- Polypropylene Nanocomposites with Graphene Oxide Containing Flame Retardant Moieties
Seung Won Lee, Huiseob Shin, Yong Hun Lee*, Jong-Chan Lee, and Jae Young Jho†

School of Chemical and Biological Engineering, Seoul National University, Seoul 08826, Korea
*Department of Application and Development, S-Oil TS&D center, Seoul 07795, Korea- 폴리프로필렌/난연제 함유 산화그래핀 복합재료
서울대학교 공과대학 화학생물공학부, *㈜에쓰-오일
Graphene oxide (GO) was
functionalized with phosphorus-containing groups to give the flame retardancy
to the polypropylene (PP)/GO nanocomposites. The composites were prepared via
melt mixing with maleic anhydride-grafted PP (MAPP) as a compatibilizer.
Through the Fourier transform infrared (FTIR) spectroscopy, and X-ray
photoelectron spectroscopy (XPS), covalently-bonded flame retardant moieties on
GO were characterized. The effect of the functionalization of GO and the use of
compatibilizer on the morphology, flame retardancy, and mechanical properties
of the PP/GO composites were investigated. SEM observation of the composites
showed that the addition of MAPP improved the exfoliation and dispersion state
of the GO sheets by improving the interfacial interaction between the filler
and the matrix. For PP/functionalized GO composites, the limiting oxygen index
(LOI), Young’s modulus, and tensile strength were increased compared with PP/GO
composites.
폴리프로필렌(polypropylene,
PP)/산화그래핀(graphene oxide, GO) 복합재료의 난연성과 기계적 성질을
향상시키기 위해 GO를 인계 난연제로 개질시켰다. 복합재료는
무수말레인산이 수식된 PP(maleic anhydride-grafted PP, MAPP)를 상용화제로
사용하여 용융 혼합 방식으로 제작되었다. 푸리에 적외선 분광학(Fourier
transform infrared, FTIR), 광전자분광법(X-ray photoelectron
spectroscopy, XPS)을 통해 난연제와 GO 사이에 공유 결합이 형성된 것을
확인할 수 있었다. GO의 기능화와 상용화제 사용이 PP/GO 복합재료의
형태학, 난연성, 기계적 성질에 미치는 영향을 평가하였다. 주사전자현미경(SEM)을 이용한 형태학 분석 결과, MAPP를 첨가함으로써 충전제와 기지재 사이의 계면 상호작용이 증가되어 GO의
박리 및 분산성이 향상되었다. PP/기능화된 GO 복합재료의
한계산소지수, 영 탄성률, 인장 강도가 PP/GO 복합재료에 비해 증가된 결과를 나타냈다.
Graphene oxide (GO) was functionalized with
phosphorus-containing groups to give the flame retardancy to the
polypropylene(PP)/GO
nanocomposites. For PP/functionalized GO composites, the limiting oxygen index,
Young¡¯s modulus, and tensile strength were increased compared with PP/GO
composites.

Keywords: polypropylene, graphene oxide, composite, flame retardant
This research was supported by the joint research of
S-Oil and Seoul National University, funded by the S-Oil, Republic of Korea.
Polypropylene (PP) is a thermoplastic polymer widely used in many fields
due to its low density, processability, and good mechanical properties.1,2
Despite these advantages, an inherent flammability issue limits applications in
some areas, such as the automotive and electrical industries, where high flame
retardancy is required.3,4 As a method for increasing the flame
retardancy of PP, a flame retardant containing halogen was widely used. Since
halogen radicals generated during combustion can remove radicals generated by
thermal decomposition of the polymer, halogen-containing flame retardants are
effective in retarding combustion. It has been applied to other polymers,
however, its use is prohibited because the halogen halide generated during
combustion is toxic and corrosive chemicals.5,6
Metal hydroxides such as aluminum trihydrate and magnesium dihydrate were
used as an alternative flame retardant. These metal hydroxides are
endothermically decomposed and release water during combustion, reducing heat
and temperature of the polymer substrate. When using metal hydroxide flame
retardants in PP, however, more than 30 wt% was required to achieve the
V-0 rating in the UL-94 test, which reduced tensile strength, impact strength,
and melt flow index.7,8 Another attempt to improve the flame
retardancy of PP involves an intumescent flame retardant (IFR) such as ammonium
polyphosphate (APP) and melamine polyphosphate (MPP). IFR is halogen-free, and
improves flame retardancy by forming an insulating barrier on the polymer
surface that prevents heat and mass transfer. This method also reduced tensile
strength, flexural strength, and impact strength because more than 25 wt% of
flame retardant was required to form a thick insulating barrier.9,10
In recent studies, interest in flame retardants has focused on phosphorus flame
retardants with phosphine oxide, such as 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide
(DOPO).
They volatilize into the gas phase to form phosphorous-centered radicals such
as PO·, PO2·, and HPO·, which act as radical scavenger. Volatilized
compounds are one of the most effective combustion inhibitors because
phosphorus-based radicals are 5 times more effective than bromine radicals and
10 times more effective than chlorine radicals.11,12 For this
reason, it is possible to improve the flame retardancy of the polymer such as
polyester and polyamide even in a small amount.13,14 In the case of
PP, however, it was difficult to apply because of the problem of dispersion due
to the large polarity difference between the flame retardant and the matrix. It
was observed in a PP/DOPO-GO composite that 30 wt% of filler was required to
achieve the V-0 rating, which reduced the tensile strength and impact strength.15
To increase the flame retardancy of PP with a small amount of flame retardants,
it is necessary to improve the dispersibility.
We desire to improve the flame retardancy and mechanical properties of PP
at the same time by attaching a phosphorus flame retardant acting as a radical
scavenger to GO, and dispersing it in PP. The flame retardants used in this
study were DOPO and triphenylphosphine oxide (TPPO), which have been known to
be excellent in thermal stability and impart flame retardancy in small amounts.
TPPO has been aminated to introduce higher amounts of flame retardants to GO
than DOPO, and to increase the dispersion state of the filler by improving the
reactivity with MAPP. Since GO is a two-dimensional sheet-like structure, it
can be used as not only reinforcing filler but also flame retardant,16,17
we chose it as a filler for composite. GO also has the problem of being
dispersed in PP due to the polarity from multiple hydrophilic groups present on
the surface. We selected the MAPP as a compatibilizer for the composite, as
MAPP was considered to interact with both PP and GO. One method often used to
increase the compatibility between PP and fillers having hydroxyl and/or amine
groups is to introduce MAPP as a compatibilizer in PP composites.18,19
The effect of functionalized GO as a flame retardant and reinforcing filler
on the flame retardancy, mechanical properties, and morphology of PP/GO
composites was investigated.
Materials. PP was a
commercial product of Sumitomo Chemical with the trade name of AY 564. GO
powder was obtained from Promico under the trade name of PRM170308. DOPO (97%)
was purchased from Tokyo Chemical Industry. MAPP (Mw ~9100,
MA 9~10 wt%) and TPPO (98%) were obtained from Sigma-Aldrich. Sulfuric
acid (H2SO4, 95%), nitric acid (HNO3, 70%),
hydrochloric acid (HCl, 35%), Tin(II) chloride anhydrous (SnCl2,
98%), sodium hydroxide (NaOH, 20 wt% aq. solution), and tetrahydrofuran (THF,
99%) were purchased from Daejung Chemicals.
Synthesis
of Aminated TPPO (TPPO-NH2). TPPO (10 g, 0.03
mol) was added to 72 mL of H2SO4 with stirring under
nitrogen condition, and solution was cooled to 0 oC with an ice
bath. HNO3 (36 mL, 0.12 mol) was added over a period of 10 min.
The reaction mixture was stood at room temperature for 4 h, and added to 1000 g
of ice. After ice was melted, the product (TPPO-NO2) was filtered,
and washed with NaHCO3 aqueous solution and deionized water before
being dried in the oven at 80 oC for 24 h. The obtained TPPO-NO2
(5 g) was placed in a round-bottomed flask with 30 g of SnCl2.
A solution of 40 mL HCl in 80 mL of ethanol was added into the flask. The
mixture was stirred at room temperature for 5 h, and 500 mL of NaOH
aqueous solution was added in order to eliminate the SnCl2. The
final product (TPPO-NH2) was filtered, and washed with deionized
water until neutral pH. The TPPO-NH2 was dried in a vacuum oven at
80 oC for 24 h.
Preparation
of Functionalized GO. GO (0.5 g)
was suspended in 200 mL of THF, and sonicated for 30 min. THF solution (50
mL) with TPPO-NH2 (1.8 g) was added with stirring. The mixture
was heated to 60 oC, and refluxed for 2 h under nitrogen
condition. After functionalization, the product (TPPO-GO) was separated by
filtration, and washed with anhydrous THF to remove unreacted TPPO-NH2.
The TPPO-GO was dried in a vacuum oven at 80 oC for 24 h. The
preparation of DOPO-GO was done by the same synthesis procedure as TPPO-GO.
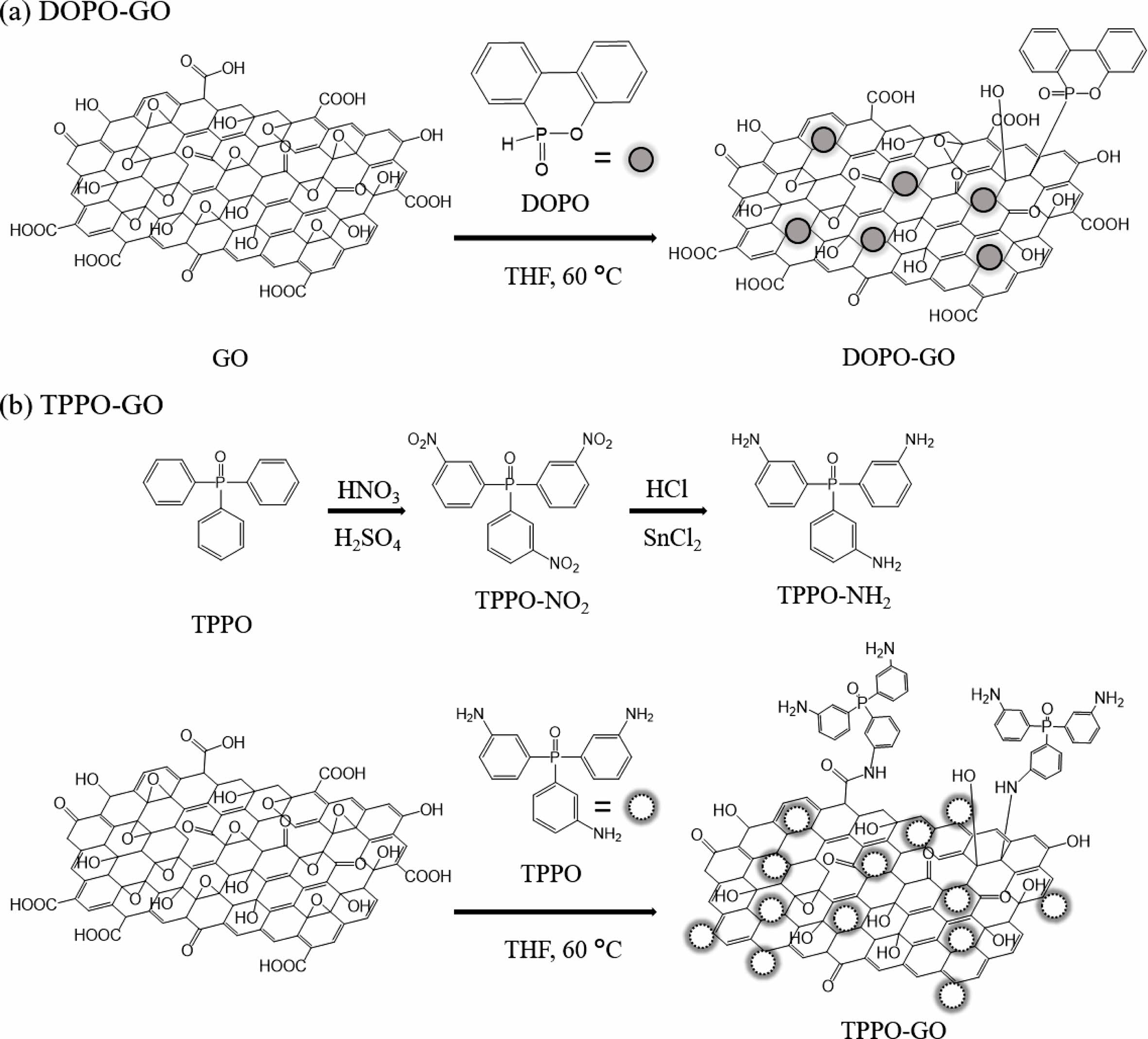
Figure 1 shows the synthesis scheme of TPPO-GO and DOPO-GO.
Preparation of PP/MAPP/Functionalized GO Nano-composites. Prior to preparation of
nanocomposites, PP/MAPP/functionalized GO master batches containing 4 wt%
of functionalized GO were prepared by reactive extrusion using an internal
mixer (Thermo scientific, Haake Polylab QC). The extrusion temperature and
rotor speed were 190 oC and 60 rpm, respectively. The master
batches were melt blended with PP in a co-rotating twin-screw extruder (Thermo
Electron, Prism TSE 16TC) with temperature profile of 190 to 200 oC
from hopper to die at the rotating speed of 200 rpm. The composites extruded
using a strand die were water-cooled, pelletized, and dried.
Characterization
and Measurement. The attenuated total reflection Fourier transform
infrared (ATR-FTIR) spectra were recorded using a FTIR spectrometer (Agilent,
Cary 600) equipped with an ATR accessory in the range from 4000 to 650 cm-1.
X-ray photoelectron spectroscopy (XPS) of each sample was recorded on X-ray
photoelectron spectrometer (Thermo scientific, K-Alpha Spectrometer) using
monochromatic Al Kα radiation as the exciting source.
Morphology was investigated with a field emission scanning electron
microscope (FE-SEM, JEOL, JSM-6701F). To investigate the phase morphology,
specimens were fractured under cryogenic conditions using liquid nitrogen.
Young’s modulus and tensile strength were determined using a universal
testing machine (UTM, LR10K, Lloyds Instruments). The tests were carried out
with a crosshead speed of 10 mm/min and specimens of
63.1 × 3.1 × 3.2 mm3 in dimension according
to the ASTM D638 Type V method.
The limiting oxygen index (LOI) was measured using an oxygen index meter
(Fire Testing Technology, Oxygen Index) according to the ASTM D2863 Type I
method, which is for specimens of 90 × 10 × 4 mm3
in dimensions.
|
Figure 1 Schematics of synthesis of (a) DOPO-GO; (b) TPPO-GO. |
Structure of TPPO-NH2 and Functionalized GOs. TPPO-NH2 was
synthesized via a two-step route. As shown in Figure 1(b), the nitro compound
(TPPO-NO2) was first obtained as an intermediate, and hydrogenated
to result in the amine compound (TPPO-NH2). Nitration and reduction
of TPPO have been well-established to obtain amino derivatives.20
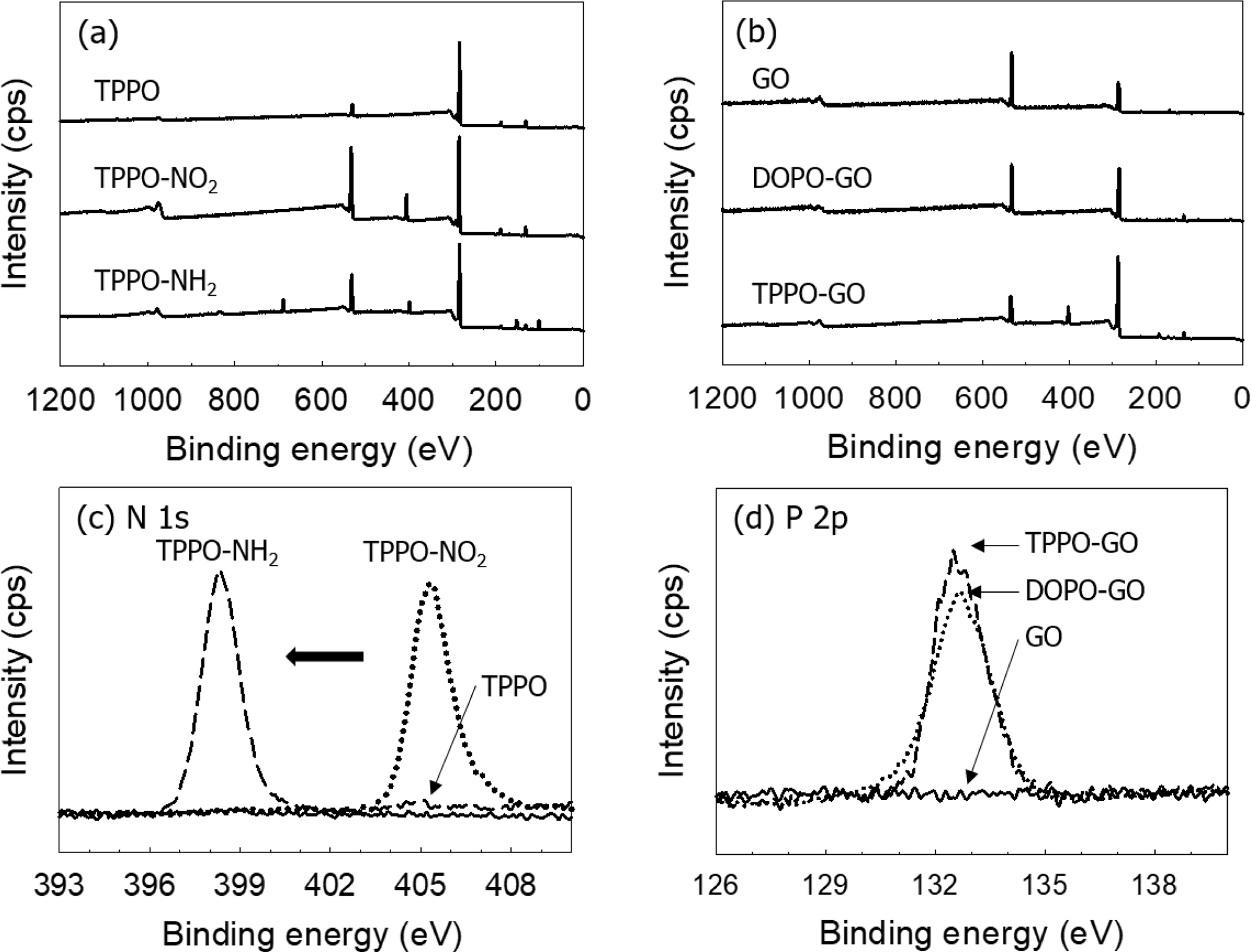
FTIR spectrum of TPPO-NH2 was shown in Figure 2(a). The formation of
aromatic primary amine group was confirmed by IR absorption peaks from 3300 to
3500 cm-1. The formation of amine was also observed by XPS N 1s peak
at 398.5 eV as shown in Figure 3(a) and 3(c). Similar results were observed for
the synthesis of bis(4-aminophenoxy)phenyl phosphine oxide.21,22
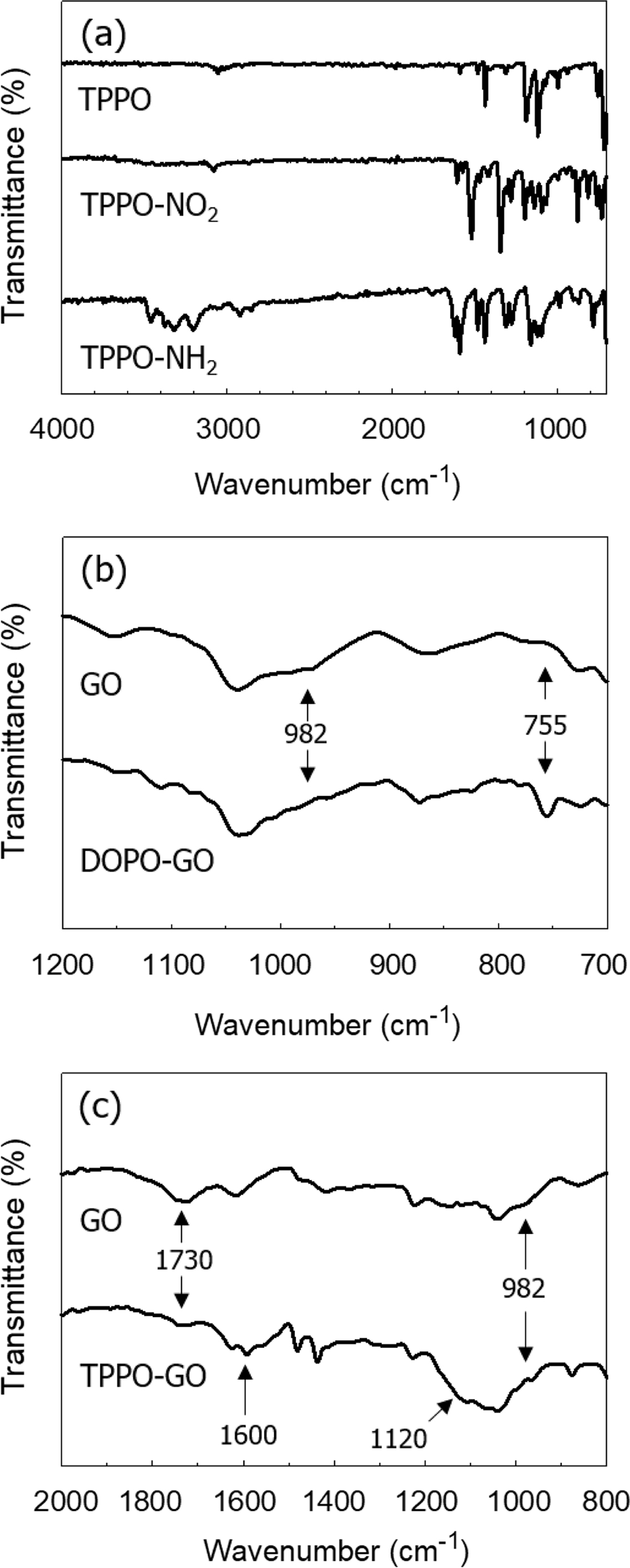
To identify the chemical reactions that took place during the
modification of GO, we investigated the FTIR spectra of GO and the
functionalized GOs, which were shown in Figure 2(b), 2(c), and 2(d). In the
spectrum of DOPO-GO, the ring-opening reaction of GO with DOPO could be
confirmed by analyzing the epoxy ring peak of GO. The asymmetrical vibration
peak of the epoxy ring of GO at 982 cm-1 was decreased when the
epoxy group reacted with DOPO (Figure 2(c)). The DOPO-GO spectrum exhibited a
new characteristic band at 755 cm-1 due to the stretching vibration
of P-O-Ph in DOPO, which was formed by the reaction between the GO and DOPO.
The same result was previously observed in the IR analysis of preparation of
phosphorous-containing epoxy resin with DOPO-GO.23 Unlike DOPO,
amine groups in the TPPO-NH2 react with both epoxy and carboxylic
acid groups in GO. As a result, absorption bands corresponding to carboxylic
acid (1730 cm-1) and epoxy (982 cm-1) were reduced
in TPPO-GO. The similar result was observed for the GO treated with p-phenylenediamine.24
Also, spectrum of TPPO-GO included peaks for the C-N (1120 cm-1),
N-H (1600, 695 cm-1), and P-Ph (1448 cm-1)
groups, implying the presence of TPPO on the GO surface.
The XPS spectra of GO and functionalized GO was shown in Figure 3(b), and
the atomic percent of each sample was summarized in Table 1. As the flame
retardants were introduced into GO, a peak of P which was not present in GO was
formed, and the ratio of C and O was changed. In the case of GO, the atomic
percent ratio of the XPS result was 7:3 for carbon and oxygen. It can be
considered that 70 carbon atoms and 30 oxygen atoms exist among 100 atoms, and
that 70 carbon atoms can constitute about 30 numbers of 6-membered rings. The
number of flame retardants introduced to the GO surface can be deduced based on
the atomic percent of C and O atoms changed by the added flame retardant, as
well as the atomic percent of newly generated P and N atoms. As a result, it is
simply calculated that one DOPO per 5.4 rings is formed on the surface of
DOPO-GO, and one TPPO per 2.5 rings on TPPO-GO. For TPPO-GO, TPPO-NH2
interacts with carboxylic acid groups and epoxy groups on the GO surface as
mentioned above. It can be confirmed that a larger amount of flame retardant is
attached on TPPO-GO surface than DOPO-GO.
Morphology. As flame
retardancy and mechanical properties of GO reinforced composites should be
dependent on the dispersion of filler including particle size as well as the
filler content, morphology of the composites was examined. The cryofractured surfaces of
the composites were shown in Figure 4. As shown in Figure 4(a) and 4(c), the
functionalized GO particles in PP/DOPO-GO and PP/TPPO-GO composites were aggregated
to a size of 20.7 and 20.6 μm, respectively. When the GO was functionalized
with flame retardants, there was no effect to improve the interfacial
interaction with the PP. For this reason, the GO was not effectively
exfoliated, resulting in the aggregation.
In comparison, smaller and thinner GO particles were found on the
fracture surface of PP/MAPP/DOPO-GO and PP/MAPP/TPPO-GO composites as shown in
Figure 4(b) and 4(d). This indicated that MAPP act as a compatibilizer. It is
considered that the polypropylene chains of the MAPP, with the same structure
as PP matrix, were exposed on the surface of functionalized GO in the
composites. These chains diffused into the PP matrix phase to form entanglement
and cocrystallization with the PP chains, creating a bridge at the interface
between the functionalized GO and the PP matrix.25,26 This
interaction led to increase compatibility between GO and PP, resulting in
effective exfoliation of GO sheets.
The size and thickness of particles in PP/MAPP/TPPO-GO was smaller than
those in PP/MAPP/DOPO-GO, which was a result of better interfacial interaction
between functionalized GO and PP. In PP/MAPP/functionalized GO composites,
maleic anhydride group in MAPP reacts with hydroxyl groups on DOPO-GO surface
and amine groups on TPPO-GO surface, and make a covalent bond between MAPP and
functionalized GO.27 The surface of DOPO-GO was functionalized with
bulky molecules (i.e. biphenyl structure of DOPO), the amount of hydroxyl
groups in DOPO-GO exposed to MAPP was relatively decreased compared to that of
the before functionalization. The amine groups on the of TPPO-GO in PP/MAPP/TPPO-GO
composite, however, exist on the outer surface of TPPO-GO, and approach to MAPP
easily. The effective exfoliation of TPPO-GO in the PP matrix is considered to
be the result of increased reactivity of MAPP, which improves the compatibility
between PP and TPPO-GO in composite.
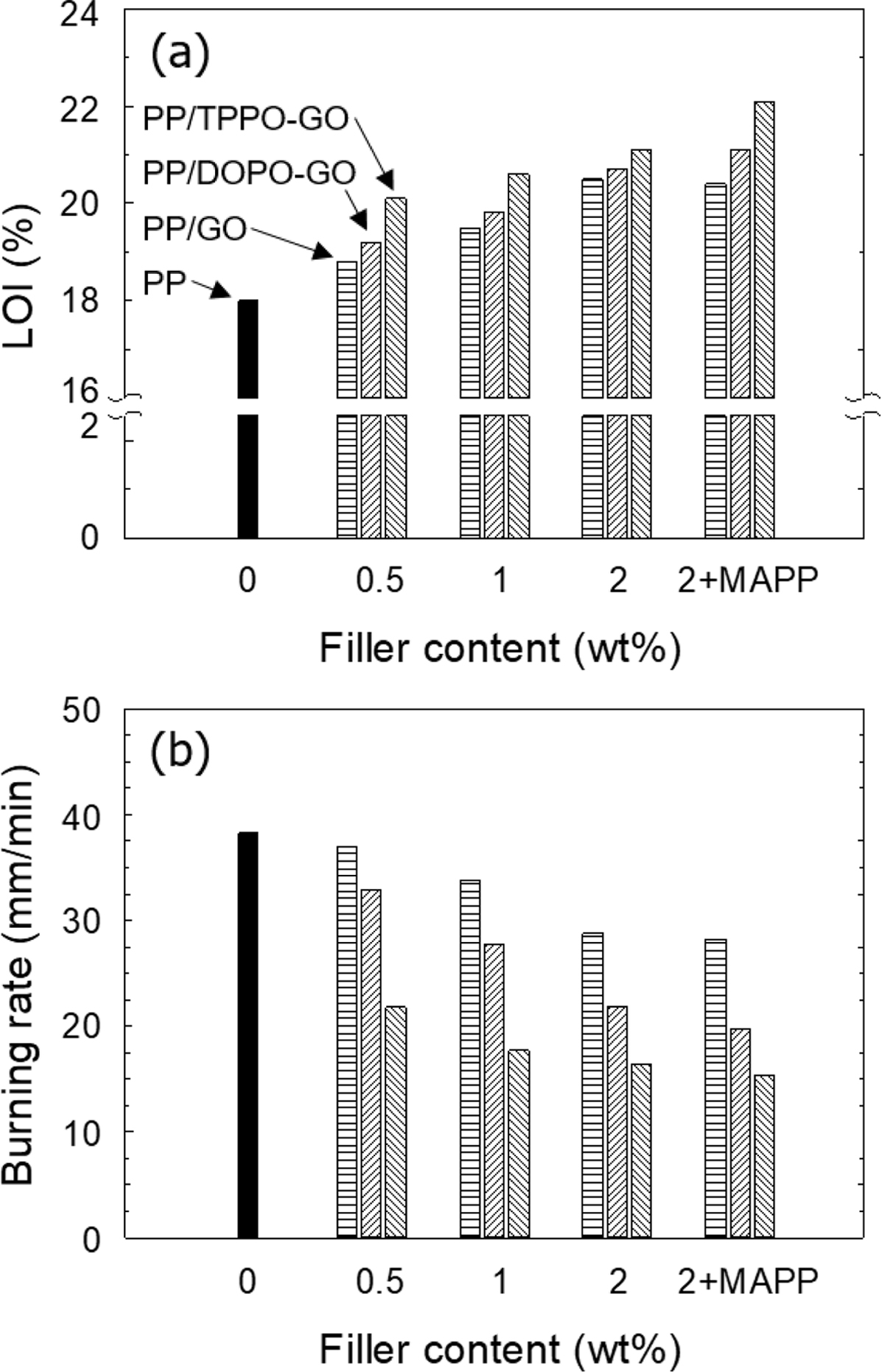
Flame
Retardancy. The LOI values of both PP/GO and PP/functionalized GO
with phosphorous flame retardant composites were increased with the filler
content increased, as shown in Figure 5(a). It has been explained that the
origin of the flame-retarding behavior of GO was thought to be its ability to
form a continuous protective layer that acts as a thermal insulator and mass
transport barrier.28-30 For PP/TPPO-GO composite, TPPO-GO was
functionalized with higher amounts of flame retardants than DOPO-GO, and the
LOI of the PP/TPPO-GO composites was higher than that of the PP/DOPO-GO
composites. The higher amount of radical scavenger formed during the combustion
process of PP/TPPO-GO composite prevented the degradation of PP more effectively
than PP/DOPO-GO composite, resulting in higher LOI. In the case of DOPO,
radicals are formed as the P-H bond is homolytically cleavaged during the
degradation process. The radicals formed in this process are stabilized by
dividing into dibenzofuran and PO radicals.31,32 For TPPO, it has
been reported that TPPO is rather poor char promoter, but are more active in
the gas phase than other phosphorous flame retardants with higher oxidation
numbers like phosphate.33 It has been also reported that as the
oxidation state of TPPO increased, the production of stable char layers
increased and the release of phosphorous-containing radicals decreased.34
The enhanced flame retardancy of the composites with GO containing phosphorous
flame retardants is considered to be a result of the radical scavenging
mechanism in the gas phase.
When the compatibilizer was used to improve the dispersion state of the
fillers in composites, the LOI value was further increased. To create an
effective protective layer with GO sheets, they must be dispersed in the
matrix, not agglomerated, so that they accumulate continuously in the condensed
phase during combustion. Effective dispersion of fillers increases the
interfacial area with matrix, which means that the amount of phosphorous flame
retardant exposed is increased. The further increase in LOI was due
to an increase in the amount of PO radicals produced as the amount of flame
retardant exposed increased.
The results of the UL-94 horizontal burning test were summarized in
Figure 5(b). The result showed that all composites showed lower burning rates
than neat PP, and the tendency was similar to that of LOI. As the filler
content increased, the burning rate gradually decreased, and showed the minimum
point when the compatibilizer was added. As mentioned above, GO formed a
protective layer during the combustion process, and phosphorus flame retardant
acted as a radical scavenger, which delayed the thermal degradation of PP. As a
result, the burning rate of the composites decreased, but all of the composites
classified as an HB rating. The specimens had to be self-extinguished to pass
the vertical burning test, which measures the time to stop combustion after
vertically igniting the specimen, but was not found in this study. It was expected
that more filler would be needed to achieve higher LOI values or UL-94 vertical
rating. The use of larger amounts of GO, however, is limited due to aggregation
of GO that reduce the tensile strength,15,27 and environmental
problems caused by the GO production process.35
Mechanical
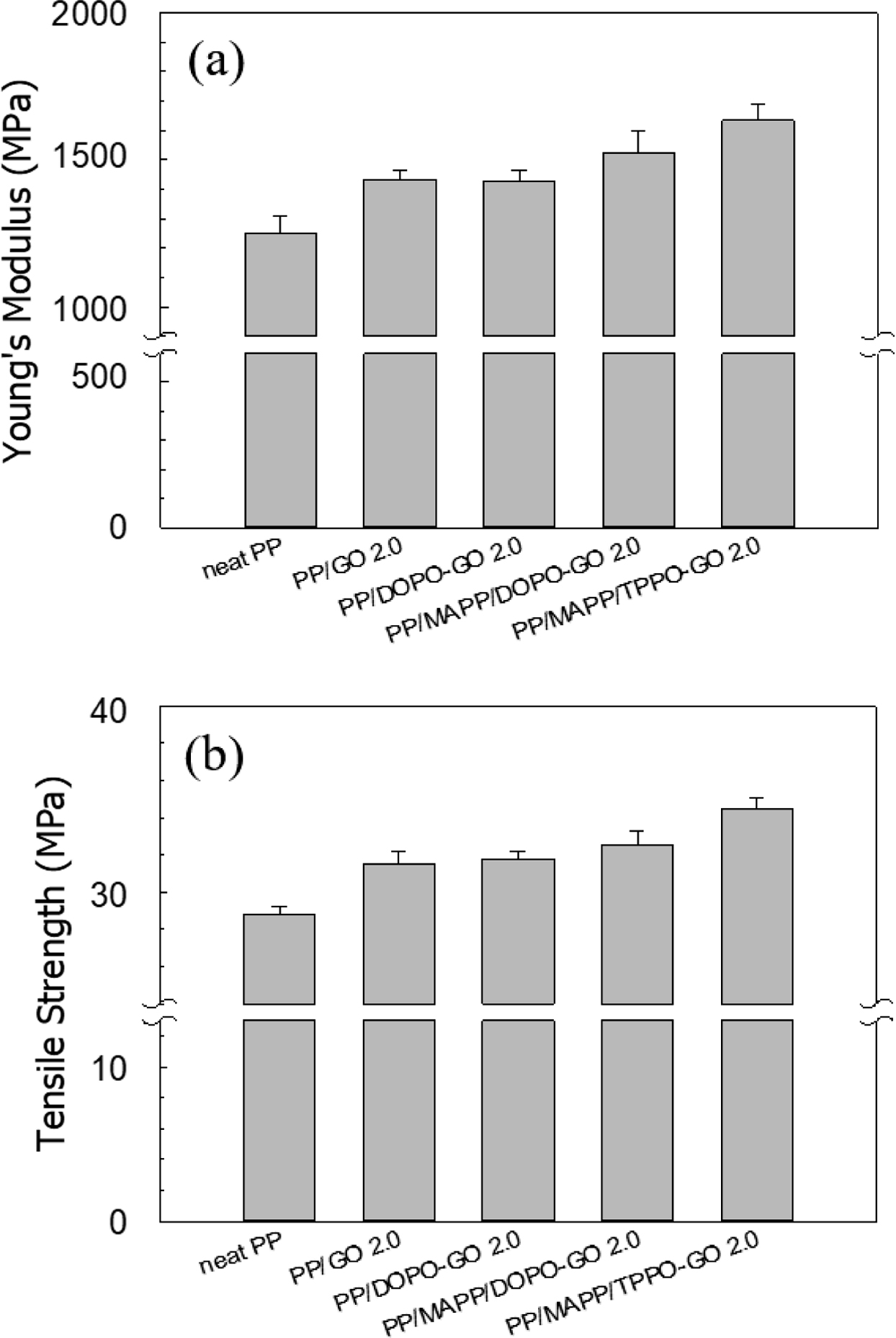
Properties. The tensile properties of PP and the composites were
shown in Figure 6. For the PP/GO composites, the Young’s modulus and
tensile strength were increased than those of neat PP, which indicated the
reinforcing effect of GO. The Young’s modulus and tensile strength of
PP/DOPO-GO composite did not show any significant increase compared to PP/GO
composite. It was expected to reduce the interaction between GO sheets by
attaching bulky DOPO to the GO surface, however, exfoliation of the GO sheets
was not effective because there was no change to improve the interfacial
interaction with PP. Since the compatibility with PP was not improved, the
dispersion state was also not improved, and there was no further increase in
mechanical properties. Recent researches reported that the mechanical
properties of a polymer composite reinforced with GO affected by dispersion
states of filler in the matrix as well as filler content.27,36
When MAPP was used as a compatibilizer, both Young’s modulus and tensile
strength were further increased. As described in the morphology part, MAPP
increased the interfacial interaction between functionalized GO and PP, which
improved the dispersion state of filler. Improved dispersion meant that the GO sheets
were effectively exfoliated and reduced in size and thickness. When the size of
the filler is decreased to nanometer size, fillers interfere with polymer chain
movement (blockage effect) that decreases the molecular mobility leading to an
increase in the stiffness and strength of the composites.37 As the
size decreased, the contact area also increased significantly compared to the
same weight or volume ratio. When the contact area increases, the stress
transfer area increases, and the total stress value measured during the tensile
test increases. Therefore, even with fillers having the same weight content,
the increased dispersion leads to an increase in modulus and strength by
effectively achieving stress transfer.38,39
It is notable that the PP/MAPP/TPPO-GO composite showed the highest
Young’s modulus and tensile strength, which meant that the dispersion state of
GO was better than the rest of the composites. The reason for this was
considered to be the better interfacial interaction between TPPO-GO and MAPP.
The hydroxyl groups in DOPO-GO were hindered by bulky molecules and blocked
from the approaching of MAPP. For TPPO-GO, however, amine groups were present
on the outer surface of TPPO-GO, making them less susceptible to steric
hindrance by bulky molecules. The improved dispersion of TPPO-GO was due to the
increased interfacial interaction with the matrix than DOPO-GO by effectively
grafting MAPP onto the surface. Similar result was reported in the
case of PP/MAPP/aminated GO composite.27 Ryu et al. have
reported that in their PP/MAPP/aminated GO with hexamethylene diamine
composite, Young’s modulus and tensile strength increased to about 35% and 14%,
respectively, on loading 2 wt% of aminated GO. When the content of more than 2
wt% was added, however, the fillers were reaggregated, and tensile strength
tended to decrease. Similar result was reported in the mechanical properties of
PP/silane coupled DOPO-GO composite. Tensile strength of PP composite decreased
from 31.0 to 28.3 MPa when DOPO-GO loaded at 10 wt%.15
|
Figure 2 FTIR spectra of (a) TPPO-NH2; (b) DOPO-GO; (c)
TPPO-GO. |
|
Figure 3 XPS survey spectra of (a) TPPO-NH2; (b) DOPO-GO and TPPO-GO. XPS high resolution spectra of (c) N 1s in TPPO-NH2; (d)
P 2p in DOPO-GO and TPPO-GO. |
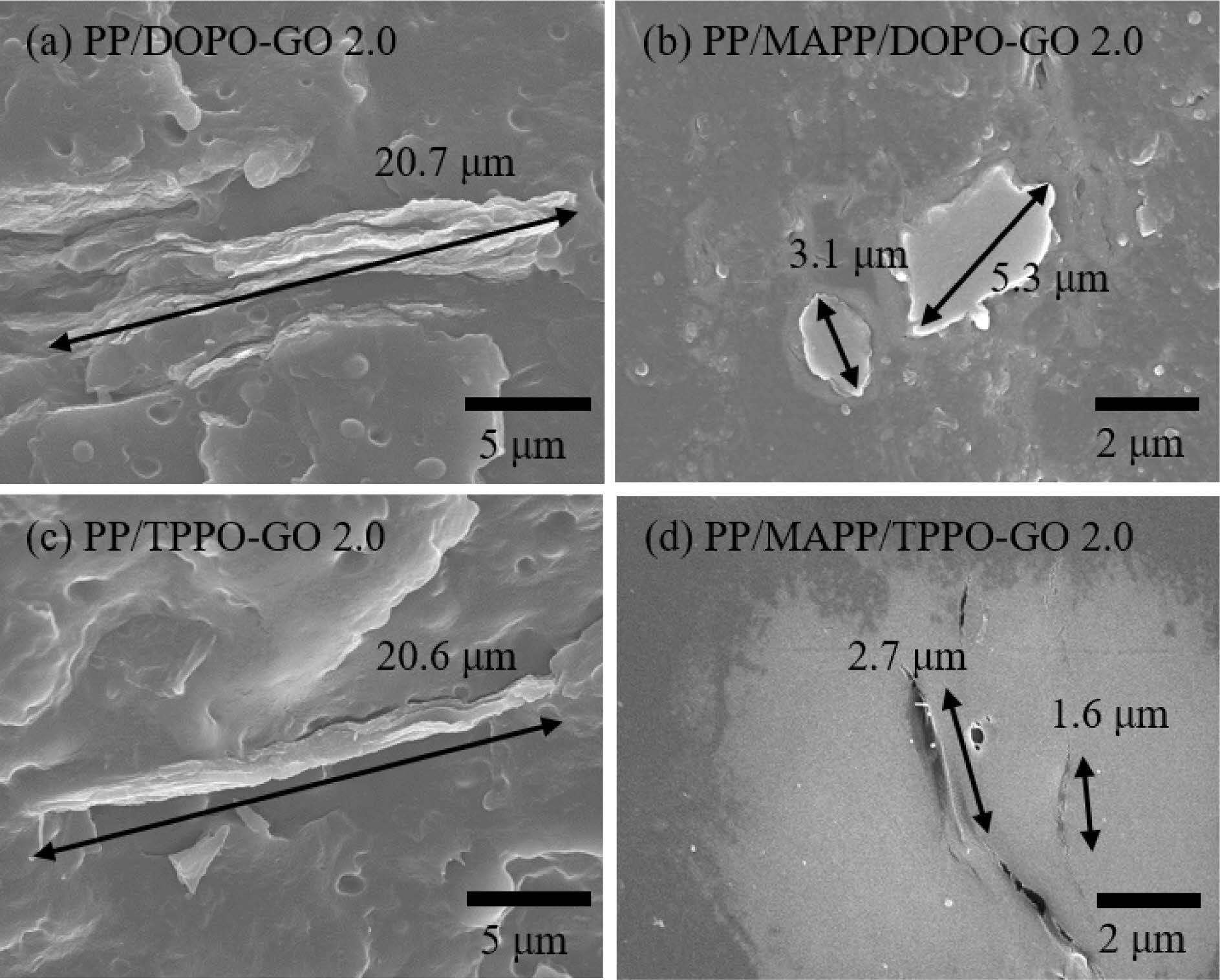
|
Figure 4 SEM images of the cryofractured surface of (a) PP/DOPO-GO; (b) PP/MAPP/DOPO-GO; (c) PP/TPPO-GO; (d) PP/MAPP/TPPOGO composites. |
|
Figure 5 (a) LOI; (b) burning rate of PP and its composites with
various filler content (wt%). |
|
Figure 6 (a) Young’s modulus; (b) tensile strength of the composites. |
PP/functionalized GO composites were prepared using MAPP as the
compatibilizer via melt blending. The DOPO and aminated TPPO were
chemically grafted onto the GO surface and its influence on the morphology,
flame retardancy, and mechanical properties was investigated. Successful
formation of covalent bonding between GO and phosphorous-based flame retardants
was corroborated using FTIR and XPS spectroscopy. Dispersion state of
functionalized GOs in PP/functionalized GO and PP/MAPP/functionalized GO
composites was confirmed by the characterization of cryofractured surface of
composites using FE-SEM. The effective interfacial interaction between TPPO-GO
and MAPP resulted in the better exfoliation and dispersion of TPPO-GO. By
reinforcing with flame retardant grafted GO fillers, the flame retardancy and
mechanical properties of PP were increased simultaneously. As the phosphorous
content of functionalized GO increased, the LOI value increased. Young’s
modulus and tensile strength of the PP/MAPP/TPPO-GO composite were higher than
those of PP and other composites due to the better interfacial interaction
between filler and matrix.
- 1. Q. T. Shubhra, A. K. Alam, and M. A. Quaiyyum, J. Thermoplast. Comp. Mater., 23, 362 (2013).
-

- 2. N. K. Jha, A. C. Misra, and P. Bajaj, J. Macromol. Sci. C, 24, 69 (1984).
-

- 3. A. Hornung, S. Donner, A. Balabanovich, and H. Seifert, J. Clean. Prod., 13, 525 (2005).
-

- 4. S. Bourbigot, M. L. Bras, and R. Delobel, Flame-retardant Polypropylene Composites, Kluwer, Dordrecht, p 255-263 (1999).
-

- 5. I. Finberg, Y. B. Yaakov, and P. Georlette, Polym. Degrad. Stabil., 64, 465 (1999).
-

- 6. L. Li, G. Wang, and C. Guo, Appl. Energy, 162, 428 (2016).
-

- 7. S. Zhang and A. R. Horrocks, Prog. Polym. Sci., 28, 1517 (2003).
-

- 8. S. Bourbigot, M. L. Bras, and R. Delobel, Polypropylene an A-Z Reference, Kluwer Publishers, Dordrecht, 1999.
-

- 9. B. Li and M. Xu, Polym. Degrad. Stabil., 91, 1380 (2006).
-

- 10. P. Song, Y. Shen, B. Du, M. Peng, L. Shen, and Z. Fang, Appl. Mater. Interface, 1, 452 (2009).
-

- 11. F. Laoutid, L. Bonnaud, M. Alexandre, J.-M. Lopez, and P. Dubois, Mater. Sci. Eng., 63, 100 (2009).
-

- 12. V. Nbushok and W. Tsang, Combust. Flame, 123, 488 (2000).
-

- 13. M. Sato, S. Endo, Y. Araki, G. Matsuoka, S. Gyobu, and D. Takeuchi, J. Appl. Polym. Sci., 78, 1134 (2000).
-

- 14. S. V. Levchik and E. D. Weil, J. Fire Sci., 24, 345 (2006).
-

- 15. F. Qi, M. Tang, N. Wang, N. Liu, X. Chen, Z. Zhang, K. Zhang, and X. Lu, RSC Adv., 7, 31696 (2017).
-

- 16. S. Liu, H. Yan, Z. Fang, and H. Wang, Comps. Sci. Technol., 90, 40 (2014).
-

- 17. B. Sang, Z.-W. Li, X.-H. Li, L.-G. Yu, and Z.-J. Zhang, J. Mater. Sci., 51, 8271 (2016).
-

- 18. F. You, D. Wang, X. Li, M. Liu, G.-H. Hu, and Z.-M. Dang, RSC Adv., 5, 8799 (2014).
-

- 19. J. U. Roh, S. W. Ma, W. I. Lee, H. T. Hahn, and D. W. Lee, Compos. B, 45, 1548 (2013).
-

- 20. Y.-L. Liu, G.-H. Hsiue, R.-H. Lee, and Y.-S. Chiu, J. Appl. Polym. Sci., 63, 895 (1997).
-

- 21. W. Liu, R. J. Varley, and G. P. Simon, J. Appl. Polym. Sci., 92, 2093 (2004).
-

- 22. Z. Tehrani, G. Burwell, M. A. M. Azmi, A. Castaing, R. Rickman, J. Almarashi, P. Dunstan, A. M. Beigi, S. H. Doak, and O. J. Guy, 2D Mater., 1, 1 (2014).
-

- 23. X.-H. Zhang, F. Liu, S. Chen, and G.-R. Qi, J. Appl. Polym. Sci., 106, 2391 (2007).
-

- 24. C. C. Caliman, A. F. Mesquita, D. F. Cipriano, J. C. C. Freitas, A. A. C. Cotta, W. A. A. Macedo, and A. O. Porto, RSC. Adv., 8, 6136 (2018).
-

- 25. Q. Li and L. M. Matuana, J. Thermoplast. Compos. Mater., 16, 551 (2003).
-

- 26. J. Duvall, C. Sellitti, C. Myers, A. Hiltner, and E. Baer, J. Appl. Polym. Sci., 52, 207 (1994).
-

- 27. S. H. Ryu and A. M. Shanmugharaj, Chem. Eng. J., 244, 552 (2014).
-

- 28. T. I. Kashiwagi, Flame Retardant Polymer Composite, Wiley, New York, 2007.
- 29. A. L. Higginbotham, J. R. Lomeda, A. B. Morgan, and J. M. Tour, Apply. Mater. Interf., 10, 2256 (2009).
-

- 30. S. Liu, Z. Fang, H. Yan, and H. Wang, RSC. Adv., 6, 5288 (2016).
-

- 31. A. Chafer, S. Seibold, W. Lohstroh, O. Walter, and M. Doring, J. Appl. Polym. Sci., 105, 685 (2007).
-

- 32. A. Konig and E. Kroke, Polym. Adv. Technol., 22, 5 (2011).
-

- 33. P. M. Hergenrother, C. M. Thompson, J. G. Smith, J. W. Connell, J. A. Hinkley, R. E. Lyon, and R. Moulton, Polymer, 46, 5012 (2005).
-

- 34. U. Braun, A. I. Balabanovich, B. Schartel, T. Knoll, J. Artner, M. Ciesielski, M. Doring, R. Perez, J. K. W. Sandler, V. Alstadt, T. Hoffmann, and D. Pospiech, Polymer, 47, 8495 (2006).
-

- 35. L. Serrano, S. Victor, C. Toledo, O. Sanahuja, A. E. Mansour, J. Abad, A. Amassian, A. M. Benito, W. K. Maser, and A. Urbina, SN Appl. Sci., 1, 179 (2019).
-

- 36. B. Yuan, C. Bao, L. Song, N. Hong, K. M. Liew, and Y. Hu, Chem. Eng. J., 237, 411 (2014).
-

- 37. E. Bahar, N. Ucar, A. Onen, Y. Wang, M. Oksuz, O. Ayaz, M. Ucar, and A. Demir, J. Appl. Polym. Sci., 125, 2882 (2012).
-

- 38. M. Moniruzzaman, J. Chattopadhyay, W. E. Billups, and K. I. Winey, Nano Letters, 7, 1178 (2007).
-

- 39. W. Noohom, K. S. Jack, D. Martin, and M. Trau, Biomed. Mater., 5, 13 (2009).
- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2020; 44(5): 725-733
Published online Sep 25, 2020
- 10.7317/pk.2020.44.5.725
- Received on Jun 2, 2020
- Revised on Jun 5, 2020
- Accepted on Jun 5, 2020
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Jae Young Jho
-
School of Chemical and Biological Engineering, Seoul National University, Seoul 08826, Korea
- E-mail: jyjho@snu.ac.kr
- ORCID:
0000-0003-4692-6362










 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.