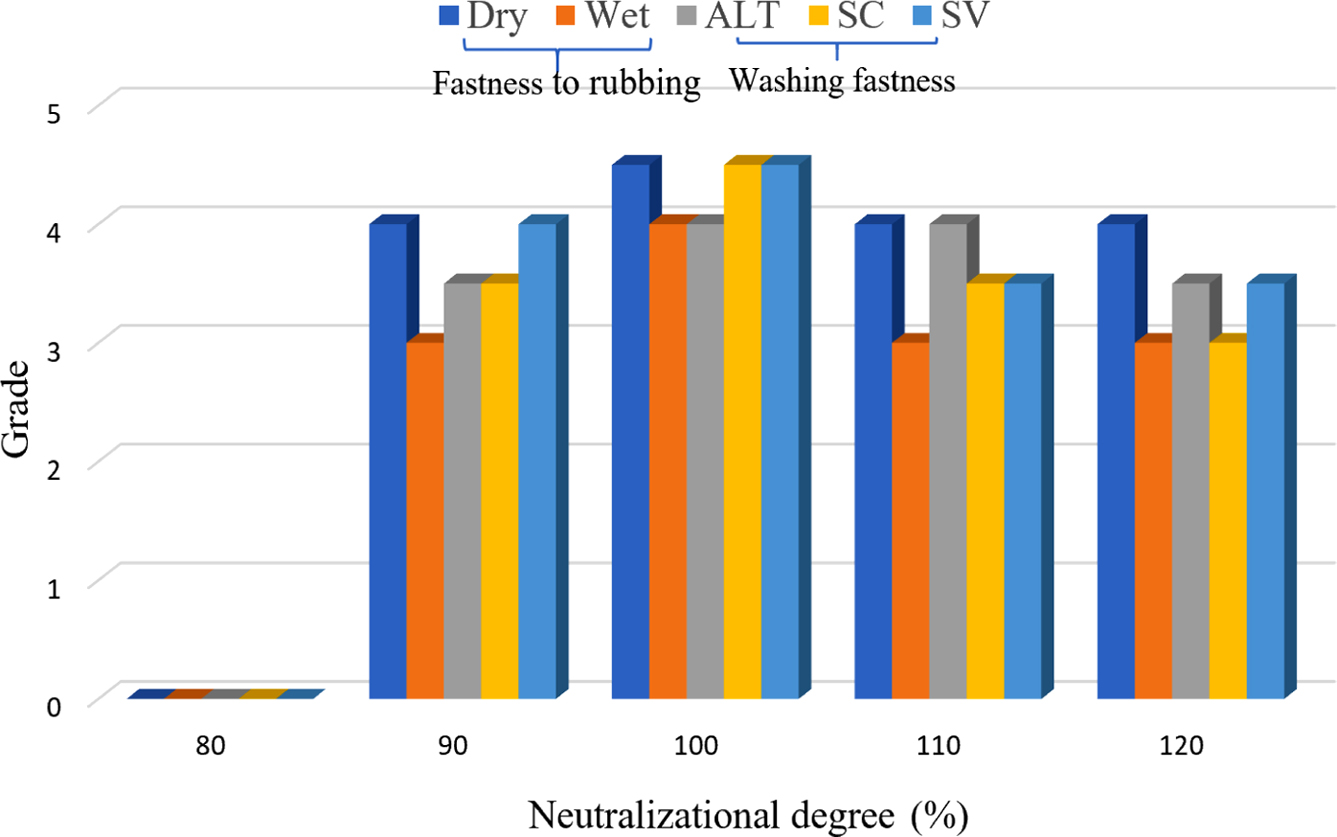
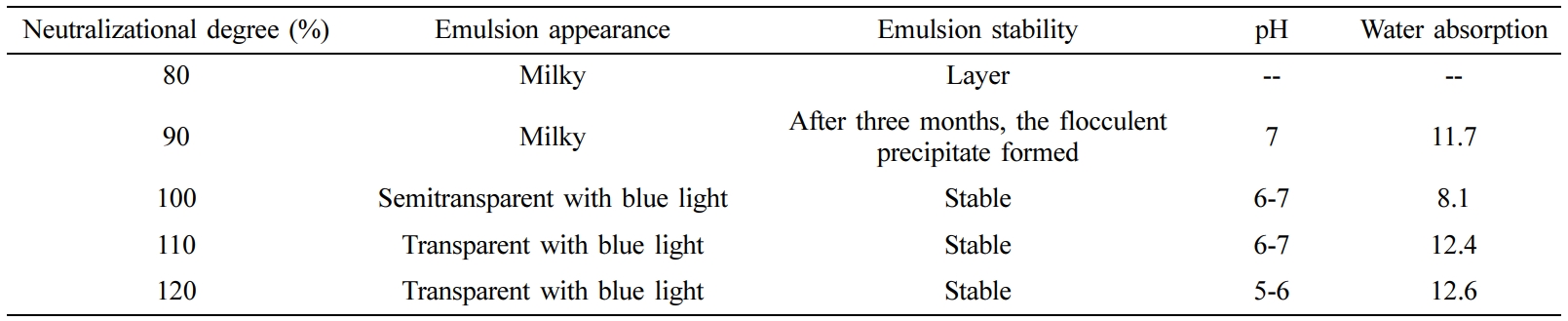
Neutralizational Degree: In the system of the synthesis of CWPU, the n (IPDI/PPG-1000) was set to 2.2, the EGDEA dosage was 7%. The influence of neutralizational degree on CWPU emulsion properties and fastness of treated cotton fabrics was investigated, as shown in Table 5 and Figure 6.
The experimental data are shown in Table 5. As the neutralizational degree increased from 80% to 120%, emulsion appearance changed from milky white to transparent pan-blue light. It can be explained by the fact that with the increases of the concentration of the quaternary ammonium cation group, the ion density on the surface of the latex particles gradually increases, and the hydrophilicity of the polyurethane prepolymer gradually increases, which improves the emulsification effect of the polymer prepolymer with water. The result is that the dispersion of the emulsion becomes more uniform and more stable and the appearance of the emulsion gradually transparent. When the neutralizational degree is low, there are fewer active centers on the polymer molecular chain, so the hydrophilicity of polyurethane prepolymer cannot be fully expressed. And when polyurethane prepolymer is dispersed in water to form larger latex particles, the denseness of the formed rubber film is poor, so the water resistance is poor. With the increases of the neutralizational degree, the concentration of the quaternary ammonium cation center on the polymer chain becomes larger, and the hydrophilicity of polyurethane prepolymer also enhances.30,31 The polyurethane prepolymer is easier to disperse in water, and the emulsion particle size is smaller. So that the adhesive film is formed with enhanced water resistance. When the neutralizational degree is larger than 100%, the content of hydrophilic group in the molecular chain will no longer change, the particle size of the emulsion will no longer change, and the excess glacial acetic acid will become an impurity in the reaction system. The water resistance will decrease as the neutralizational degree increases. As depicted in Figure 6, the point with the best fastness was known when the neutralizational degree is 100%. This is attributed to the large particle size of polyurethane emulsion when neutralizational degree is less than 100%. This leads to poor film formation of polyurethane on the fiber. When the neutralizational degree exceeds 100%, the fixing agent solution has a lower pH than the neutralizational degree of 100%, and the number of dye molecules forming covalent bonding with the fiber is reduced. Taken together, the neutralizational degree is 100%.
In a subsequent discussion, CWPU used in the test and characterization was prepared by the optimized process (n (IPDI/PPG-1000) was 2.2, EGDEA dosage was 7%, neutralizational degree was 100%).
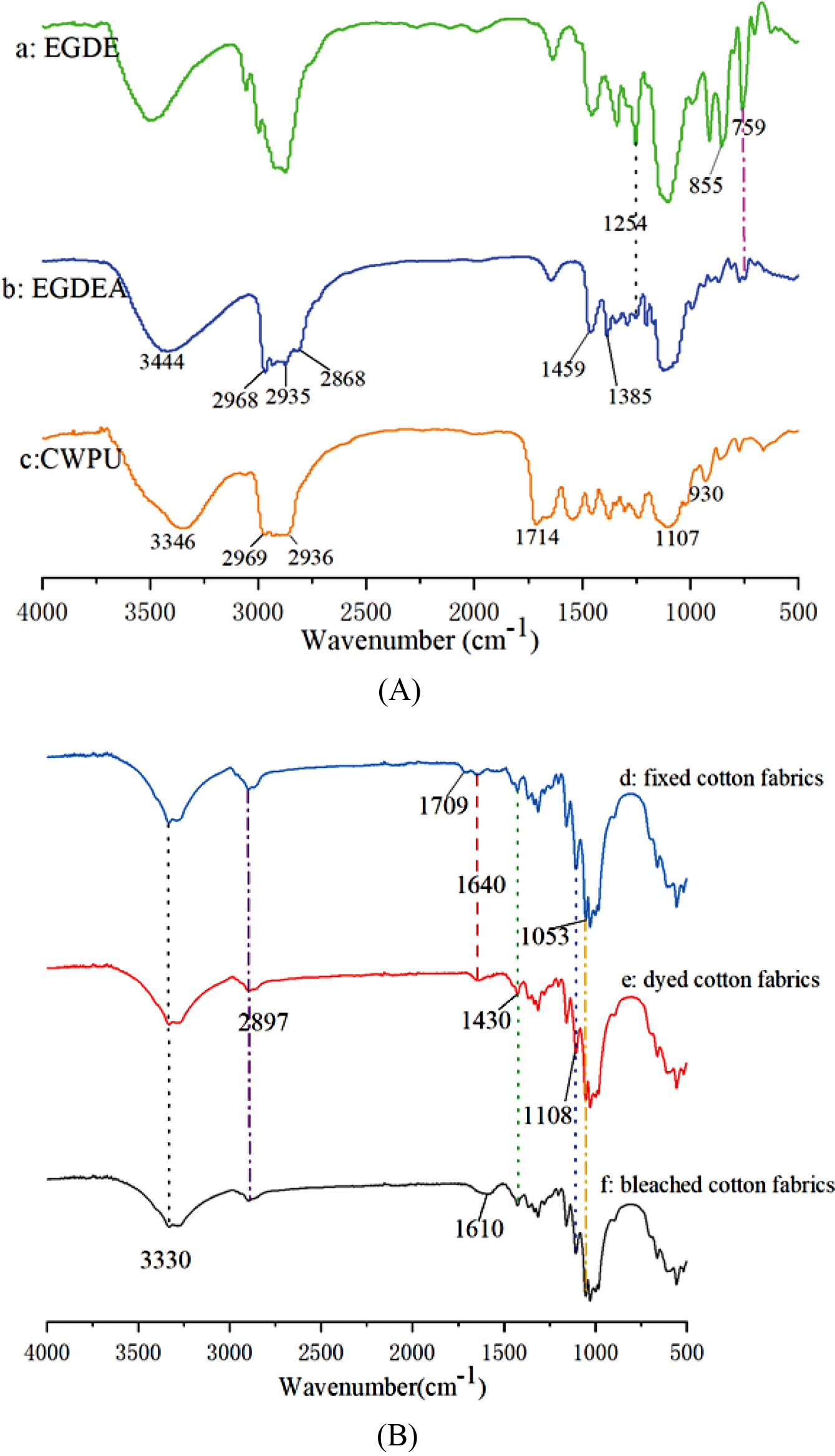
Characterization of CWPU. FTIR Spectroscopy Analysis: The chemical structures of EGDE, EGDEA and CWPU were confirmed by FTIR as Figure 7(A) shows. As shown in Figure 7(a), EGDE exhibited the characteristic absorption peaks at 1254, 855 and 759cm-1 which were assigned to epoxy groups. In Figure 7(b), the stretching vibrational peaks at 2968, 2935 and 2868cm-1 corresponded to the -CH3 and -CH2 groups; Symmetric and asymmetric bending vibration absorption peaks of methyl groups at 1459 and 1385cm-1, respectively. Compared with Figure 7(a), the characteristic absorption peaks of epoxy groups at 1254, 855, and 759cm-1 disappear or were weakened. In addition, the -OH frequency-doubled stretching vibration absorption peak at 3444 cm-1 was strengthened. Indicating that a ring-opening reaction has occurred and the desired cationic chain extender has been formed. In Figure 7(c) of the CWPU, the -NH in -NHCOO- had a stretching vibration peak at 3346 cm-1 and the characteristic absorption peaks at 2969 and 2936cm-1 were assigned to the -CH3, -CH2 stretching vibrations, respectively. The C-O-C stretching vibration peaks near 1107 and 930cm-1 indicated that the target product CWPU was generated.
In Figure 7(B), the spectrum of bleached cotton fabrics shows some peaks at around 3330, 2897, 1610, 1430 and 1053cm-1, which are attributed to the -OH stretching, C-H stretching, -OH of water absorbed from cellulose, CH2- symmetric bending and C-O stretching, respectively.32 Compared to cotton fabrics, the spectrum of dyed cotton fabrics shows a new peak at around 1640 cm-1 is attributed to the C=N stretching vibration, and the vibration peak of C=O bond appeared at 1708cm-1 on the fixed cotton fabrics. This phenomenon is caused by the s-triazinyl group on the dye and the carbamate on the CWPU, respectively. At the same time, compared with bleached cotton fabrics, the absorption peaks of dyed cotton fabrics and fixed cotton fabrics at 1108 and 1053cm-1 are obviously enhanced, and the fixed cotton fabrics have greater enhancement. This is due to the reaction of dyes and fibers to form ether bonds, C-O-C of CWPU itself and the -OH on the dye reacts with the -NCO on the CWPU to form -COONH-.
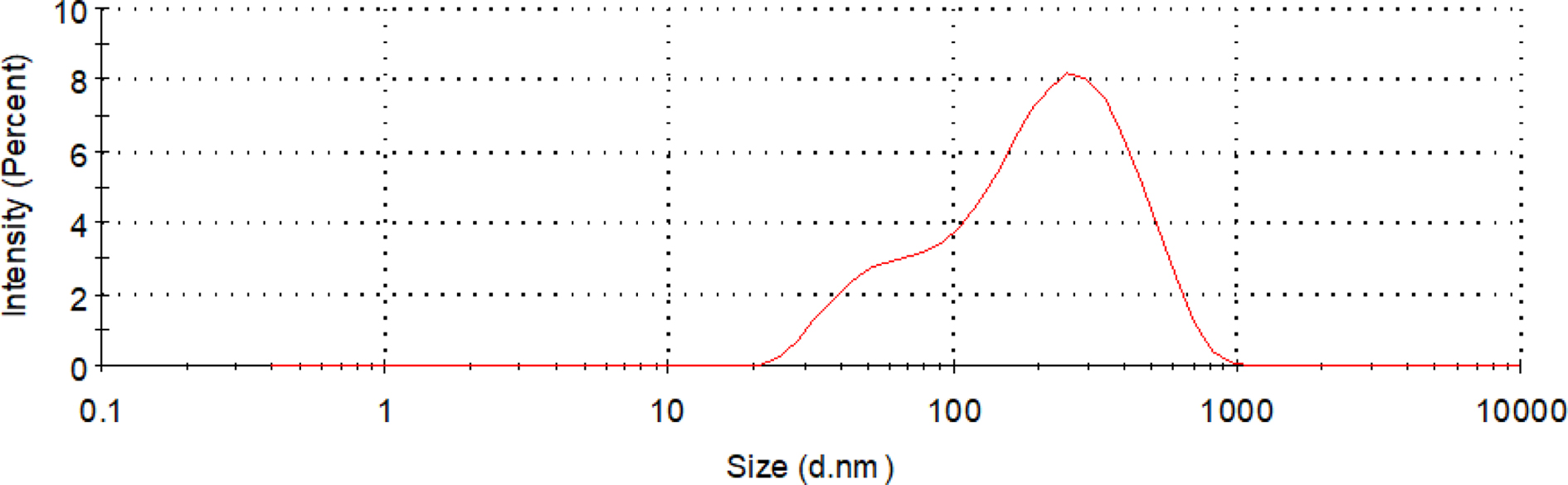
Particle Size, Zeta Potential, Particle Size Distribution: CWPU was synthesized according to the optimized synthesis process. The main physical properties of the emulsion were observed and measured. The results are shown in Table 6 and Figure 8.
As can be seen from Table 6 and Figure 8, the average particle size of the emulsion particles is 125 nm, and the emulsion particle size distribution is narrow. This shows that the particle size is relatively uniform. The average Zeta potential of the emulsion was 33.1 mV, and its absolute value was greater than 30 mV. It has a higher positive charge, and the electrostatic repulsion between the emulsions can keep the emulsion particles in the system stable, and the emulsion stability is better.
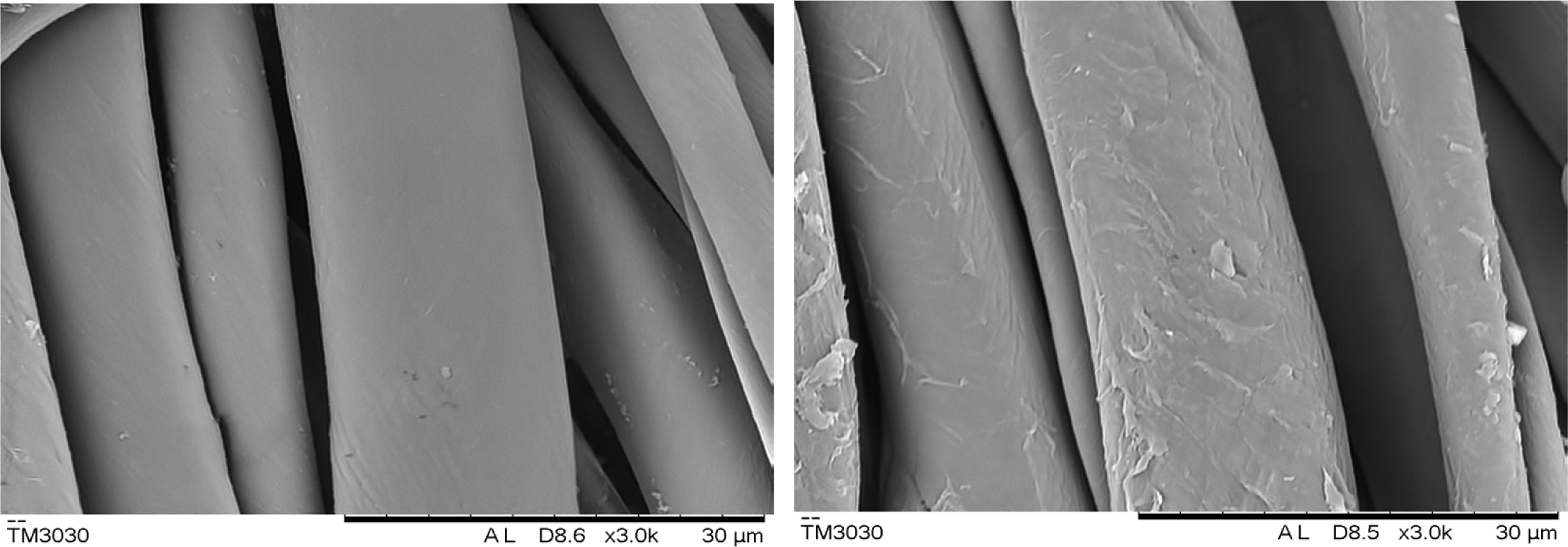
SEM Analysis: SEM micrographs of pristine and CWPU coated cotton fabrics were illustrated in Figure 9. It can be seen from Figure 9 that the surface of the fixed cotton fiber is covered with a film formed by baking the fixing agent. This layer of fixing agent film increases the smoothness of the fabric surface. And the film wraps the dye on the fiber to prevent the dye from falling off during the rubbing process and improve the rubbing fastness and soaping fastness.
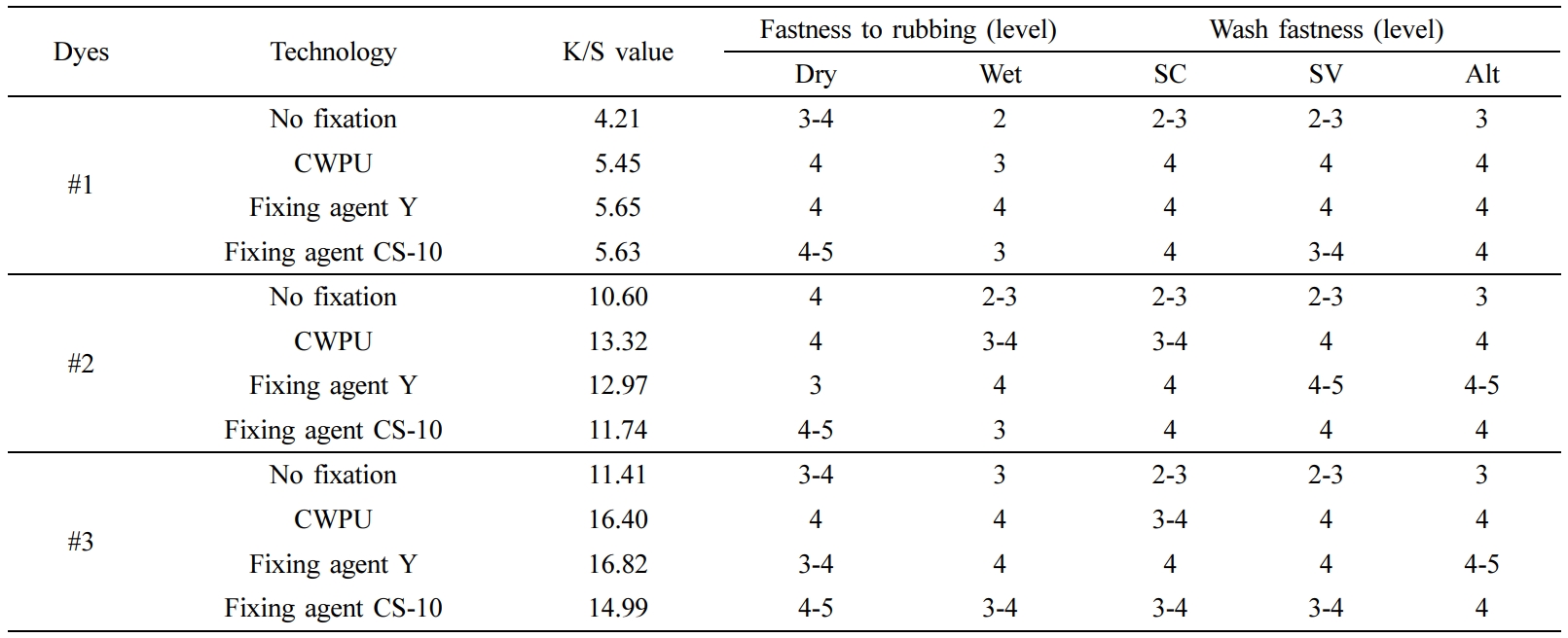
Comparisons of Fixing Effect. The CWPU fixing agent was synthesized by the above optimum process conditions. It is applied to the cotton fabrics dyed with reactive dyes. The CWPU treated fabric was compared with the unfixed fabric, and the fabric treated with fixing agent Y or cationic fixing agent (copolymer of dimethyldiallylammonium chloride and diallylamine), and the results are shown in Table 7.
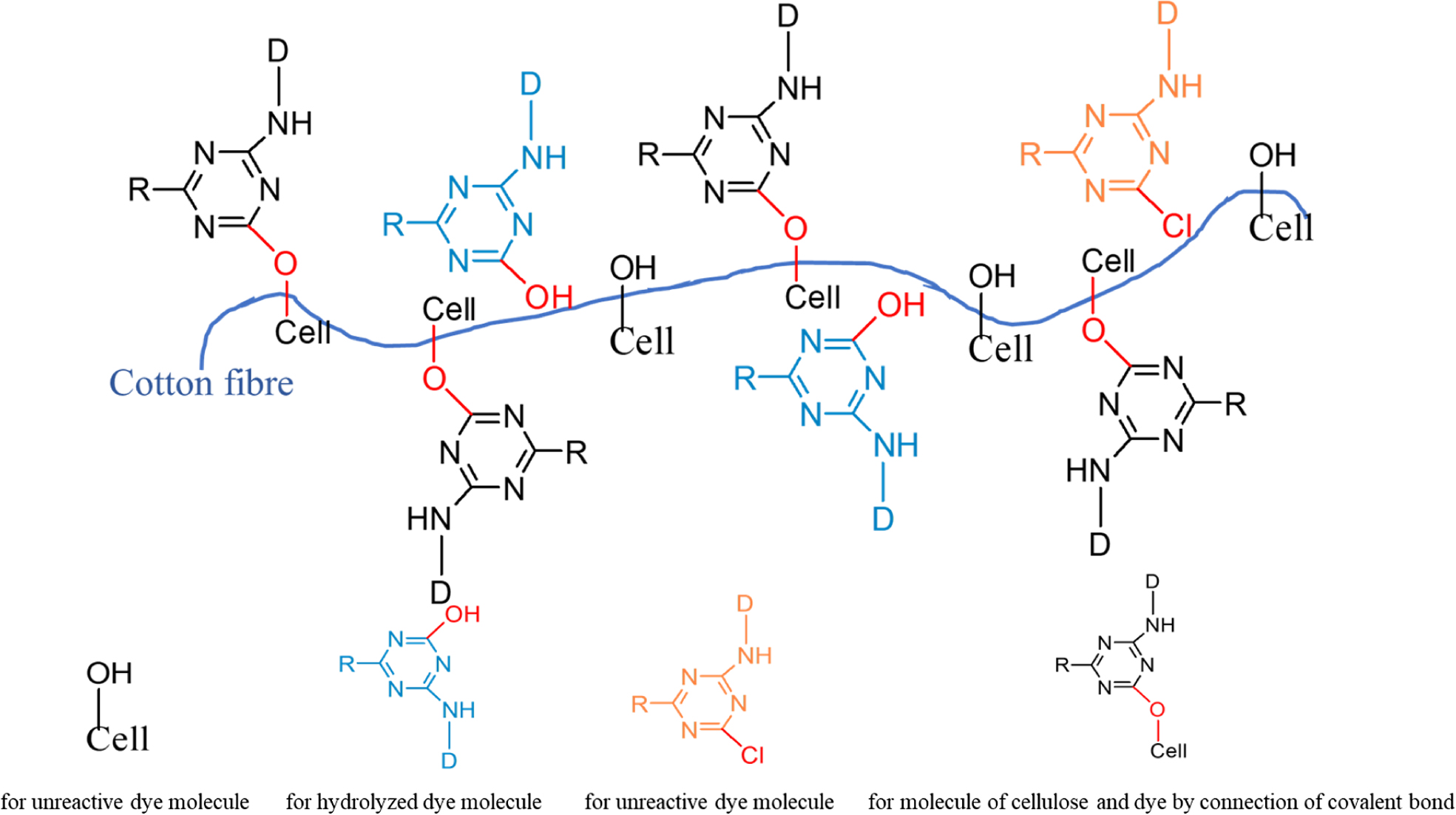
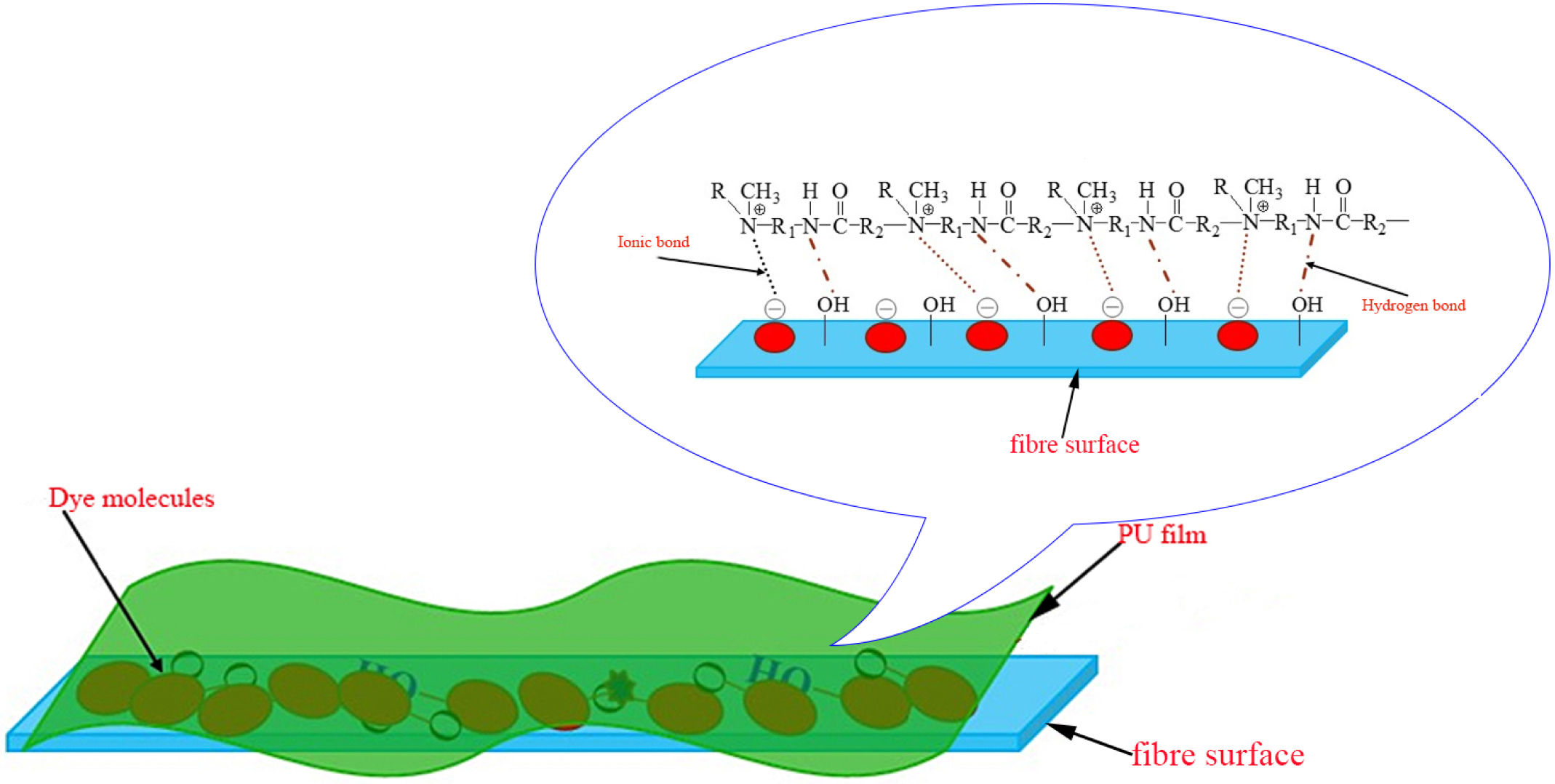
As we can be seen from Table 7, the dry rubbing fastness of fabrics treated with CWPU can be improved by 0 to 0.5 grade, the wet rubbing fastness can be improved by 0.5 to 1 grade, and the fade and staining can be improved by 0.5 to 1 grade. The fixing effect of the fixing agent Y on wet rubbing fastness is better than CWPU, but the advantage of CWPU is that it does not contain formaldehyde. Comparing the K/S value of the fabric after fixing, it can be seen that the fabric treated with CWPU fixing color is darker and the dyeing effect is better. The excellent color fixing performance of CWPU can be attributed to the following three reasons (the illustration was shown in Figure 10).
a) The quaternary ammonium ion of CWPU can combine with the anionic group of reactive dye by ionic bond, so that the dyes will form an insoluble lake in the fixing agent and settle on the fiber, reduce its water solubility and improve the color fastness of dyed fabrics.33,34
b) During the curing and baking process, the ‘protected -NCO group’ is slowly released as the temperature rises, and reacts with the active groups on the fabric or dye (such as -OH, -NH2).35 The CWPU molecule is combined with the fabric or dye in the form of a covalent bond, which improves the fixing effect of the fabric.
c) The CWPU can form a layer of protective film similar to the net on the surface of the fiber due to its high polymer nature, which can prevent some dyes from directly contacting the friction head.20,25 It can reduce the degree of dissolution and hydrolysis of the dye in the soap solution, thereby can reduce the staining of the dye on the cloth.
|
Figure 4 Fastness properties of the different n (IPDI/PPG-1000). Alt: color change of dyed sample; SC: staining on cotton; SV: staining on viscose, wash-scale (1-5). |
|
Figure 5 Fastness properties of the different EGDEA dosage (%). Alt: color change of dyed sample; SC: staining on cotton; SV: staining on viscose, wash-scale (1-5). |
|
Figure 6 Fastness properties of the different neutralizational degree (%). Alt: color change of dyed sample; SC: staining on cotton; SV: staining on viscose, wash-scale (1-5). |
|
Figure 7 (A) FTIR spectra of EGDE(a), EGDEA(b), and CWPU(c); (B) FTIR spectra of bleached cotton fabrics, dyed cotton fabrics and fixed cotton fabrics. |
|
Figure 8 Particle size and distribution of CWPU emulsion. |
|
Figure 9 SEM image of cotton fibers before and after fixing. |
|
Figure 10 The fixing mechanism of CWPU. |
Cationic chain extender EGDEA was synthesized by ethylene glycol diglycidyl ether and diethylamine. Using the self-emulsifying method, the n (IPDI/PPG-1000) was 2.2, the EGDEA dosage was 7%, and the neutralizational degree was 100%, which can produce an aqueous polyurethane emulsion with excellent performance, good appearance and stability. FTIR indicates the synthesis of the target product, and SEM indicates that the target product is finished on the surface of the fabric. After CWPU treatment on cotton fabrics dyed with reactive dyes, the dry rubbing fastness can be improved by 0 to 0.5, the wet rubbing fastness can be improved by 0.5 to 1, and the soaping fastness can be improved by 0.5 to 1. Compared with two commercial fixing agents, the fixing agent has similar fixing, has less influence on the color of the fabric and environment-friendly.
- 1. Mashaly, H. M.; Hauser, P. J. #Cold Pad Batch Cationization and Dyeing of Linen Fabric with Reactive Dyes. #Res. J. Text. Apparel #2012, 16, 111-118.
-

- 2. Burkinshaw, S.; Mignanelli, M.; Froehling, P.; Bide, M. #The Use of Dendrimers to Modify the Dyeing Behaviour of Reactive Dyes on Cotton. #Dyes Pigm. #2000, 47, 259-267.
-

- 3. Hashem, M. M. #Development of a One-stage Process for Pretreatment and Cationisation of Cotton Fabric. #Color. Technol. #2006, 122, 135-144.
-

- 4. Evans, G. E.; Shore, J.; Stead, C. V. #Dyeing Behaviour of Cotton after Pretreatment with Reactive Quaternary Compounds. #J. Soc. Dyers Colour. #1984, 100, 304-315.
-

- 5. Lee, C. H.; Tang, A. Y. L.; Wang, Y.; Kan, C. W. #Effect of Reverse Micelle-encapsulated Reactive Dyes Agglomeration in Dyeing Properties of Cotton.# Dyes Pigm. #2019, 161, 51-57.
-

- 6. Siddiqua, U. H.; Ali, S.; Iqbal, M.; Hussain, T. Relationship between Structure and Dyeing Properties of Reactive Dyes for Cotton Dyeing. J. Mol. Liq. 2017, 241, 839-844.
-

- 7. Rahman Bhuiyan, M. A.; Hossain, M. A.; Zakaria, M.; Islam, M. N.; Zulhash Uddin, M. #Chitosan Coated Cotton Fiber: Physical and Antimicrobial Properties for Apparel Use. #J. Polym. Environ. #2017, 25, 334-342.
-

- 8. Shu, D.; Fang, K.; Liu, X.; Cai, Y.; Zhang, X.; Zhang, J. #Cleaner Coloration of Cotton Fabric with Reactive Dyes Using a Pad-batch-steam Dyeing Process. #J. Clean Prod. #2018, 196, 935-942.
-

- 9. Chen, L.; Wang, B.; Ruan, X.; Chen, J.; Yang, Y. #Hydrolysis-free and Fully Recyclable Reactive Dyeing of Cotton In Green, Non-nucleophilic Solvents for a Sustainable Textile Industry. #J. Clean Prod. #2015, 107, 550-556.
-

- 10. Riaz, B.; Dilshad, H.; Muhammad, N.-U.-H.; Rajput, A. W.; Rana, #A. Eco-friendly Route for Dyeing of Cotton Fabric Using Three Organic Mordants in Reactive Dyes.# Ind. Textila. #2019, 70, 25-29.
-

- 11. Luo, Y.; Pei, L.; Zhang, H.; Zhong, Q.; Wang, J. #Improvement of the Rubbing Fastness of Cotton Fiber in Indigo/Silicon Non-Aqueous Dyeing Systems. #Polymers #2019, 11, 1854-1863.
-

- 12. El-Molla, M.; Helmy, M.; El-Mahdey, F. A.-E.# Preparation and Utilization of Fixing Agents for Dyed Cotton Fabrics. #Egypt. J. Chem. #2020, 63, 37-50.
-

- 13. Nallathambi, A.; #Venkateshwarapuram Rengaswami, G. D. Salt-free Reactive Dyeing of Cotton Hosiery Fabrics by Exhaust Application of Cationic Agent. #Carbohydr. Polym. #2016, 152, 1-11.
-

- 14. Choi, H. J.; Choe, H.; Seo, W. J.; Kim, J. H. #Physical Properties of Flexible Polyurethane Foams Manufactured by Varying Toluene Diisocyanate Contents.# Polym. Korea #2019, 43, 532-539.
-

- 15. Liu, L.; Yao, J.# Salt-free Dyeability of Thiourea Grafted Cotton Fabric. #Fiber. Polym. #2011, 12, 42-49.
-

- 16. Javaid Mughal, M.; Saeed, R.; Naeem, M.; Aleem Ahmed, M.; Yasmien, A.; Siddiqui, Q.; Iqbal, M. #Dye Fixation and Decolourization of Vinyl Sulphone Reactive Dyes by Using Dicyanidiamide Fixer in the Presence of Ferric Chloride.# J. Saudi Chem. Soc. #2013, 17, 23-28.
-

- 17. Liu, Y.; Zhao, X.# Preparation of a Cationic Environment-friendly Fixing Agent. #Fibres Text. East. Eur.# 2017, 25, 96-102.
-

- 18. Akindoyo, J. O.; Beg, M. D. H.; Ghazali, S.; Islam, M. R.; Jeyaratnam, N.; Yuvaraj, A. R. #Polyurethane Types, Synthesis and Applications - A Review.# RSC Adv.# 2016, 6, 114453-114482.
-

- 19. Xinrong, S.; Nanfang, W.; Kunyang, S.; Sha, D.; Zhen, C. #Synthesis and Characterization of Waterborne Polyurethane Containing UV Absorption Group for Finishing of Cotton Fabrics. #J. Ind. Eng. Chem.# 2014, 20, 3228-3233.
-

- 20. Liang, H.; Liu, L.; Lu, J.; Chen, M.; Zhang, C.# Castor Oil-based Cationic Waterborne Polyurethane Dispersions: Storage Stability, Thermo-physical Properties and Antibacterial Properties. #Ind. Crop. Prod. #2018, 117, 169-178.
-

- 21. Santamaria-Echart, A.; Fernandes, I.; Ugarte, L.; Barreiro, F.; Arbelaiz, A.; Corcuera, M. A.; Eceiza, A. #Waterborne Polyurethane-urea Dispersion with Chain Extension Step in Homogeneous Medium Reinforced with Cellulose Nanocrystals. #Compos. Pt. B-Eng. #2018, 137, 31-38.
-

- 22. Jiménez-Pardo, I.; Sun, P.; van Benthem, R. A. T. M.; Esteves, A. C. C. #Design of Self-dispersible Charged-polymer Building Blocks for Waterborne Polyurethane Dispersions. #Eur. Polym. J. #2018, 101, 324-331.
-

- 23. Špírková, M.; Hodan, J.; Kredatusová, J.; Poręba, R.; Uchman, M.; Serkis-Rodzeń, M. #Functional Properties of Films Based on Novel Waterborne Polyurethane Dispersions Prepared without a Chain-extension Step.# Prog. Org. Coat. #2018, 123, 53-62.
-

- 24. Li, R.; Ton Loontjens, J. A.; Shan, Z. #The Varying Mass Ratios of Soft and Hard Segments in Waterborne Polyurethane Films: Performances of Thermal Conductivity and Adhesive Properties.# Eur. Polym. J.# 2019, 112, 423-432.
-

- 25. Sardon, H.; Irusta, L.; González, A.; Fernández-Berridi, M. J. #Waterborne Hybrid Polyurethane Coatings Functionalized with (3-aminopropyl)triethoxysilane: Adhesion Properties. #Prog. Org. Coat. #2013, 76, 1230-1235.
-

- 26. Wang, Z.; Gao, D.; Yang, J.; Chen, Y. #Synthesis and Characterization of UV-curable Waterborne Polyurethane-acrylate Ionomers for Coatings. #J. Appl. Polym. Sci. #1999, 73, 2869-2876.
- 27. Dong, C.; Xin, W.; Luo, Y. #Synthesis and Application of a Cationic Waterborne Polyurethane Fixative Using Quaternary Ammonium Diol as a Chain Extender. #RSC Adv. #2018, 8, 42041-42048.
-

- 28. Morel, A.; Salaün, F.; Bedek, G.; Dupont, D.; Giraud, S. #Water Vapor Permeability of Thermosensitive Polyurethane Films Obtained from Isophorone Diisocyanate and Polyester or Polyether Polyol.# J. Mater. Sci. #2017, 52, 1014-1027.
-

- 29. Guo, Y.; Guo, J.; Miao, H.; Teng, L.; Huang, Z.# Properties and Paper Sizing Application of Waterborne Polyurethane Emulsions Synthesized with Isophorone Diisocyanate. #Prog. Org. Coat. #2014, 77, 988-996.
-

- 30. Yang, W.; Cheng, X.; Wang, H.; Liu, Y.; Du, Z. #Surface and Mechanical Properties of Waterborne Polyurethane Films Reinforced by Hydroxyl-terminated Poly(fluoroalkyl methacrylates). #Polymer #2017, 133, 68-77.
-

- 31. Ge, Z.; Luo, Y. #Synthesis and Characterization of Siloxane-modified Two-component Waterborne Polyurethane. #Prog. Org. Coat. #2013, 76, 1522-1526.
-

- 32. Xu, L.-L.; Guo, M.-X.; Liu, S.; Bian, S.-W. #Graphene/Cotton Composite Fabrics as Flexible Electrode Materials for Electrochemical Capacitors. #RSC Adv. #2015, 5, 25244-25249.
-

- 33. Liu, Z.; Tian, Y.; Kang, S.; Zhang, X. #Synthesis and Characterization of Novel Epoxy-modified Waterborne Polyurethanes and Their Use in Carbon Fiber Sizing. #J. Appl. Polym. Sci. #2012, 125, 3490-3499.
-

- 34. Chang, J.; Wang, X.; Shao, J.; Li, X.; Xin, W.; Luo, Y. #Synthesis and Characterization of Environmentally-Friendly Self-Matting Waterborne Polyurethane Coatings. #Coatings #2020, 10, 494-505.
-

- 35. Wang, H.-H.; Lin, M.-S. #Poly(urea-urethane) Polymers with Multi-functional Properties.# J. Polym. Res. #2000, 7, 81-90.
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2021; 45(1): 1-11
Published online Jan 25, 2021
- 10.7317/pk.2021.45.1.1
- Received on May 26, 2020
- Revised on Aug 3, 2020
- Accepted on Sep 1, 2020
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Xiangdong Zhou
-
*College of Textile and Clothing Engineering, Soochow University, Suzhou 215021, China **National Engineering Laboratory for Modern Silk, Suzhou 215123, China
- E-mail: zhouxiangdong@suda.edu.cn
- ORCID:
0000-0002-9743-0060











 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.