- Effect of Ionic Natural Polysaccharides on the Functional Enhancement of Porous Hydrogel Contact Lenses
Department of Optometry & Vision Science, Catholic University of Daegu, Hayang-Ro 13-13, Gyeongsan, Gyeongbuk 38430, Korea
- 이온성 천연다당류가 다공성 수화젤 콘택트렌즈의 기능 향상에 미치는 영향
대구가톨릭대학교 안경광학과
The purpose of this study was
to examine the effect of ionic natural polysaccharides on the physico-chemistry
properties of contact lenses by cross-linking ionic natural polysaccharides
after fabricating porous hydrogel contact lenses. Sodium carbonate was used as
a foaming agent in the preparation of porous hydrogels, and ionic natural
polysaccharides such as alginate, chitosan, and agarose were used. Porous
hydrogels have significantly improved water content, oxygen permeability, and
wettability than ordinary hydrogels (polymacon). Natural polysaccharide
cross-linked contact lenses have improved wettability and antibacterial
properties in all porous contact lenses, regardless of their ionic type.
Protein adsorption of contact lenses was affected by the ionicity of
polysaccharides. It was confirmed that the ionic natural polysaccharide
contributes to the improvement of the function of the contact lens by being
bound to the hydrogel contact lens.
다공성 수화젤 콘택트렌즈를 제작한 후 이온성 천연다당류를 가교시켜 이온성 천연다당류가 콘택트렌즈의 물리적∙화학적 성질에 미치는 영향을 알아보고자 하였다. 다공성 수화젤은 발포제로서 sodium carbonate를 사용하였으며 이온성 천연다당류로서 alginate, chitosan, agarose를 사용하였다. 다공성 수화젤은 일반 수화젤보다 함수율, 산소투과율, 습윤성이 매우 향상되었다. 천연다당류가 결합된 콘택트렌즈는 이온성의 종류에 관계없이
모든 다공성 콘택트렌즈에서 습윤성과 항균성을 향상시켰다. 콘택트렌즈의 단백질 흡착은 다당류의 이온성에 영향을 받았다. 이온성 천연다당류가 수화젤 콘택트렌즈에 결합됨으로써 콘택트렌즈의 기능향상에 기여함을 확인하였다.
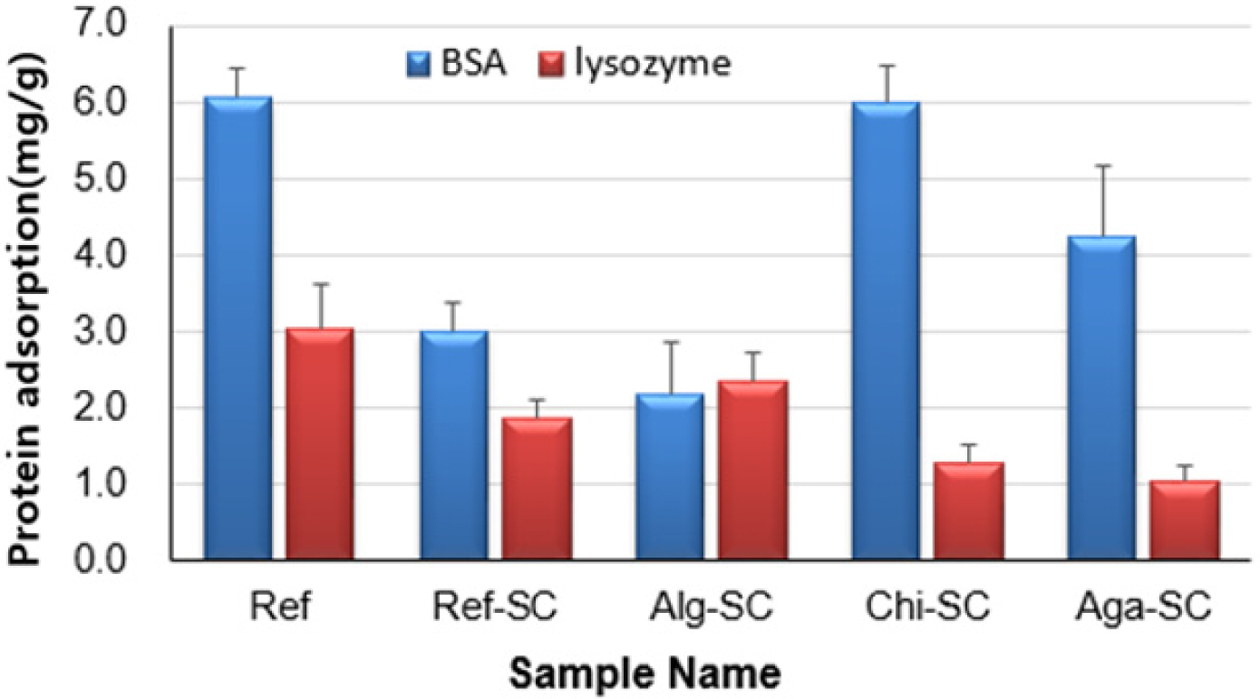
The negatively charged BSA protein is highly adsorbed on
the cationic Chic-SC, and the negatively charged Alg-SC has the lowest
adsorption amount. On the other hand, the positively charged lysozyme protein
is highly adsorbed to anionic Alg-SC and less adsorbed to Chi-SC and Aga-SC.
Keywords: contact lens, foaming agent, natural polysaccharide, oxygen permeability, porous hydrogel
This work was supported by research grants from Daegu Catholic University
in 2018.
Superporous hydrogel (SPH) has an ‘open channel’ system
interconnected by a 3D hydrophilic polymer network with multiple pores with
diameters from µm to mm. Therefore, when a part of the hydrogel is
exposed to water or aqueous fluids, the fluids are absorbed immediately through
the open space.1,2 Consequently, SPH has a very fast
swelling rate and absorbs a large amount of water compared to general
hydrogels. However, because SPH has weak mechanical properties due to the high
expansion rate, polysaccharides including hydrophilic polymers such as sodium
alginate, pectin, and chitosan or hybrid agents such as polyvinyl and alcohol
are added to increase the mechanical strength.3
Recently, due to the growing interest
in natural polysaccharides, they are being used to develop hydrogels for
biomedical and pharmaceutical applications.4 Natural polysaccharides have various compositions and properties that
cannot be easily imitated compared to synthetic polymers. Hydrogels
based on natural polysaccharides are being used for various applications due to
properties such as biocompatibility, biodegradability, and irritation reaction.5
The polysaccharides, which are now widely used in biomedical and
pharmaceutical fields, include alginate, chitosan, dextran, carrageenan, and
agarose.6,7
Alginate is a seaweed polysaccharide which is
a natural anionic polymer extracted from brown algae or produced by bacteria
and consists of (1-4)-linked b-D-mannuronate
(M) and its C-5 epimer a-L-guluronate
(G) residues.8 Alginate is a
natural polysaccharide that is widely used because of its gelling properties
due to the interaction between carboxylic groups and metal ions. Hydrogels
obtained from alginate have properties similar to the extracellular matrix and
are used in tissue engineering and regenerative medicine applications due to
their biocompatibility and low toxicity.9
Chitosan is a cationic polysaccharide made from alkaline N-deacetylation
of natural polysaccharide chitin extracted from the outer coverings of
crustaceans, and is composed of glucosamine and N-acetyl glucosamine.10
Chitosan is widely studied in the fields of wound dressing, anticoagulants,
drug delivery systems, biomedicine, and pharmaceutical research due
to properties such as antimicrobial activity,11
biodegradability,12 and biocompatibility.13
In addition, as chitosan contains a large number of hydroxy groups (-OH), which
helps hydrophilicity and nucleophilic amine groups (-NH) to facilitate bonding
with other functional groups, it is used for various biomedical applications.14
Agarose is a linear polysaccharide extracted from red
seaweed, and is composed of alternating D-galactose and 3,6-anhydro-L-galactopyranose
linked by α-(1→3)
and β-(1→4) glycosidic bonds and includes several ionized sulfate groups.
Agarose is widely used as an agar culture medium in gel-type microbiology and
in the food industry, and is utilized in biomedical applications,
jellification, biocompatibility, and natural biodegradability.15
These natural polysaccharides have useful functions but are difficult to
apply to hydrogel production because of the limitations in reactivity and
processability.16 To overcome this problem, there is a method of
bridging natural polysaccharides to existing polymers by an interpenetrating
polymer
network
(IPN).
An IPN is a polymer comprising two or more networks which
are at least partially interlaced on a polymer scale but not covalently bonded
to each other. As a polymer chain from one network is physically intertwined
with another network, it is difficult to separate each network.17
An IPN can produce highly incompatible polymers in a homogeneous form, such as
organic-inorganic mixtures,18 and can increase the
mechanical strength and toughness of the final product by using the individual
properties of each network.19
Hydrogel contact lenses are preferred by many because of their flexible
and comfortable fit, but low oxygen permeability can cause eye health problems.
Silicone hydrogels containing silicone monomers have higher oxygen permeability
compared to hydrogel contact lenses, but have limitations such as reduced
wettability due to hydrophobic surfaces and easy adsorption of proteins and
lipids. Accordingly, there is a need to develop high-performance contact lens
materials to overcome problems such as low oxygen permeability of conventional
hydrogel contact lenses and reduced surface wettability of silicone hydrogel
contact lenses.
The purpose of this study is to prepare a porous contact lens using a
foaming agent in order to compensate for the disadvantages of the hydrogel and
silicone hydrogel contact lenses and make use of the advantages. In order to
improve the function of the porous hydrogel, ionic natural polysaccharides are
introduced. In addition, the effects of the ionic properties of natural
polysaccharides on the physical properties of contact lenses were also
investigated.
Reagent
and Sample Preparation.
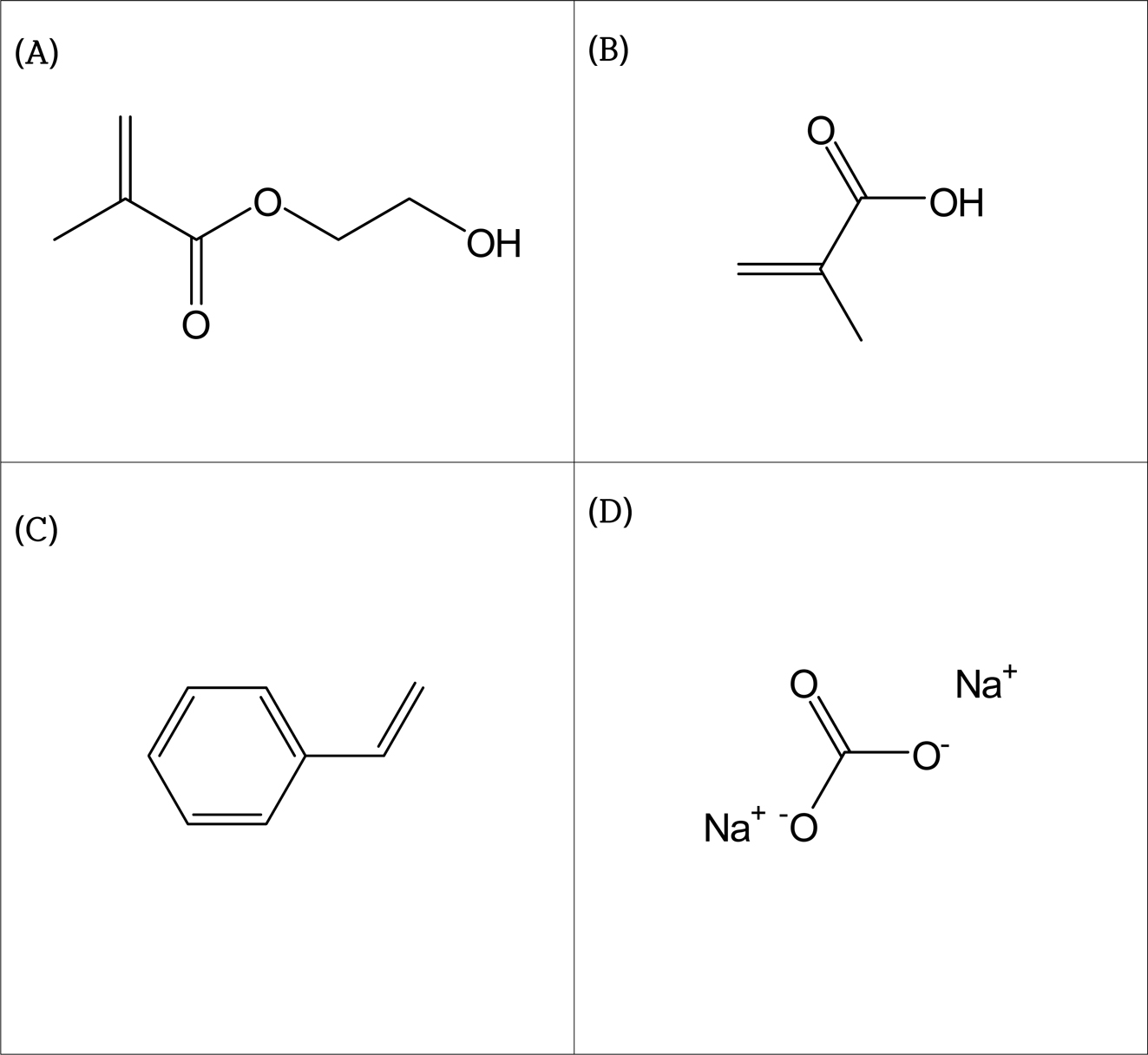
This study used 2-hydroxyethylmethacrylate (HEMA, Sigma-Aldrich)
which is a basic monomer, methacrylic acid (MAA, JUNSEI) which
is a hydrophilic monomer, and styrene (Sigma-Aldrich)
which is a hydrophobic monomer as the reagents to make the hydrogel contact
lenses. In addition, sodium carbonate (SC, Sigma-Aldrich)
was used as the foaming agent, ethylene glycol dimethacrylate (EGDMA, Sigma-Aldrich)
as the cross-linking agent, and 2,2-azobis(isobutyronitrile) (AIBN, JUNSEI)
as the initiator (Figure 1). Natural polysaccharides such as anionic alginate,
neutral agarose, and cationic water-soluble chitosan were used to improve the
function of the fabricated porous hydrogel. The water-soluble chitosan was
supplied by BIOPOLYTECH Co., Ltd. (Figure 2). In terms of IPN, N,N'-methylenebisacrylamide
(MBAA) was used as the cross-linking agent and ammonium persulfate
(APS)
as the initiator, which are Sigma-Aldrich products.
Thermal polymerization was performed on the polymacon
contact lens without the foaming agent and on the porous hydrogel contact lens
with the foaming agent at 80 °C for two hours according to the
casting mold method. The general hydrogel contact lens without the foaming
agent was referred to as Ref, and the contact lens with sodium carbonate as the
foaming agent was referred to as Ref-SC (Table 1).
The IPN solutions were prepared by producing alginate,
agarose, and water-soluble chitosan as a 1% solution, respectively, and adding
0.3% of MBAA (cross-linking agent) and APS (initiator). The IPN procedure was
performed by immersing Ref-SC contact lenses in each IPN solution for 24 h
at 37 oC
(Figure
3). The samples that were IPN-treated with alginate,
agarose, and chitosan were referred to as Alg-SC, Aga-SC, and
Chi-SC, respectively.
Physical
Properties. The physical and chemical properties of the fabricated
samples such as the water content, refractive index,
contact
angle, light transmittance, and oxygen permeability were
measured according to ISO 18369-4:2017. For each measurement item, we averaged
five measurements for each sample.
The refractive index was measured by an Abbe Refractometer (ATAGO DR-A1)
after washing each IPN-treated sample twice in phosphate-buffered saline (PBS)
and removing water with a wiper.
The water content was measured according to ISO
18369-4:2017 and was calculated by using the following eq.
(1).
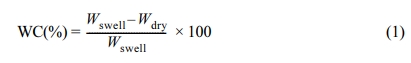
WC(%) is the water content in an equilibrium state, Wswell
is the weight of the sample swelled for 24 h, and Wdry is
the weight of the sample dried in an oven for 16 h.
The contact angle of the samples hydrated for 24 h was measured by
using a DSA30 from Kruss GmbH by the sessile drop method. We dropped PBS solution
3 µL
on the surface
of the lens to measure the angle of the water droplet twice per second for 10 s.
The light transmittance was measured in the range of 200~800 nm, and
we used Agilent’s Cary 60 UV-Vis as the measuring instrument.
The oxygen permeability was measured by the polarographic method and we
calculated the DK/t by measuring the current value using Rehder’s 201T. The
thickness of the samples was measured by using a low-pressure dial gauge
(Mitutoyo, VL-50-B). The curvature radius of the polarographic cell was 8.7 mm
and
the current value was measured after stabilizing at 35±0.5 oC
(equal to the temperature of the eye) for at least one hour.
Protein
Quantification. In terms of protein, this study used anionic protein bovine
serum
albumin (BSA) and
cationic protein lysozyme, which are similar in shape and chemical properties
to human albumin. First, we measured the hydrated weight of each sample to
absorb protein and prepared each protein in a solution of 5 mg/mL
in PBS. Then, we poured the protein solution into vials and
immersed each sample at 37 oC for an incubation time of 24 h
to absorb the protein. Next, each sample was washed twice with PBS and added to
a solution of 3% sodium dodecyl sulfate (SDS)
dissolved in distilled water and heated to 95 oC
for 15 min.
The proteins attached to the samples were desorbed by shaking the solution
gently with a vortex for three minutes. The absorbance was measured by using
Agilent’s Cary 60 UV-Vis, and we confirmed the absorbance value at 280 nm,
which is the maximum absorption wavelength of protein. The molar
extinction coefficient e
of BSA is 3.35 (mg-cm/g), and the e
of lysozyme is 13.2 (mg-cm/g), and the amount of protein
adsorbed was calculated by using eq. (2)
below.20

Q is the protein adsorption amount (mg/g)
v is the volume of the solution (mL)
c is the protein concentration in the solution
m is the mass of adsorbent
Antimicrobial
Activity. This study used E. coli (ATCC 10536) to examine
the antimicrobial activity of the natural polysaccharides with ionic
properties. The strain was provided by the Korea culture center of microorganisms
(KCCM). The liquid medium used for the antimicrobial activity test was prepared
by mixing 2 g of peptone and 1.2 g of beef in 40 mL of distilled water and
performing an autoclave sterilization treatment at pH 7.0,
and the E. coli was primary cultured in the liquid medium. Each sample
was added to the vial containing the liquid medium, and 1 µL
of the cultured E. coli was added and incubated at 35 oC
for 16 h, and subsequently diluted 10000 times. 1 mL
of the diluted solution was smeared to dry film and incubated at 35 oC
for 24 h.
E. coli 3M petrifilmTM was used as the dry film
medium.
The antibacterial test was repeated three times and expressed as an average
value.
|
Figure 1 The chemical structure of monomers: (A) 2-hydroxyethylmethacrylate (HEMA); (B) methacrylic acid (MAA); (C) styrene;
(D) sodium carbonate (SC). |
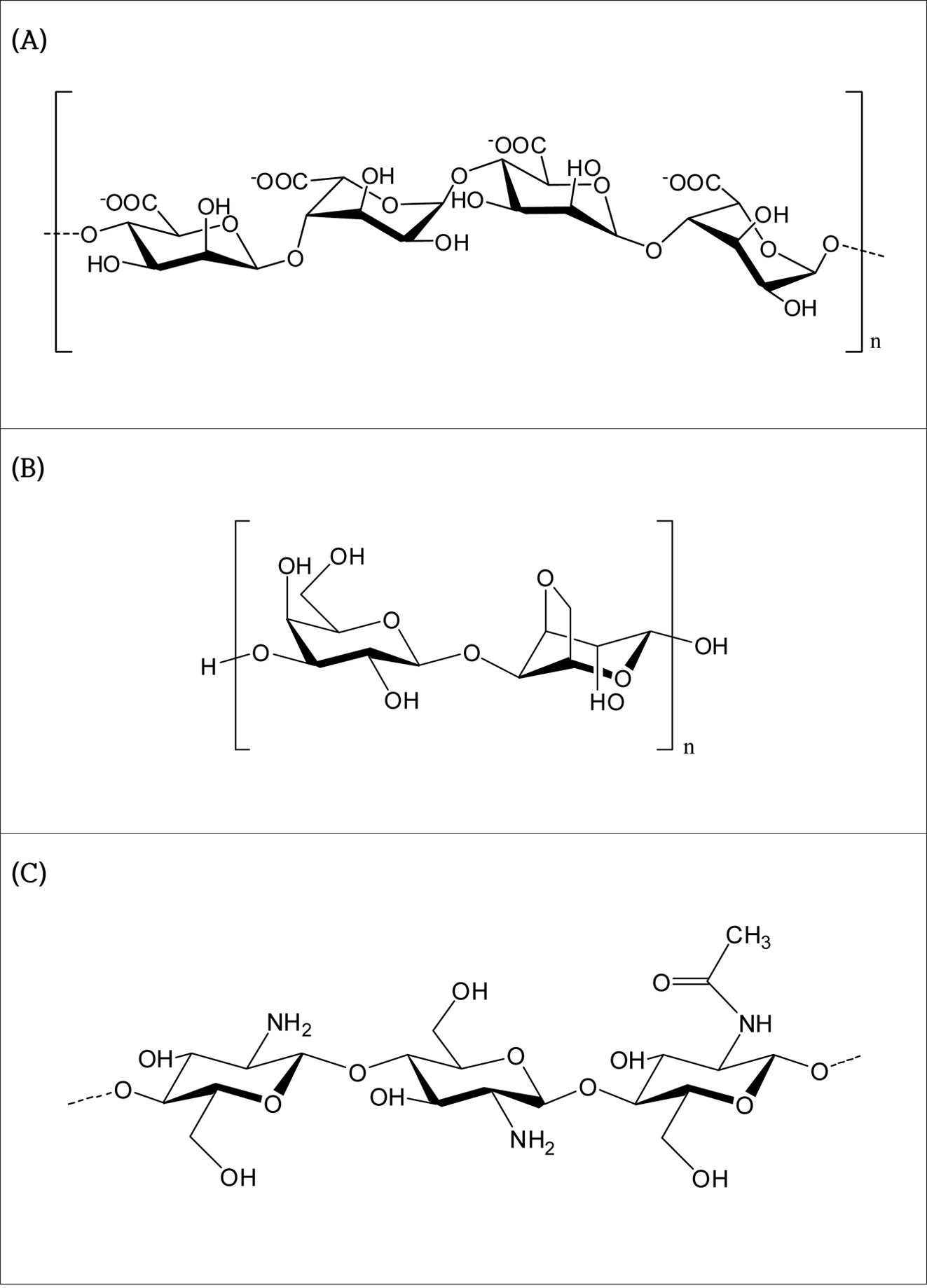
|
Figure 2 The chemical structure of natural ionic polysaccharides:
(A) alginate; (B) agarose; (C) chitosan. |
|
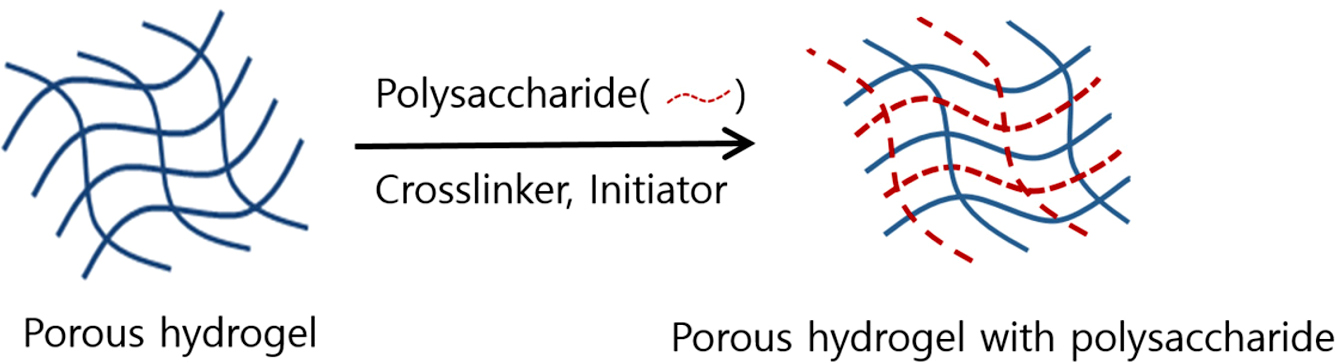
Figure 3 Scheme for preparing a porous hydrogel contact lenses
with ionoic polysaccharide such as alginate, agarose, and chitosan. |
|
Table 1 Composition of Hydrogel Contact Lens and Nomenclatures of Samples (wt%) |

The
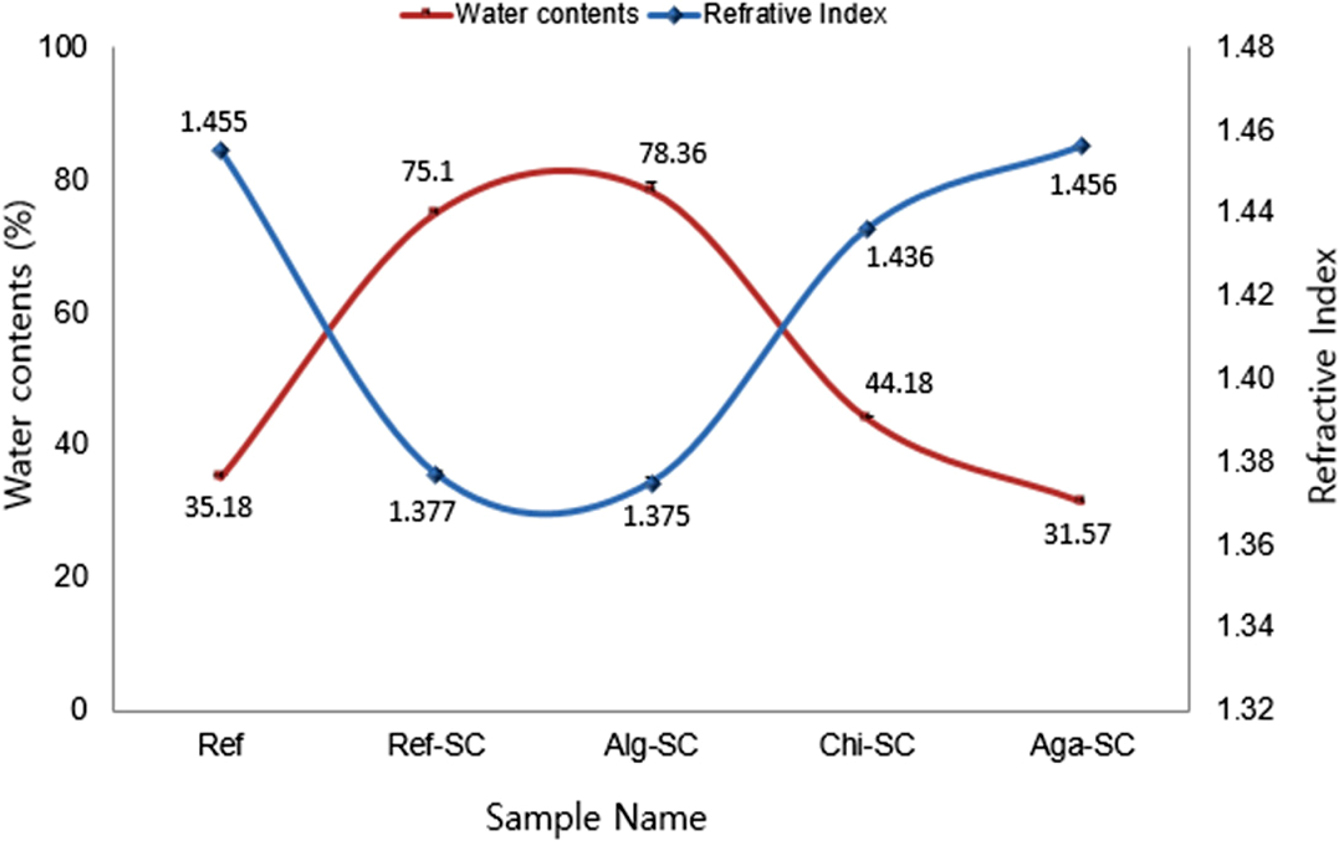
Physical Properties of the Porous Contact Lenses. Water Content and Refractive Index: Figure 4 shows the
results of measuring the water content and the refractive index of the hydrogel
contact lenses polymerized with the composition ratios shown in Table 1. As a
result of comparing the water content of Ref (polymacon contact lens) and Ref-SC
(porous hydrogel using a foaming agent), the water content of Ref-SC was more
than two times higher than that of Ref. This is because SC (the foaming agent)
produced carbon dioxide and formed pores in the contact lens, which absorbed a
large amount of water.21
The water content of Alg-SC cross-linked with alginate
was the highest at 78.36%, while that of Aga-SC cross-linked with agarose was
the lowest. The water content of Chi-SC and Aga-SC was 44.18 and 31.57%,
respectively, which was lower than that of Ref-SC by 41.17 and 57.96%,
respectively. Aga-SC even showed a decrease in water content compared
to the sample before using the foaming agent. Chavda et
al.22 reported that the expansion slowed down and the
equilibrium expansion rate decreased as the concentration of chitosan increased
in superporous hydrogels. It was also reported that the flexibility of the
polymer chains was greatly limited by the entanglement with cross-linked
chitosan networks. In addition, the water content is lowered as the expansion
rate decreases23 due to the cross-link between the
amino groups of chitosan and the carboxyl groups of MAA of adjacent chains.
According to the study by Vardar et al.,24
agarose exhibited a relatively lower water content compared with
IPNs with alginate and chitosan, which is probably due to the absence of electric
charge and low polarity.
The refractive index of Aga-SC was highest at 1.456,
which is similar to Ref, while that of Alg-SC was the lowest at 1.375. The
refractive index is related to structural aspects such as the molecular
arrangement and density in the contact lens and is generally inversely related
to the water content. In cross-links with high substitution rates, swelling
hardly occurs because of the high density.25
Therefore, the refractive index of Aga-SC with the lowest water content was
high and the refractive index of Alg-SC with the highest water content was the
lowest.
Light
Transmittance: The light transmittance is one of
the most important conditions that must be satisfied for optical lenses. Figure
5
shows the results of measuring the light transmittance in this study. The light
transmittance is classified into UV-B (280~315 nm), UV-A
(315~380 nm), and visible light (380~780 nm)
according to ISO standards. In terms of the light transmittance of the visible
light spectrum for each sample, the transmittance of Ref-SC without
cross-linking polysaccharides was 93.85%, and the visible light
transmittance of Alg-SC, Chi-SC, and Aga-SC cross-linked with
natural polysaccharides were 90.49, 91.72, and 88.55%, respectively. The
transmittance of all of the samples with polysaccharides slightly decreased,
but satisfied a transparency of more than 88%, which is the basic requirement
for contact lenses according to ANSI Z80.20: 2004.
Oxygen
Permeability: Since the
cornea has no blood vessels, most of the oxygen to the eye is supplied from the
external environment. Consequently, wearing contact lenses acts as a barrier
which reduces the oxygen transfer rate to the eye.26 As low oxygen
permeability causes side effects such as hypoxia, neovascularization,
corneal edema, and endothelial polymegethism,27 contact lenses
should have a high oxygen permeability to offer a higher level of
safety and comfort.28 The oxygen transmissibility of the
lens is expressed by DK ((cm2/sec)·(mLO2/mL·mmHg)·10-11)) and the oxygen
perme-ability
(transmissibility level) is expressed as the DK per thickness
of the lens, DK/t ((cm/mLO2)/(sec·mL·mmHg)·10-9)).
As shown in Figure 6, the
DK/t of Ref-SC with the foaming agent was 28.95×10-9,
which increased significantly by 74.8%, compared to Ref without the foaming
agent. This shows that using the foaming agent increased the oxygen
permeability in addition to the water content. Among the samples that were
IPN-treated with polysaccharides, the DK/t of Alg-SC cross-linked with alginate
were the highest at 30.43×10-9. In the
case of hydrogel contact lenses, oxygen is transferred by the water contained
in the lens. Consequently, the oxygen permeability is mainly determined by the
water content and the thickness.29 Therefore, if the water
content is high, the oxygen permeability also increases exponentially. The
porous hydrogel contact lenses showed higher oxygen permeability than the
non-porous contact lenses because of the higher water content. In addition, we
confirmed that the hydrogels cross-linked with natural polysaccharides were
significantly influenced by the water content. The DK/t of Chi-SC were the
lowest at 10.28×10-9, which were 64.5% lower than
that of Ref-SC. Consistent with previous studies that report the low oxygen and
carbon dioxide permeability of chitosan and that the oxygen permeability
depends on the water content,30 the oxygen permeability of
Chi-SC cross-linked with chitosan was also reported as low in this study due to
the low water content.
Wettability: As
contact lenses are directly affected by the wettability of the hydrogel because
they are in close contact with the tear film, the stability of the tear film is
also affected by the wettability. If the stability of the tear film is
destroyed by the use of contact lenses, symptoms such as dry eyes will occur.31
Therefore, contact lenses need to maintain a hydrophilic surface to maintain a
stable tear film. The contact angle is the angle that is formed when a liquid
comes into contact with a solid surface. Large contact angles lead to
hydrophobic properties and small contact angles lead to hydrophilic properties
and better wettability. Contact lenses with good wettability tend to reduce
dehydration and cause fewer tears.32
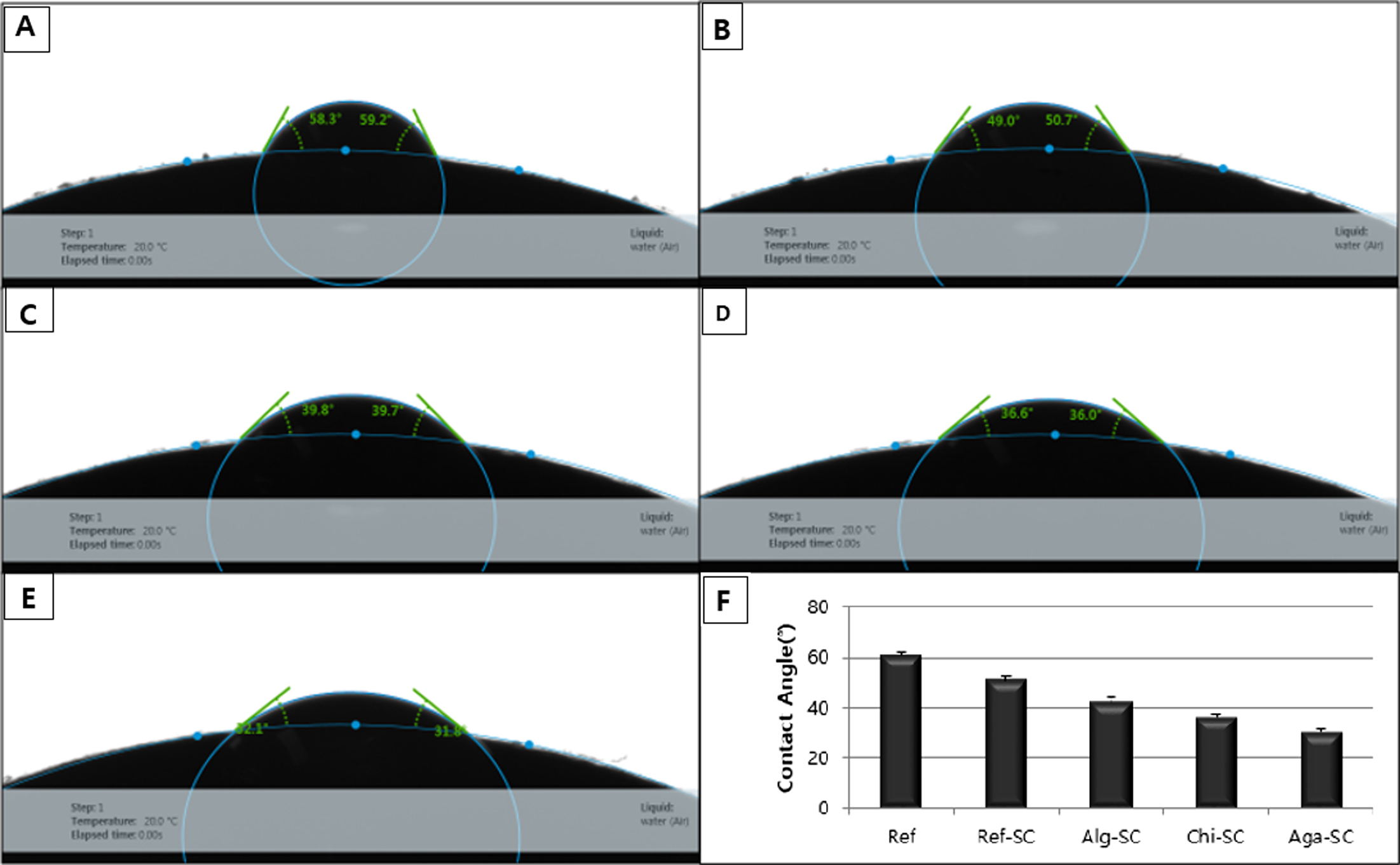
As a result of measuring the contact angles in this
study, the contact angle of Ref was highest at 60.8° and the contact angle of
Ref-SC was 51.0°, which is about 16% lower than that of Ref. This shows that
adding the foaming agent increased the wettability. The
contact angles of Alg-SC, Chi-SC, and Aga-SC cross-linked with natural
polysaccharides were 42.0°, 36.3°, and 30.1°, respectively, which were about
17.6%, 28.8%, and 41.0% lower, respectively, than that of Ref-SC. In
particular, Aga-SC exhibited the highest wettability (Figure 7).
The chitosan used for cross-linking contains a large number of hydroxy groups
(-OH) and amine groups (-NH2), and shows hydrophilicity due to
strong hydrogen bonds with water. In addition, the hydrophilicity of agarose is
composed of excessive hydroxy groups with 3 hydroxyl radicals per unit of
agarose. Therefore, the covalent bond between the atoms of the -OH group
becomes extremely high due to the significant difference between hydrogen and
oxygen, and this polarity is reported to interact with polar water molecules
through hydrogen bonds to impart strong hydrophilicity to the compound.33
The
Antimicrobial Activity of Contact Lenses with Ionic Natural Polysaccharides. The
contact lens surface is a suitable substrate for bacterial adhesion and biofilm
formation.34 Contact lenses are biomaterials that are in direct
contact with the eyes, and bacterial contamination which leads to biofilm
formation on the surface due to long-term wear is a major problem.35
Organic materials and bacteria quickly spread toward the contact lens surface
immediately after contact with lens care solutions or body fluids.36
Typical eye-related bacteria include E. coli, Pseudomonas aeruginosa,
and Staphylococcus aureus, and these pathogens cause bacterial
conjunctivitis and corneal ulcers.37
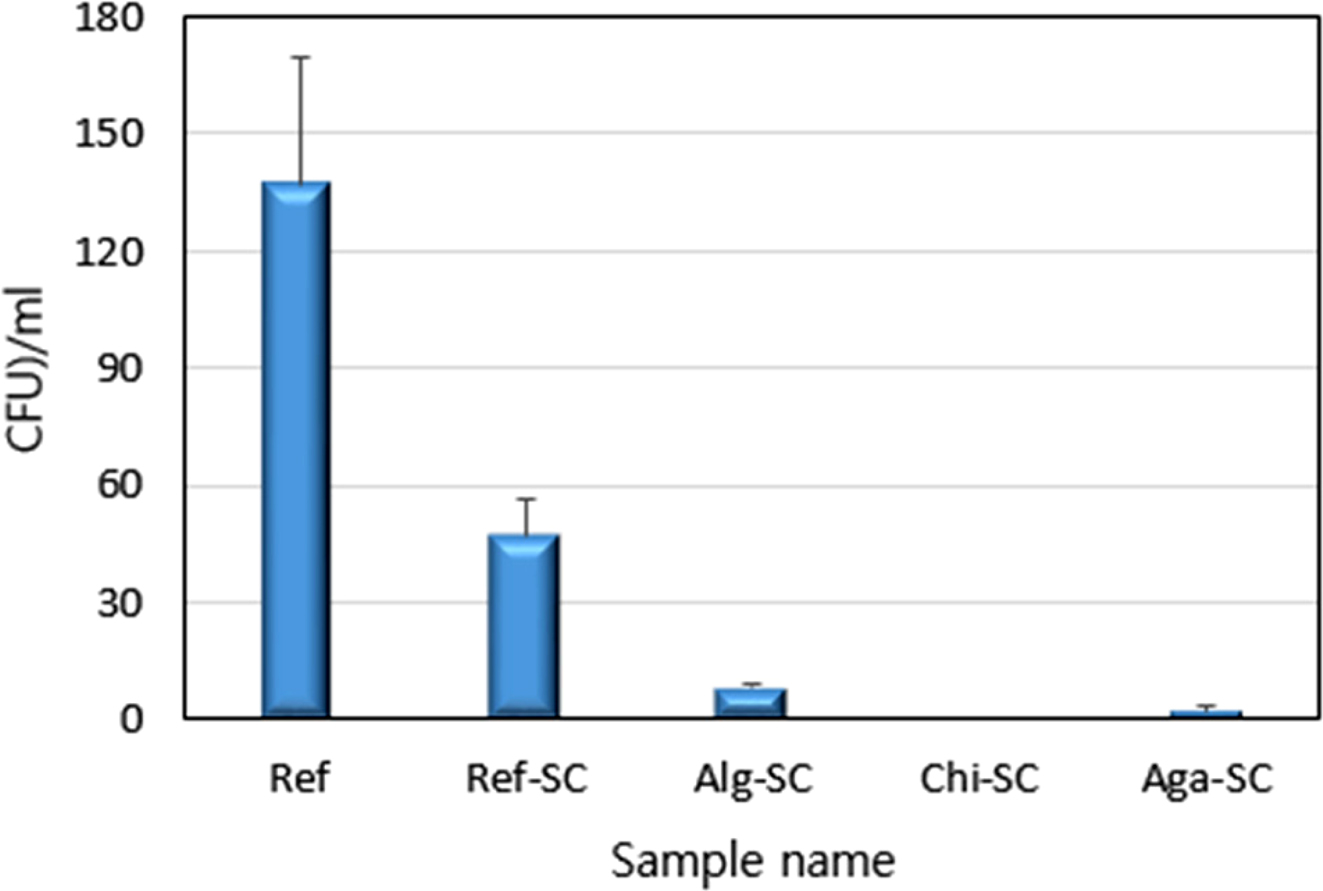
This study found that all of the samples cultured with
contact lenses had more antimicrobial activity compared to the Base cultured
without contact lenses (Figure 8). The introduction of charged
moieties into the polymer network by copolymerizing HEMA with anionic monomers
such as MMA improves cell adhesion and proliferation and enhances the
biological performance of the material.38 Based on
this, the increase of antimicrobial activity in all of the samples containing
MMA has been considered to be enhanced under the presence of MMA. In addition,
the hydrogels with SC exhibited antimicrobial activity, and research results
have shown that increasing the amount of SC enhances the antimicrobial
activity.39 The number of E. coli in Ref, a normal
hydrogel lens, is 138 CFU, and the porous hydrogel lens is reduced to 47 CFU.
Porous hydrogel lenses cross-linked with natural polysaccharides have
significantly improved antibacterial properties (Figure 8). The
porous hydrogel contact lenses containing chitosan exhibit more antimicrobial
activity than those containing alginic acid and agarose.
Surface treatment by natural polysaccharides or polysaccharides has been
used as a promising means to cope with implant-related biofilm infections and
many natural polymers exhibit antibiotic properties.35 Alginic acid
has been proven to have antibacterial effects against S. aureus and E.
coli,38 and chitosan is well known for its superior
antibacterial properties.39 It has been reported that the D-glucosamine
of chitosan exhibits immune activities by increasing the activity of natural
killer cells involved in the defense of the immune system. Moreover, positively
charged chitosan oligosaccharide molecules selectively destroy only the
bacterial cell membranes without destroying neutral normal cell membranes due
to the electrostatic interaction with negatively charged bacterial membranes.40
The antimicrobial activity of water-soluble chitosan was higher than that of
chitosan, and the antimicrobial activity against E. coli of N–N-propyl-N
and N-dimethyl chitosan was reported to be 20 times than that of
chitosan.41
Protein
Adsorption of Contact Lenses According to Ionic Properties of Natural Polysaccharides. The human
tear film contains many proteins and lipids. Contact
lenses are highly affected by tears and cause various side effects such as
decreased comfort and visual acuity due to the adsorption of proteins contained
in the tear components.42 Hydrogel lenses consist of
hydrophobic or hydrophilic, negatively charged or positively charged monomers
and have a significant effect on the amount of protein deposition.43
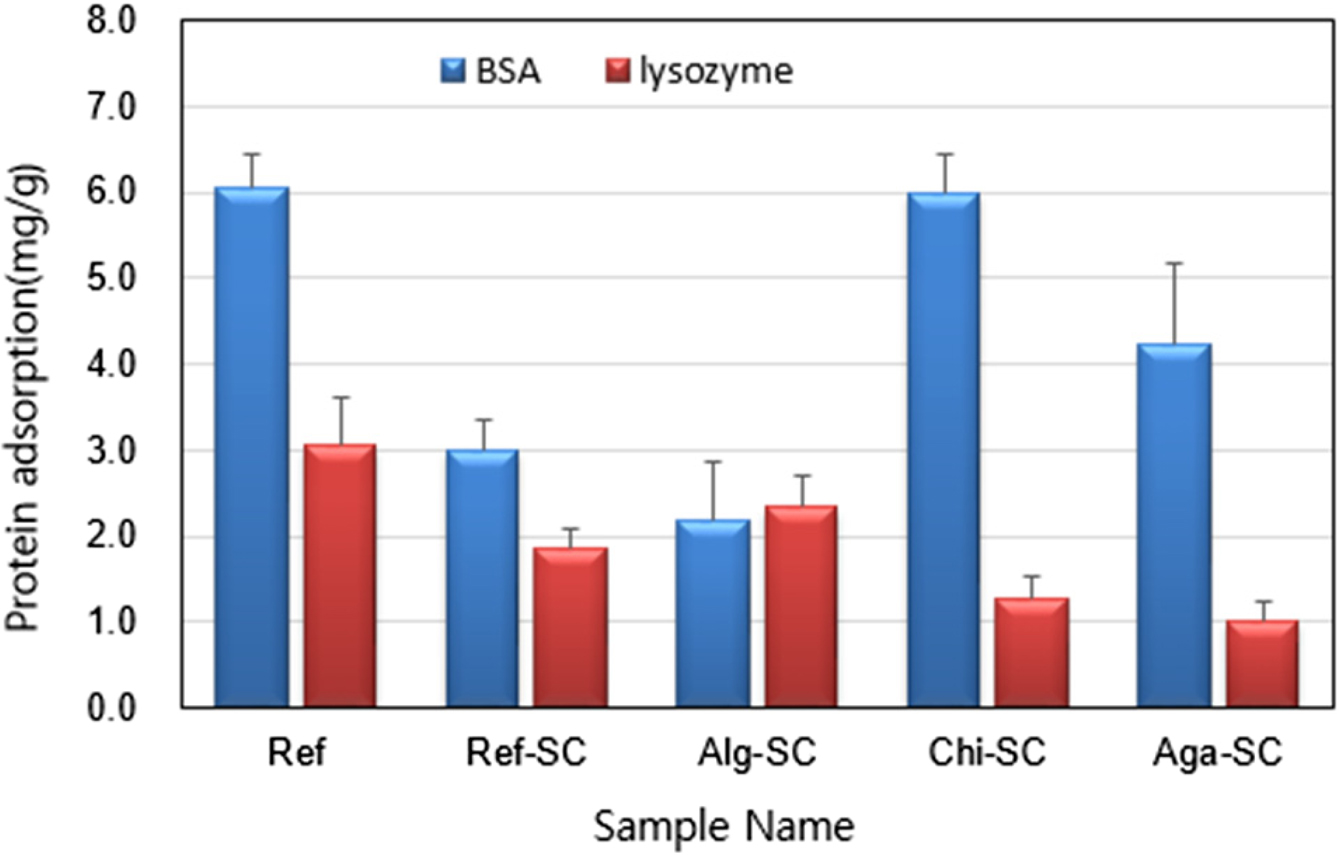
In the case of anionic Alg-SC, the adsorption of
negatively charged BSA was the least and the adsorption of positively charged
lysozyme was the most. In addition, cationic Chi-SC showed
the most adsorption of BSA, while nonionic Aga-SC showed the least adsorption
of lysozyme (Figure 9). Albumin, a protein in tears, is
negatively charged and has a high molecular weight. Therefore, the protein
adhesion is reduced by the charge repulsion effect when penetrating into the
negatively charged hydrogel matrix. On the other hand, lysozyme has a low
molecular weight and has a positive charge at physiological pH, which
facilitates penetration into hydrogels containing negatively charged monomers.44
The negatively charged BSA protein is highly adsorbed on the cationic Chic-SC,
and the negatively charged Alg-SC has the lowest adsorption amount. On the
other hand, the positively charged lysozyme protein is highly adsorbed to
anionic Alg-SC and less adsorbed to Chi-SC and Aga-SC. BSA adsorbed more
than lysozyme in all samples except Alg-SC. Therefore, we confirmed that the
ionic properties of natural polysaccharides affect the type and amount of
proteins adsorbed in porous hydrogel contact lenses.
|
Figure 4 Comparison of water contents (red line) and refractive
index (blue line) of porous hydrogels contact lenses with ionic polysaccharide. |
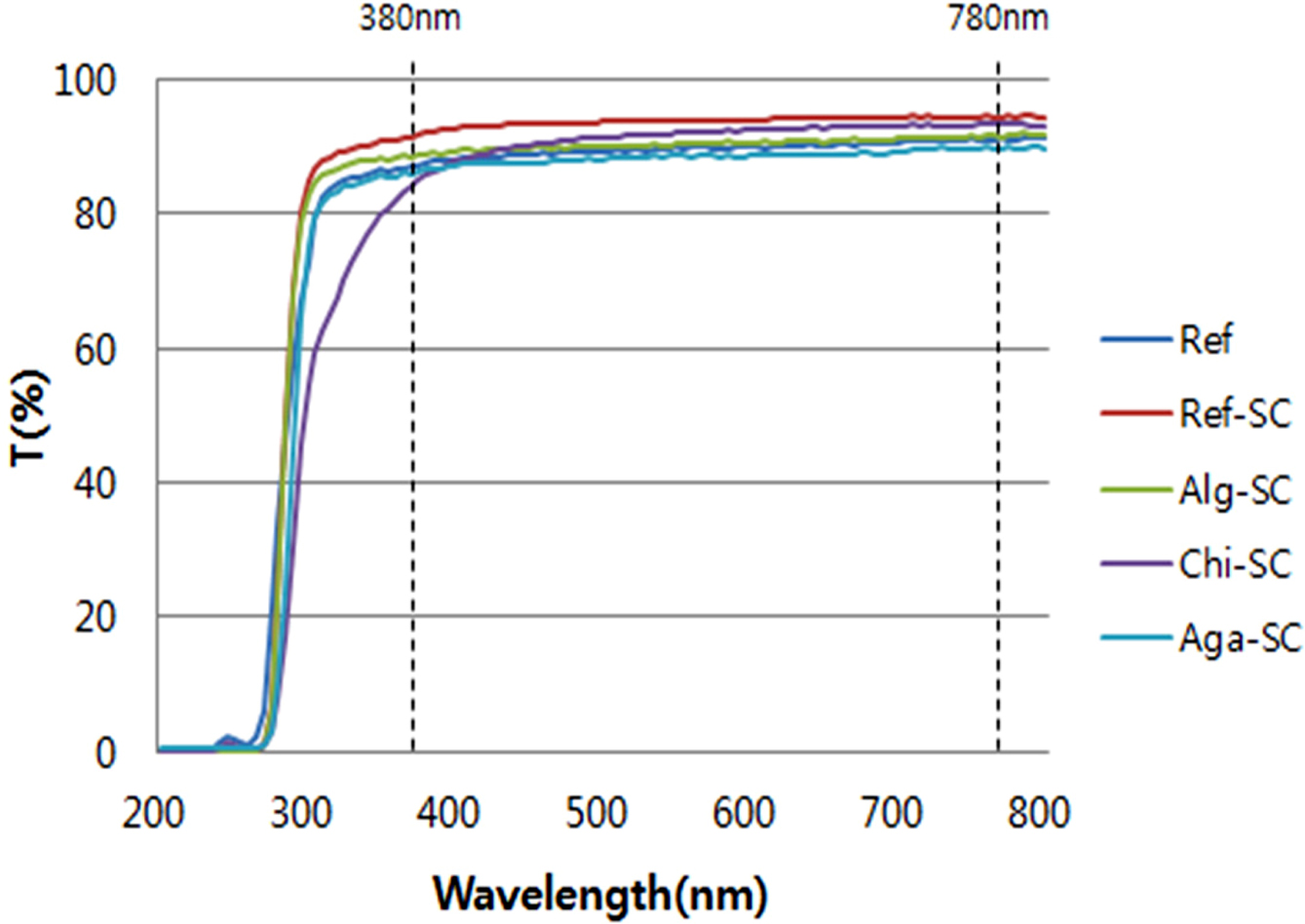
|
Figure 5 Transmittance of porous hydrogel contact lenses with
ionic polysaccharides. |
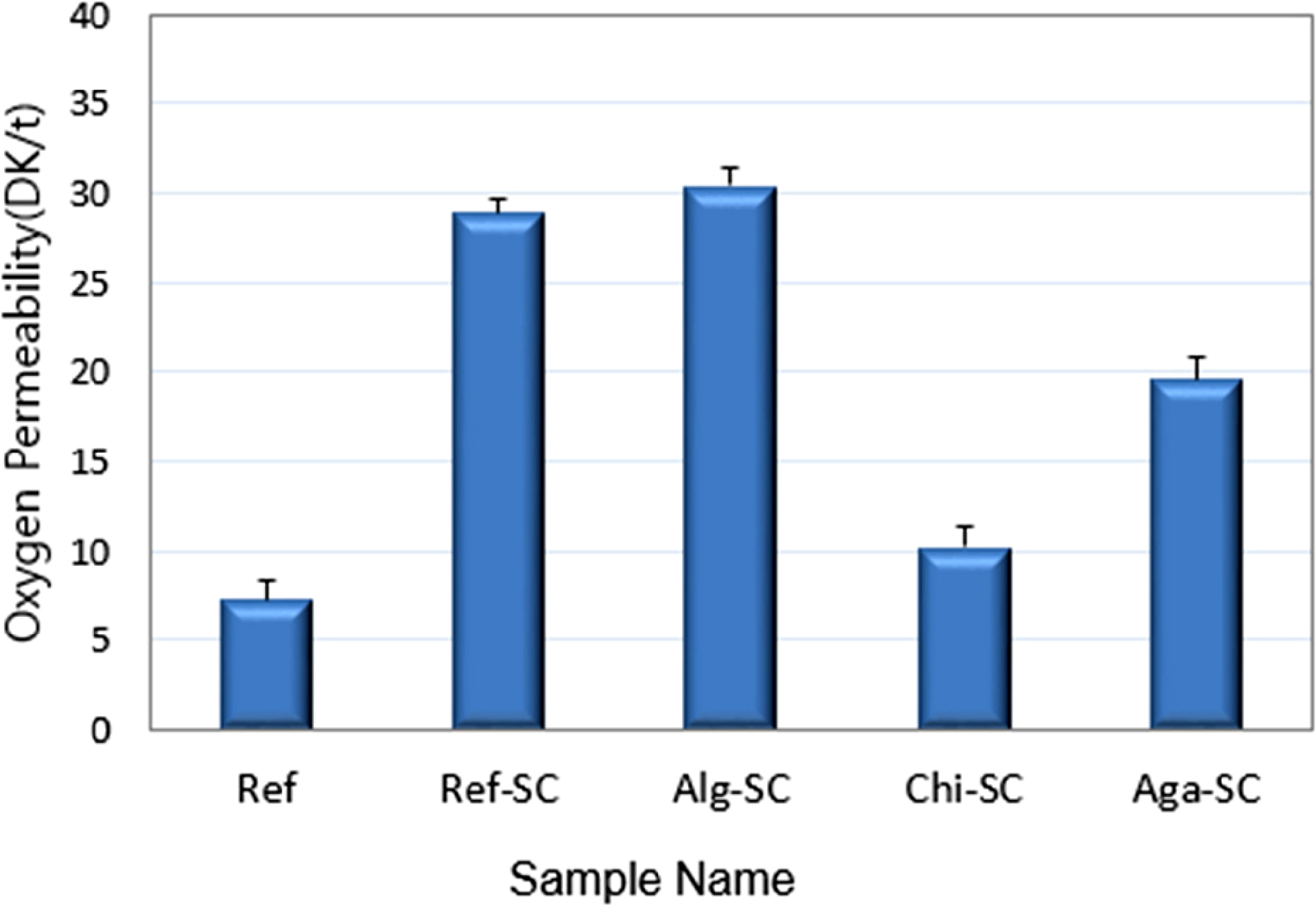
|
Figure 6 Comparison of oxygen transmissibility and oxygen
permeability of porous hydrogel contact lenses. |
|
Figure 7 Contact angle image of nonporous and porous hydrogels: (A) Ref; (B) Ref-SC; (C) Alg-SC; (D) Chi-SC; (E) Aga-SC; (F) contact
angle graph of porous hydrogel contact lenses. |
|
Figure 8 Antibacterial activities for E. coli of porous hydrogel contact lenses. |
|
Figure 9 Protein adsorption of BSA (blue) and lysozyme (red) to
porous hydrogel contact lenses. |
Porous hydrogels cross-linked with natural polysaccharides have better
contact lens properties than non-porous hydrogels. In addition, the wettability
and antimicrobial activities were significantly improved regardless of the type
of ionic polysaccharide. Protein adhesion is highly affected by the type of
ionic polysaccharide. In conclusion, this study found that natural
polysaccharides have a significant contribution to the functional enhancement
of porous hydrogel contact lenses.
- 1. J. Chen, H. Park, and K. Park, J. Biomed. Mate. Res., 44, 53 (1999).
-

- 2. H. Omidian, J. G. Rocca, and K. Park, J. Control. Release, 102, 3 (2005).
-

- 3. M. Nagpal, S. K. Singh, and D. Mishra, Acta Pharm. Sci., 53, 7 (2011).
- 4. M. Kumari and G. S. Chauhan, J. Appl. Polym. Sci., 363, 119 (2011).
-

- 5. V. K. Thakur and M. K. Thakur, J. Clean. Prod., 82, 1 (2014).
-

- 6. C. C. Lin and A. T. Metters, Adv. Drug Deliv. Rev., 58, 1379 (2006).
-

- 7. M. Hamidi, A. Azadi, and P. Rafiei, Adv. Drug Deliv. Rev., 60, 1638 (2008).
-

- 8. K. Y. Lee and D. J. Mooney, Prog. Polym. Sci., 37, 106 (2012).
-

- 9. L. Shapiro and S. Cohen, Biomaterials, 18, 583 (1997).
-

- 10. H. S. Whang, W. Kirsch, Y. H. Zhu, C. Z. Yang, and S. M. Hudson, J. Macromol. Sci. Polym. Rev., 45, 309 (2005).
-

- 11. R. C. Goy, D. D. Britto, and O. B. Assis, Polímeros, 19, 241 (2009).
-

- 12. I. Makarios-Laham and T. C. Lee, J. Environ. Polym. Degrad., 3, 31 (1995).
-

- 13. N. D. Thorat, S. V. Otari, R. M. Patil, R. A. Bohara, H. M. Yadav, V. B. Koli, and R. S. Ningthoujam, Dalton T., 43, 17343 (2014).
-

- 14. D. de Britto and O. B. Assis, Carbohydr. Polym., 69, 305 (2007).
- 15. F. Rossi, M. Santoro, T. Casalini, P. Veglianese, M. Masi, and G. Perale, Int. J. Mol. Sci., 12, 3394 (2011).
-

- 16. S. J. Kim, S. J. Park, and S. I. Kim, React. Funct. Polym., 53, 55 (2003).
-

- 17. Y. Zhao, J. Kang, and T. Tan, Polymer, 47, 7702 (2006).
-

- 18. M. Sangermano, W. D. Cook, S. Papagna, and S. Grassini, Eur. Polym. J., 48, 1796 (2012).
-

- 19. H. Omidian, J. G. Rocca, and K. Park, Macromol. Biosci., 6, 703 (2006).
-

- 20. B. Gao, H. Hu, J. Guo, and Y. Li, Colloid Surfaces B, 77, 206 (2010).
-

- 21. S. Ghazali and N. Adnan, Indian J. Sci. Technol., 10, 1 (2017).
- 22. H. Chavda and C. Patel, J. Pharm. Bioallied Sci., 2, 124 (2010).
-

- 23. A. Ávila, K. Bierbrauer, G. Pucci, M. López-González, and M. Strumia, J. Food Eng., 109, 752 (2012).
-

- 24. E. Vardar, M. Vert, J. Coudane, V. Hasirci, and N. Hasirci, J. Biomater. Sci. Polym. Ed., 23, 2273 (2012).
-

- 25. W. E. Hennink, H. Talsma, J. C. H. Borchert, S. C. De Smedt, and J. Demeester, J. Control. Release, 39, 47 (1996).
-

- 26. V. Compan, A. Andrio, A. Lopez-Alemany, E. Riande, and M. F. Refojo, Biomaterials, 23, 767 (2002).
-

- 27. A. S. Hoffman, Adv. Drug Deliv. Rev., 54, 3 (2002).
-

- 28. N. Efron, P. B. Morgan, I. D. Cameron, N. A. Brennan, and M. Goodwin, Optom. Vis. Sci., 84, E328 (2007).
-

- 29. J. Pozuelo, V. Compañ, J. M. González-Méijome, M. González, and S. Mollá, J. Membrane Sci., 452, 62 (2014).
-

- 30. A. Ito, M. Sato, and T. Anma, Macromol. Chem. Phys., 248, 85 (1997).
- 31. A. S. Bruce, J. C. Mainstone, and T. R. Golding, Biomaterials, 22, 3249 (2001).
-

- 32. Y. Iwasaki and K. Ishihara, Sci. Technol. Adv. Mat., 13, 064101 (2012).
-

- 33. A. Awadhiya, S. Tyeb, K. Rathore, and V. Verma, Eng. Life Sci., 17, 204 (2017).
-

- 34. M. J. Elder, F. Stapleton, E. Evans, and J. K. Dart, Eye, 9, 102 (1995).
-

- 35. G. A. Junter, P. Thébault, and L. Lebrun, Acta Biomater., 30, 13 (2016).
-

- 36. Y. F. Dufrêne, C. J. P. Boonaert, and P. G. Rouxhet, Colloids Surfaces B, 7, 113 (1996).
-

- 37. I. Jalbert, M. D. Willcox, and D. F. Sweeney, Cornea, 19, 116 (2000).
-

- 38. H. M. Lee, J. K. Kim, and T. S. Cho, Bull. Kor. Chem. Soc., 32, 4239 (2011).
-

- 39. T. T. Cushnie, V. E. Hamilton, and A. J. Lamb, Microbiol. Res., 158, 281 (2003).
-

- 40. M. E. I. Badawy and E. I. Rabea, Int. J. Carbohydr. Chem., 2011, 460381 (2011).
-

- 41. Z. Jia and W. Xu, Carbohydr. Res., 333, 1 (2001).
-

- 42. D. Luensmann and L. Jones, Contact Lens Anterior., 31, 179 (2008).
-

- 43. D. Luensmann and L. Jones, Contact Lens Anterior., 35, 53 (2012).
-

- 44. C. E. Soltys-Robitaille, D. M. Ammon Jr., P. L. Valint Jr., and G. L. Grobe, Biomaterials, 22, 3257 (2001).
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2020; 44(5): 625-632
Published online Sep 25, 2020
- 10.7317/pk.2020.44.5.625
- Received on Apr 3, 2020
- Revised on May 31, 2020
- Accepted on Jun 7, 2020
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Hyun Mee Lee
-
Department of Optometry & Vision Science, Catholic University of Daegu, Hayang-Ro 13-13, Gyeongsan, Gyeongbuk 38430, Korea
- E-mail: hmlee@cu.ac.kr
- ORCID:
0000-0001-6668-5864










 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.