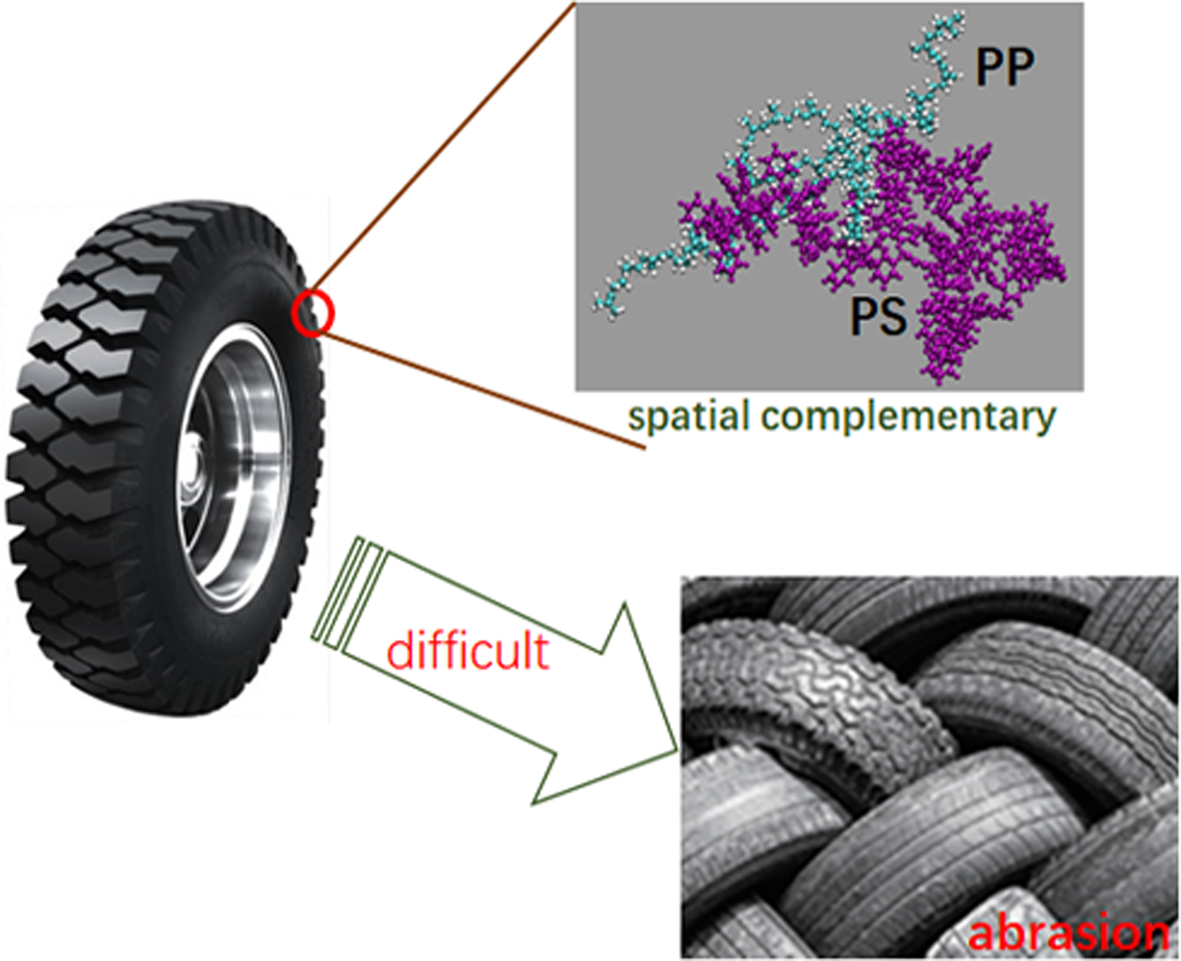
- Molecular Dynamics Study of Polyisoprene-polystyrene Composites: Spatial Complementary Behaviour
Doublestar Group Co., Ltd., Qingdao 266400, China
*College of Chemical and Biological Technology, Taiyuan University of Science and Technology, Taiyuan 030021, China- 폴리이소프렌-폴리스티렌 복합재료의 분자 동력학 연구: 공간 보완 거동
Various additives have been
applied to adjust the properties of rubber in the tire industry. As an
important environmental waste, plastic is a potential additive to be added to
rubber to blend aiming at forming abrasive and deformation resistance
elastomers. However, the molecular details remain unclear, especially for their
assembly structure. Using all-atom molecular dynamics simulations, we have
studied the assembly structures and processes of polyisoprene and polystyrene
complex, focusing on the spatial complementary behavior. The simulation results
indicate that polyisoprene and polystyrene can form tight entangled structure.
The polyisoprene can adjust their conformations to fill up the cavity generated
from polystyrene self-aggregation. The formed cross-linked and spatial
polystyrene complementary structures can improve the plasticity and abrasive
resistance, which is superiority in tire design. Our results provide an
important understanding of the rubber application and tire industry and give a
possible idea to deal with abandoned plastics.
The polyisoprene (PP) can adjust their conformations to
fill up the cavity generated from polystyrene (PS) self-aggregation and form
tight entangled structures. The formed cross-linked and spatial polystyrene
complementary structures can improve the plasticity and abrasive resistance,
which is superiority in tire design.
Keywords: tire industry, polyisoprene-polystyrene composites, assembly structure, spatial complementary behavior
We are grateful for financial support
from The Key Research and Development Projects of Shanxi
Province (201903D121025) and National Key R&D Program
of China (Grant No.2018YFC1902605).
The Supporting Information is
available regarding the additional simulation results, including
the glass transition temperature for PP and PS, time evolutions
of potential energy, radial distribution function of PP-PS and
PP-PP or PP:PS=20:5 and 20:10, time evolutions of LJ potential energy and density for different mixed ratios, and mean
square displacement of system and PS for different mixed ratios.
The materials are available via the Internet at http://journal.
polymer-korea.or.kr.
PK_2020_044_06_835_Supporting_Information_template.pdf (300 kb)
Supplementary Information
The amount of car has been expanding rapidly since the beginning of the
20th century, which is projected to increase to 1500 million by 2050.
The spectacular expansion of car usage has inevitably increased the number of
car accidents, with millions of people are suffering from the severe injuries
every year.1 Many reasons can lead to car accidents,2,3
such as driver careless and vehicle defects. Among important vehicle defects,
tire defect has become a serious problem.4 Tire defects can generate
from two main reasons, including physical damage and thermal damage, both often
occurring in high-speed driving.
Rubber, a polymer material, is the most important and the largest portion
of the tire. Previous studies indicate that rubbers can undergo the deformation
if existing external deformation force and stress.5,6 Experiencing
long-time driving, the tire may abrade and generate irreversible deformation,
which will increase the risk of car accident. Thus, increasing the abrasive and
deformation resistance of rubber need further study.
Several additives have been selected to improve performance, such as
abrasive and deformation resistance. These additives contain nanoparticles7-12
and polymers.13,14 Plastic, such as polystyrene (PS), is a
long-chain structure same as rubber. Plastic is easily synthesized and even can
be obtained from environmental waste, thus some researchers focus on using
plastic to adjust the properties of rubber. Rheological properties like shear
viscosity, shear modulus, die swell, and extrudate surface have been monitored
for numerous thermoplastic elastomeric systems over the years.15-19
For example, Banerjee et al. have investigated the interaction parameter
of polyamide 6/ fluoroelastomer blends through rheological measurement by
estimating the interfacial tension between the dispersed rubber phase and the
continuous matrix of thermoplastic.20 As we know, structure
determines the properties, but no study paid attention to the structure of the
rubber and plastic mixture.
In this study, we have studied the assembly structures and processes of
polyisoprene (PP) and PS complex by all-atom molecular dynamics simulations.
The simulation results indicate that PP and PS can form tight entangled
structure, with PP adjusting their conformations to fill up the cavity of PS
self-aggregation. Depending on the adding ratio, adding PS can slightly
increase the gyration and increase the density of the complex. The formed
cross-linked and spatial complementary structures decrease the molecular
diffusion and can potentially improve the plasticity and abrasive resistance.
Gromos force field21 was selected to simulate the assemble
process of PP and PS and obtain final molecular structures, which has been
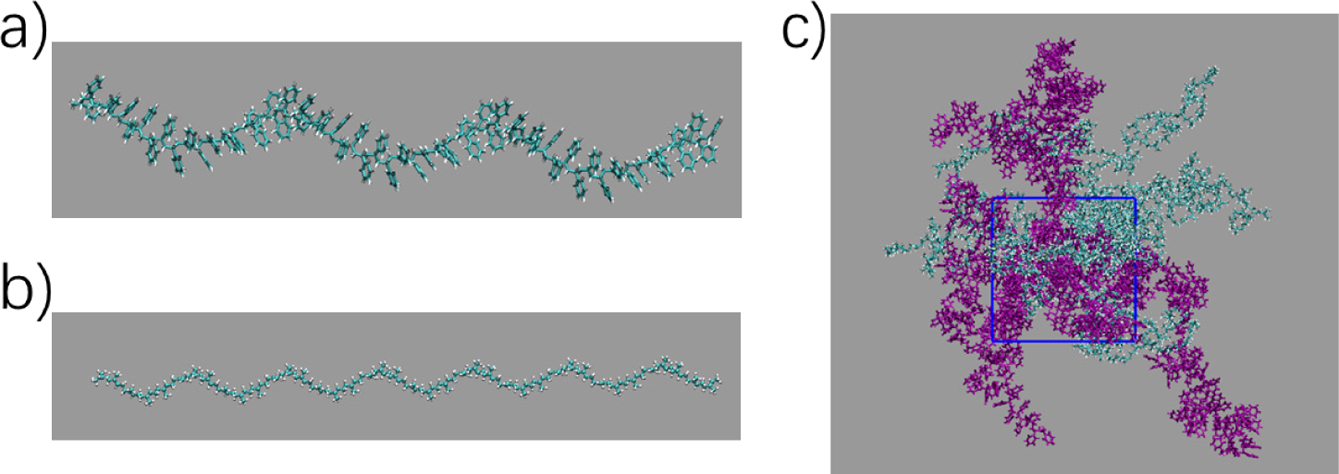
widely used in polymer simulations. The models of PP and PS were generated by
using ATB tool (Figure 1 (a), (b)).22 Each PP and PS both contain 40
repeated units. The system setup included 20 PP molecules and PS molecules with
different number, varying from 0 to 20. Both PP and PS monomers were inserted
randomly into the box (Figure 1(c)). Period boundary conditions were applied in
all three dimensions. The system energy was firstly minimized using the
steepest descent algorithm, and then followed by 1 ns simulation under NVT
ensemble condition using conjugate gradient algorithm. Isotropic Berendsen
barostat was used with a coupling constant of τP = 4
ps. The compressibility was 5×10-5 bar-1. The time step
of simulations was 10 fs, and the neighbor list was updated every 10
steps. The temperature was maintained at 298.15 K by v-rescale temperature
coupling. Simulations in NPT ensembles lasted until up to the equilibrium
state. All simulations were performed using GROMACS 4.6.7.23
Snapshots were rendered by VMD.24
|
Figure 1 Models for our simulations: (a) polystyrene; (b) polyisoprene; (c) initial mixed system. |
Structure
Characterization of PP and PS Assembly. To testify our
models of PP and PS, we have added extra simulations to calculate the glass
transition temperature for PP and PS. The system was prepared with the chains
in a stretched configuration. The system was firstly relaxed in melt at 540 K,
and then cooled down to 300 K (PS) and 120 K (PP) with a constant colling rate
of 0.01 K/ps. The glass transition temperature of PP and PS are 217 K and
406 K, respectively (Figure S1). The values agree well with the glass
transition temperatures in previous researches,25,26 thus our models
are appropriate. Four different mixed ratios of PP and PS were performed to
finish the assembly, experiencing 50 ns NPT equilibrium. From Figure S2, the
potential energy of different mixed ratios all finally reaches a plateau. Final
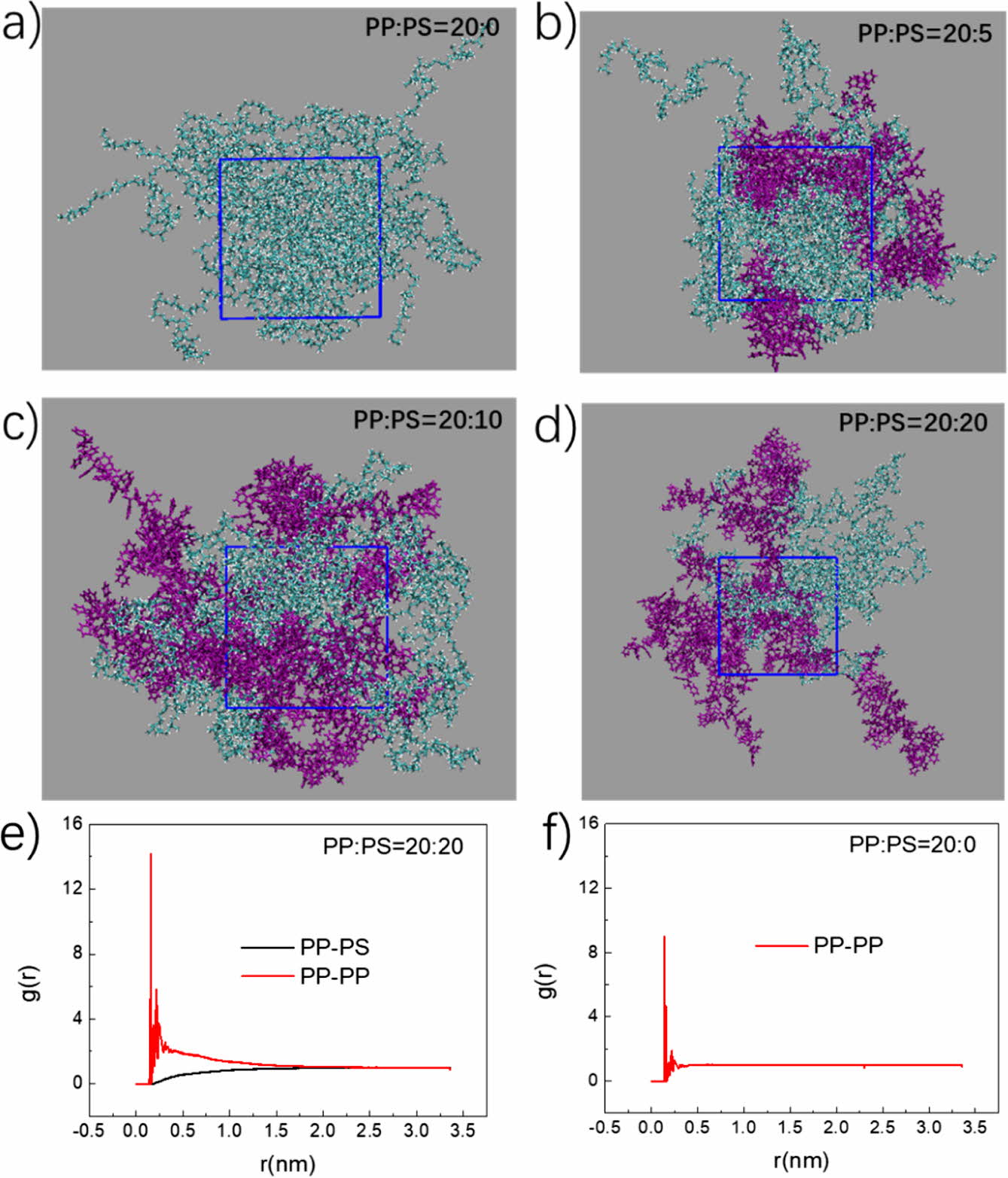
assembly structure was shown in Figure 2. For the pure PP system, initial
discrete PP finally formed an aggregation state (Figure 2(a)), corresponding to
a high peak of radial distribution function at 0.2 nm (Figure 2(f)). While
adding PS into the system, PP and PS can first assemble into aggregation via
cross-linking (Figure 2(b)-(d)). The peak at 0.2 nm for PP - PP contact
increases especially for the ratio of 20:20 (Figure 2(e) and Figure S3). This
means PS assembly can make PP themselves contact more tightly. LJ interaction
energy for PS-PP and PP-PP were shown in Figure 3(a) and Figure S4. PP-PP
interaction decreases while the PS-PP interaction increases. This means
additional PS molecules can contact the PP aggregation and finally form mixed
assembly structures. The separated PP and PS assemblies were shown in the
Figure 3(b) and 3(c), which exhibit amorphous structures. This indicates that
PS and PP can form irregular cross-linking structures. The cross-linking degree
is dependent on the adding ratio of PS. A higher adding ratio can make more PS
contact the PP. If no extra PS was added into rubber, the interaction of PP-PP
just kept at a stable level.
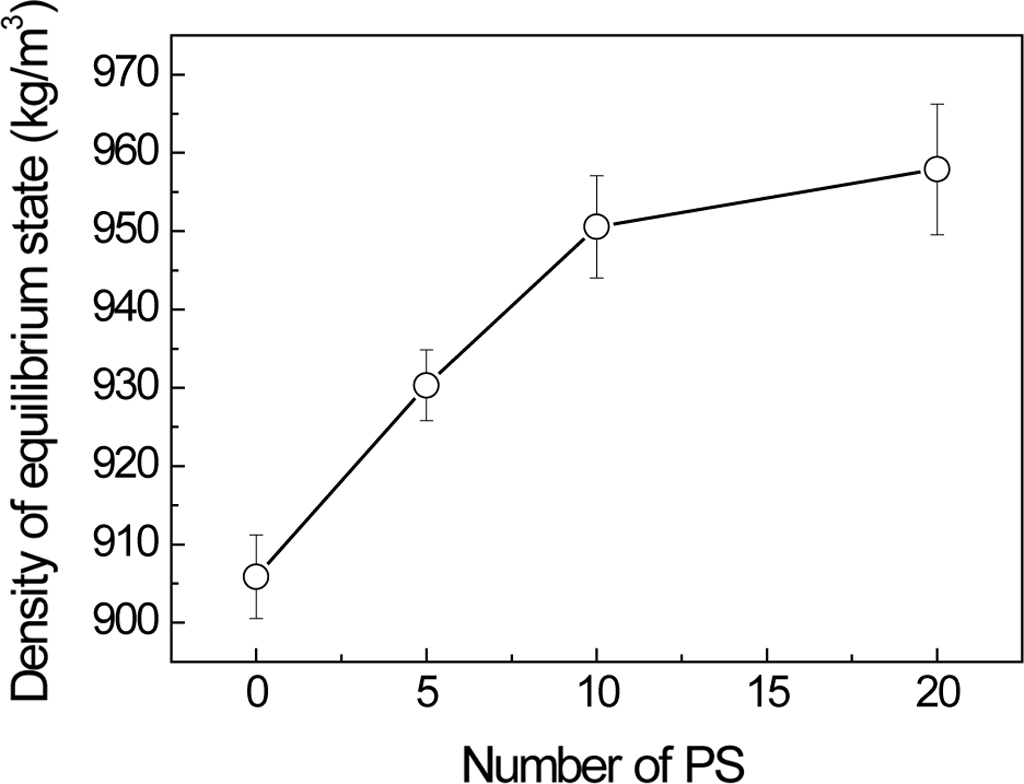
Adding
Ratio of PS Mediated Density and Diffusion Variation of PP and PS Assembly. From the above
analysis, PP and PS finally formed crossing-linking structures. With the
increase of adding ratio, the density of complex increases gradually. If the
adding ratio is smaller than 33.3%, the density increases linearly. However,
continuous adding PS cannot increase the density always via linear
relation. Adding PS up to the ratio of 20:20, the density is only a little
higher than the ratio of 20:10, because nearly all of the cavities of PP
aggregation were filled by the PS (Figure 4 and Figure S5).
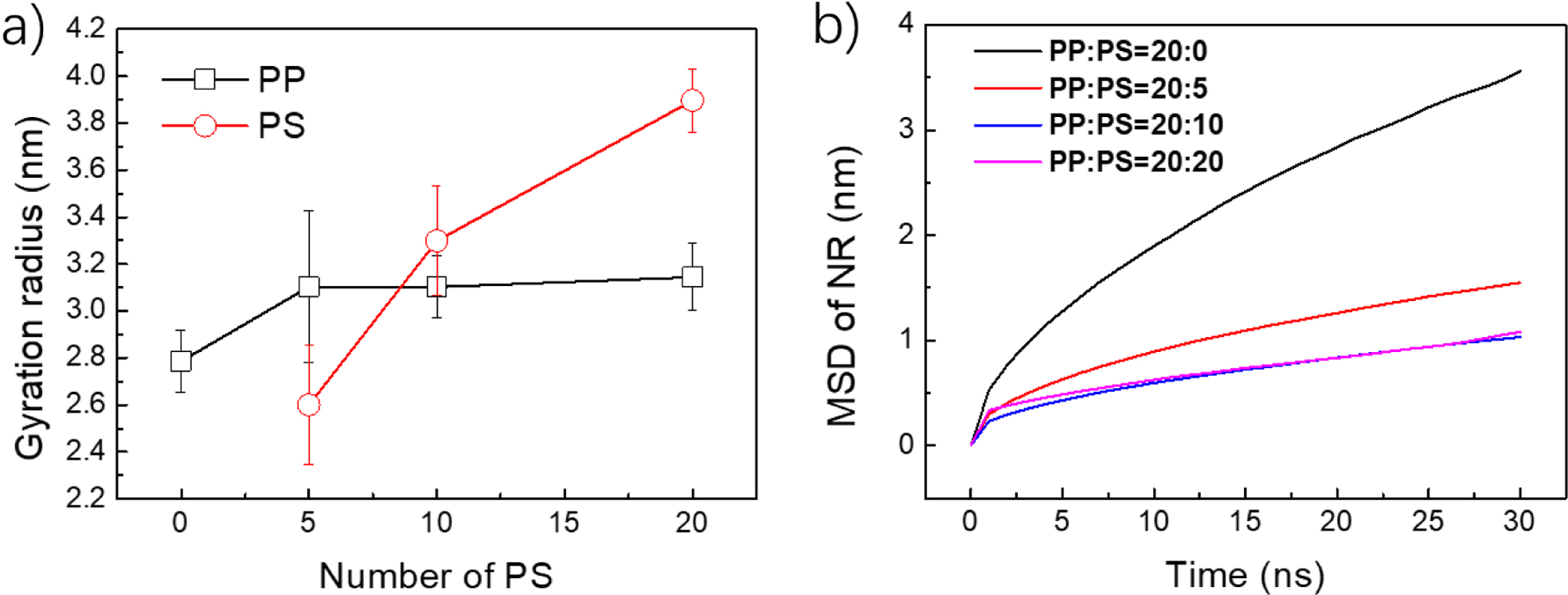
The gyration radius of different systems were calculated and shown in
Figure 5. For the pure PP system, the final gyration radius of single PP was
2.8 nm. After adding PS molecules, the gyration radius increases to
3.1 nm, no matter what the adding ratios are. It was expected for PS that
the gyration radius increases with the increase of adding ratio, because the PS
inserts into the PP assembly, which makes PS more amorphous. The cross-linking
structure also makes the diffusion of PP decreases, from the analysis of mean
square displacement (Figure 5(b)). Likewise, adding PS also can decrease the
diffusion rate of the whole system, which in turn proves the crossing-linking
structures (Figure S6(a), S6(b)).
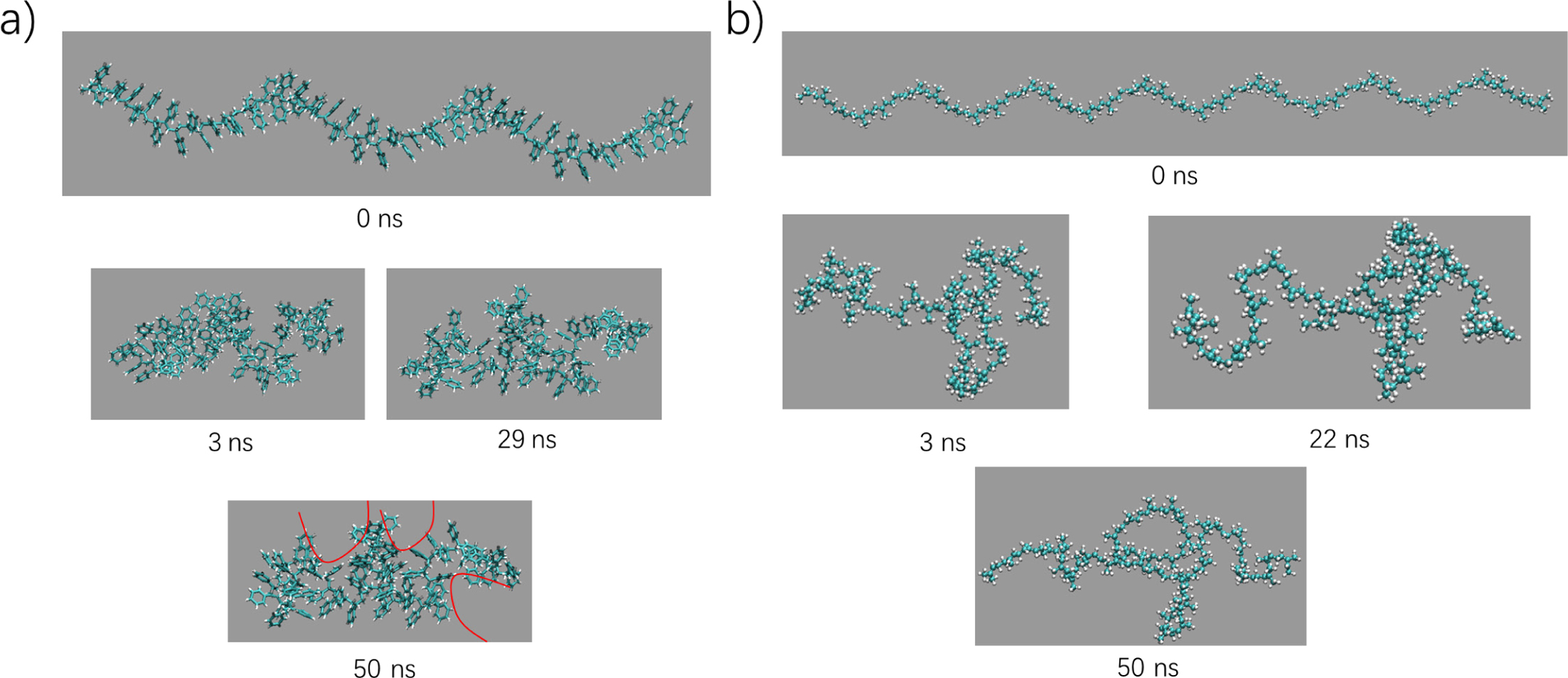
Spatial
Complementary Behavior of PS and PP. Comparing to experimental
investigations, molecular dynamics simulations can give structure details of
the molecular level. Time evolutions of typical snapshots for single PP and PS
were shown in Figure 6. For PS chain, it can shrink itself to aggregation by
hydrophobic and π-π interactions. But because of the steric hindrance and
bending rigid, it’s difficult for the long-chain to adjust itself to form a
compact structure. Inevitably, there are some defects on the surface of formed
aggregation, which have
been labeled using the red lines (Figure 6(a)). Lacking in the π-π interactions but
higher rigid, PP chain is more difficult to bend itself to form aggregation,
only some local bending exists during the whole chain.
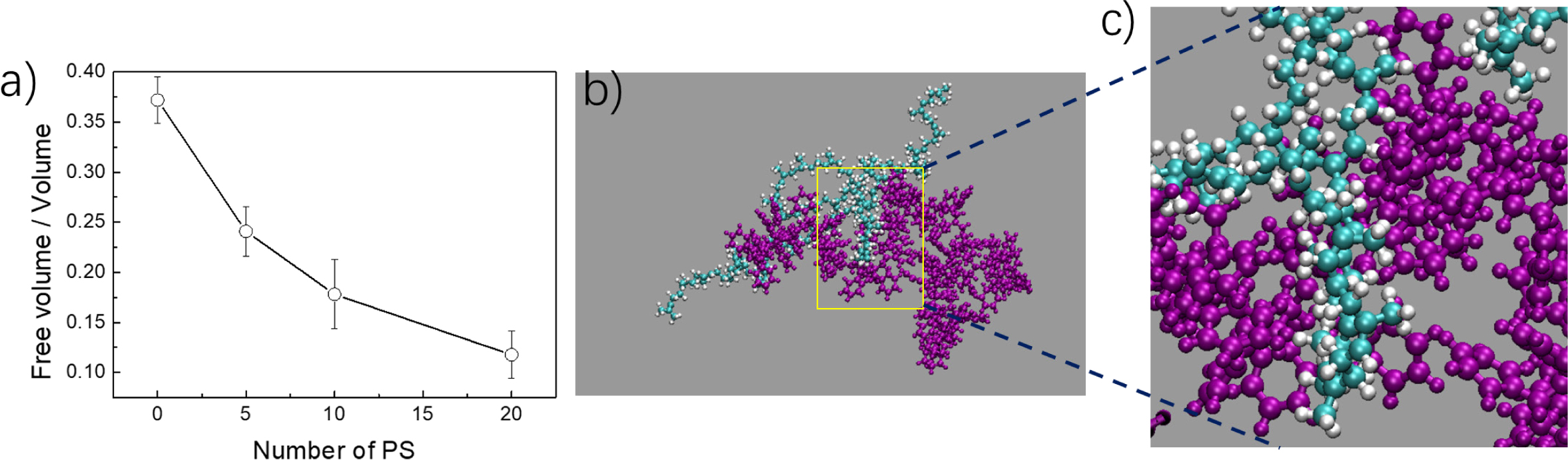
Bearing in our mind that the density increases after adding PS into PP
phase, the free volume was calculated to explore the reason (Figure 7(a)). In
Gromacs software, the program of free volume tries to insert a probe with a
given radius into the simulations box and if the distance between the probe and
any atom is less than the sums of the van der Waals radii of both atoms, the
position is considered to be occupied. Relative to the whole volume, free
volume decreases gradually with the ratio of PS increasing. It means that the
gaps among the PP aggregation is filled with the PS chain. The molecular
structure was captured and shown in Figure 7(a) and 7(b). The side chain is
embedded in the surface defects of PS aggregation exactly. In the past decades,
many additives have been added to PP to improve the abrasive and deformation
resistance. For example, natural rubber filled with carbon black can form a
higher degree of entanglement, causing higher Young’s modulus and hardness.12
Another report revealed a strong connection between the resorcinol formaldehyde
latex fiber coating and the peroxide-cured rubber matrix.13 The
mechanisms of different additives are similar, both forming a complex structure
of entanglement state. In our work, the complex structure of PP and PS shows a
typical spatial complementary behavior, which can increase the entanglement at
a very high degree, thus it will improve the abrasive and deformation
resistance highly.
Effect
of Spatial Complementary Behavior in Car Industry. The above simulations indicate
that the spatial complementary of PP and PS can increase the cross-linking
degree and decrease the surface defects. If using pure PP to produce the tire,
it is easier to generate deformation while adding high shear force and stress.
Also, due to the surface defects or gaps, friction between tire and road can
more easily cause the tire abrasion. On the contrary, the complex of PP and PS
can form a tight cross-linking structure, which may resist higher external
force. Due to improving the compactness of tire, the surface of the tire can
resist more stress, without increasing the intensity of pressure. In a word,
adding PS is a potential solution to increase abrasive and deformation
resistance. The possible impact mechanism was described in Figure 8 vividly.
|
Figure 2 Characterizations of assembly structures. Final snapshots
of PP-PS complex of different mixed ratios for PP:PS=20:0 (a);
20:5 (b); 20:10 (c); 20:20 (d). Radial distribution function of PP-PS
and PP-PP for PP:PS=20:20 (e); 20:0 (f). |
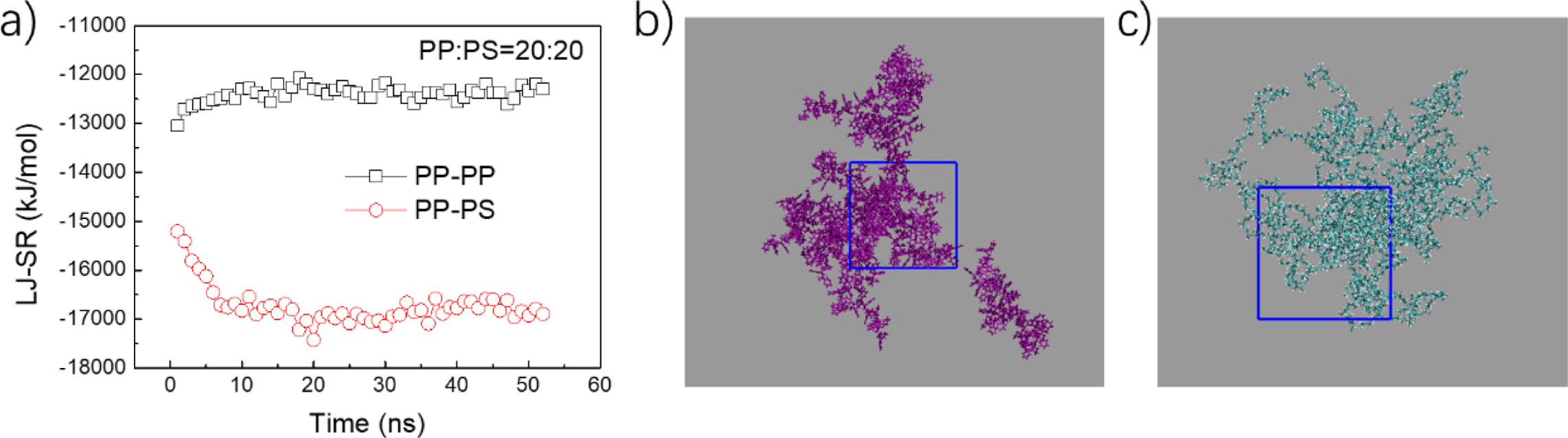
|
Figure 3 (a) Time evolutions of LJ potential energy for PP-PP and PP-PS. Stable conformations of PS (b); PP (c). The mixed ratio is 20:20. |
|
Figure 4 Density of equilibrium state for different mixed ratios. |
|
Figure 5 (a) Gyration radius of PP and PS under different mixed ratios; (b) Mean square displacement of PP for different mixed ratios. |
|
Figure 6 Time evolutions of typical snapshots depicting the conformation transformations of single PP (a); PS (b). |
|
Figure 7 (a) The ratio of free volume among whole volume for different mixed systems. (b) Typical embedded structure exhibiting spatial
complementary behavior. (c) Partial enlarged detail corresponding to (b). |
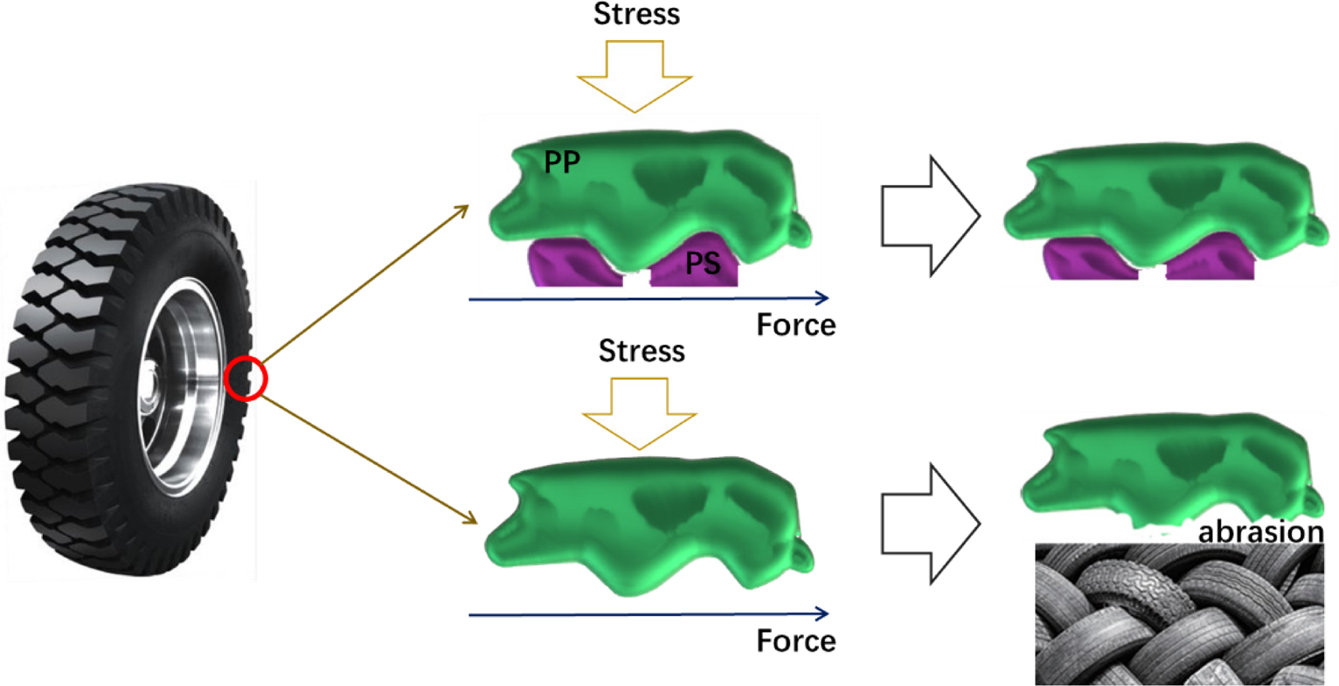
|
Figure 8 Sketch map of possible impact mechanism for using PP
and PS mixture producing tires. |
The studies of PP and PS blends have been performed in the past years,
but the molecular mechanism remains unclear. Using all-atom molecular dynamics
simulations, we have studied the assembly structures and processes of PP and PS
complex. The simulation results indicate that PP and PS can form a tight
entangled structure. The PP can adjust their conformations to fill up the
cavity generated from PS self-aggregation, which will offset the original
defects. After adding PS, the density of complex increases, which is
corresponding to the decrease of free volume. Besides, the diffusion rate for
PP decreases apparently because some chains of PP are inserted into PS
aggregations. The formed cross-linked and spatial complementary structures can
improve the plasticity and abrasive resistance, which is superiority in tire
design.
Our results demonstrate the complementary structure of PP-PS complex,
which in turn provides an important understanding for rubber application and
tire industry. Besides, plastic waste has become a serious environmental
problem. Using plastic as potential materials to modify the tire abrasive
resistance may be an effective solution to deal with dumped plastic. Although
our results reveal the spatial complementary behavior of PS and PP, many
problems are requiring study. For example, how do the kinds of plastic affect
the PP properties? In any event, our recent results give a theoretical
explanation for future PS applications in the tire industry.
- 1. H. Van Der Heijden and W. Garn, Eur. J. Oper. Res., 225, 420 (2013).
-

- 2. P. Thiffault and J. Bergeron, Accid. Anal. Prev., 35, 381 (2003).
-

- 3. L. Aarts and I. Van Schagen, Prev., 38, 215 (2006).
-

- 4. R. Grogan and T. Watson, J. Forensic. Sci., 14, 165 (1974).
-

- 5. G. J. Osanaiye, A. I. Leonov, and J. L. White, J. Non-Newton Fluid, 49, 87 (1993).
-

- 6. O. Muratoglu, A. Argon, R. Cohen, and M. Weinberg, Polymer, 36, 921 (1995).
-

- 7. A. B. Irez, E. Bayraktar, and I. Miskioglu, Mechanics of Composite, Hybrid and Multifunctional Materials, Springer, Vol 5, p 67 (2019).
-

- 8. A. Kinloch, R. Mohammed, A. Taylor, C. Eger, S. Sprenger, and D. Egan, J. Mater. Sci., 40, 5083 (2005).
-

- 9. B. Ahmadi-Moghadam, M. Sharafimasooleh, S. Shadlou, and F. Taheri, Mater. Design, 66, 142 (2015).
-

- 10. X. He, X. Shi, M. Hoch, and C. Gögelein, Polym. Compos., 39, 3212 (2018).
-

- 11. L. Qu, G. Yu, X. Xie, L. Wang, J. Li, and Q. Zhao, Polym. Compos., 34, 1575 (2013).
-

- 12. S. Salaeh and C. Nakason, Polym. Compos., 33, 489 (2012).
-

- 13. M. Bhattacharya, M. Maiti, and A. K. Bhowmick, Polym. Eng. Sci., 49, 81 (2009).
-

- 14. B. Karaağaç, Polym. Compos., 35, 245 (2014).
-

- 15. S. Saha and A. K. Bhowmick, Polymer, 103, 233 (2016).
-

- 16. A. Jha and A. Bhowmick, Polym. Degrad. Stabil., 62, 575 (1998).
-

- 17. L. Lin, S. Liu, Q. Zhang, X. Li, M. Ji, H. Deng, and Q. Fu, ACS Appl. Mater. Inter., 5, 5815 (2013).
-

- 18. W. Sengers, P. Sengupta, J. W. Noordermeer, S. Picken, and A. Gotsis, Polymer, 45, 8881 (2004).
-

- 19. A. Mousa, U. Ishiaku, and Z. M. Ishak, Polym. Test., 19, 193 (2000).
-

- 20. S. S. Banerjee, K. D. Kumar, and A. K. Bhowmick, Mater. Eng., 300, 283 (2015).
-

- 21. L. D. Schuler, X. Daura, and W. F. Van Gunsteren, J. Comput. Chem., 22, 1205 (2001).
-

- 22. A. K. Malde, L. Zuo, M. Breeze, M. Stroet, D. Poger, P. C. Nair, C. Oostenbrink, and A. E. Mark, J. Chem. Theory. Comput., 7, 4026 (2011).
-

- 23. B. Hess, C. Kutzner, D. Van Der Spoel, and E. Lindahl, J. Chem. Theory. Comput., 4, 435 (2008).
-

- 24. W. Humphrey, A. Dalke, and K. Schulten, J. Mol. Graph. Model., 14, 33 (1996).
-

- 25. P. Sharma, S. Roy, and H. A. Karimi-Varzaneh, J. Phys. Chem. B, 120, 1367 (2016).
-

- 26. D. Hudzinskyy, A. V. Lyulin, A. R. Baljon, N. K. Balabaev, and M. A. Michels, Macromolecules, 44, 2299 (2011).
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2020; 44(6): 835-840
Published online Nov 25, 2020
- 10.7317/pk.2020.44.6.835
- Received on Jun 20, 2020
- Revised on Aug 8, 2020
- Accepted on Aug 27, 2020
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Supporting Information
Introduction
Methods
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Yongbing Xue
-
*College of Chemical and Biological Technology, Taiyuan University of Science and Technology, Taiyuan 030021, China
- E-mail: tykjdxxyb@163.com
- ORCID:
0000-0001-9084-7288










 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.