- A Novel Approach to Disentangle Carbon Nanotubes Using Viscous Sugar Syrup
Dong Wook Chae†
 , Stephen C. Hawkins* , Chi P. Huynh**, Youngho Eom***, Youkyung Han****, Bong-Seob Shin, and Jong-Young Jeon
, Stephen C. Hawkins* , Chi P. Huynh**, Youngho Eom***, Youkyung Han****, Bong-Seob Shin, and Jong-Young JeonDepartment of Textile Engineering, Kyungpook National University, Sangju 37224, Korea
*School of Mechanical and Aerospace Engineering, Queen’s University Belfast, BT9 5AH, UK
**Lintec of America, INC. Inc. R&D Division Nano-Science and Technology Center, Richardson, Texas 75081, USA
***Department of Polymer Engineering, Pukyong National University, Busan 48513, Korea
****School of Convergence & Fusion System Engineering, Kyungpook National University, Sangju 37224, Korea- 점착성 설탕시럽을 이용하여 탄소나노튜브의 얽힘을 푸는 새로운 접근방법
채동욱†
 · Stephen C. Hawkins* · Chi P. Huynh** · 엄영호*** · 한유경**** · 신봉섭 · 전종영
· Stephen C. Hawkins* · Chi P. Huynh** · 엄영호*** · 한유경**** · 신봉섭 · 전종영경북대학교 섬유공학과, *School of Mechanical and Aerospace Engineering, Queen’s University Belfast **Lintec of America, INC. Inc. R&D Division Nano-Science and Technology Center ***부경대학교 고분자공학과, ****경북대학교 융복합시스템공학부
The disentanglement of
multi-walled carbon nanotube (MWNT) bundles by using a sticky sugar syrup was
explored for the first time. The syrup was prepared by mixing sucrose, glucose,
fructose, and distilled water in a 1:1:1:0.8 weight ratio. A disk-type mixer
suitable for high-viscosity materials was built to mix this syrup with MWNTs.
To find out the optimum mixing conditions for effective disentanglement of
MWNTs, the content ratio of MWNTs to sugar syrup was varied at each mixing
step. Digital image processing technique was used with optical micrographs to
quantitatively estimate the dispersion of nanotubes in sugar syrups. This
revealed that the most homogeneous dispersion of MWNTs in the sugar syrup was
achieved by gradually adding the syrup at each mixing step rather than all at
once at the initial mixing step. The dynamic viscosity (η') of the mixtures was
measured to evaluate the overall dispersion state of MWNTs. The incorporation
of MWNTs increased the η' of sugar syrup, exhibiting a decreased η' with an
increase in the degree of dispersion. To validate this novel dispersion method
for polymer composites, disentangled MWNTs were transferred into poly(vinyl
alcohol) (PVA) and polyacrylonitrile (PAN); they exhibited uniform dispersion
in each polymer matrix, without reagglomeration occurred.
끈적끈적한 설탕시럽을 사용한 다중벽 탄소나노튜브(MWNT) 번들의 풀림이 연구되었다. 시럽은 수크로스, 글루코스, 프럭토스와 증류수를
1:1:1:0.8의 무게 비율로 혼합하여 제조되었다. 고점도의 물질을 위한 디스크형태의
믹서가 시럽과 MWNTs를 혼합하기 위하여 제작되었다. MWNT의
효과적인 풀림을 위한 최적의 혼합조건을 알기 위하여 MWNT/설탕시럽 함량 비율을 각 제조단계에서 다양하게
하였다. 설탕시럽에서 나노튜브의 분산상태를 정량적으로 평가하기 위해 디지털영상처리기술이 광학현미경 이미지에
사용되었다. 이것은 MWNT의 균일한 분산이 시럽을 한 번에
첨가하기보다는 각 단계에서 점차적으로 첨가함으로써 얻어진다는 것을 보여주었다. 혼합물의 동적점도가 MWNT의 전체적인 분산상태를 평가하기 위해서 측정되었다. MWNT 첨가가
시럽의 동적점도를 증가시켰고 분산정도가 증가하면서 동적점도가 감소함을 보였다. 이 새로운 분산방법을
고분자 복합체에 적용가능한지 입증하기 위해서 분산된 MWNT가 폴리비닐알콜과 폴리아크릴로니트릴에 옮겨졌고
각 매트릭스에서 재뭉침없이 균일하게 분산됨을 보였다.
Sticky sugar syrup with crystal-inhibiting characteristics
was used to disentangle MWNT bundles. The ratio of black area to the entire
area in the optical micrographs
was calculated to quantitively estimate the dispersion degree of nanotubes. The
effective disentanglement of MWNTs in the sugar syrup was achieved by gradually
adding the syrup at each mixing step.
Keywords: sugar syrup, disentanglement, multi-walled carbon nanotube, dynamic viscosity, composite
This research was supported by Basic Science Research
Program through the National Research Foundation of Korea (NRF) funded by the
Ministry of Education (NRF-2017R1D1A1B03035939).
Carbon nanotubes (CNTs) have a fiber-like structure with diameters in the
nanometer scale and lengths up to tens of microns. Due to their various
benefits such as exceptional mechanical, electrical, magnetic, and thermal
properties,1-6 many researches have been devoted to exploring their
potential applications in a large scale. Currently advances in the catalytic
process have allowed the production of multi-walled carbon nanotubes (MWNTs) at
the commercial scale. However, most of the manufacturing processes result in
highly entangled and aggregated structures.7 CNTs tend to cluster
together due to the strong van der Waals attraction between them, so it is
extremely difficult to homogeneously disperse them in various media. This can
prevent the achievement of their superior properties in applications such as
reinforcement materials and membranes. Therefore, the homogeneous dispersion of
CNTs in the desired medium is a prerequisite for their practical applicability.
Previous dispersion works on CNTs in continuous phase were focused on the
chemical modification of the nanotube surface by strong acids, gas plasma or
functionalization, polymer dispersants, surfactant-assisted processing, etc.8-14
In addition, most of the dispersion methods operate through chemical treatments
and strong, long-lasting sonication. These generate defects on the nanotube
walls and makes the nanotubes short with open ends, which might worsen the
electrical and mechanical properties.15
The full and easy dispersion
method of CNTs with a little energy input and in a large scale is favored in
the industrial field. Furthermore, noncovalent approaches appear more
attractive than the covalent ones since they maximize the preservation of the intrinsic
properties of CNTs.16 However, it does not seem that there is a
simple way to obtain fully disentangle nanotubes with a little limit to their
further applications. Thus, the present study proposes a novel method to
conveniently disentangle nanotubes in their manufactured state without addition
of chemicals nor functionalization of the nanotube surface. Further, fully
dispersed nanotube suspensions were applied to the fabrication of
CNT-reinforced polymer composites. MWNTs were mixed with a sticky sugar syrup,
which was used as the dispersing medium to disentangle them prior to
resuspending them in the desired solvents. To discover the effective mixing
conditions to disentangle the MWNTs three different mixing ways were tested by
varying the ratio of sugar syrup to MWNTs in each mixing step. The versatility
of this method was clearly demonstrated by transferring disentangled MWNTs into
polyacrylonitrile (PAN) and poly(vinyl alcohol) (PVA) matrices, soluble in
dimethylformamide (DMF) and water with very different degrees of affinity
toward nanotubes, respectively.
Materials. MWNTs (average
diameter = 40–50 nm and length = ~500 μm)
produced via a chemical vapor deposition (CVD) method were used for the
experiments. As a dispersing medium, viscous sugar syrup was prepared by mixing
sucrose, glucose, fructose, and distilled water in a 1:1:1:0.8 weight ratio.
PVA (degree of hydrolysis = 99.5%, degree of polymerization = 1700) and PAN
(molecular weight = 150000 g/mol) were purchased from DuPont and
Sigma-Aldrich, respectively.
Disentanglement
of MWNTs in Sugar Syrup. A disk-type mixer
was designed to give high shear to viscous material. The gap distance between
the mixing plates was fixed to 0.2 mm and one of them, having a diameter
of 65 mm, was rotated at 20 rpm. The disentanglement of the MWNTs occurs
by passing the mixture through between these two plates.
Dispersion
of MWNTs in PAN and PVA. The sugar syrup
was removed by washing with distilled water and the filtered MWNTs in a wet
state were rinsed with DMF and water, (i.e., the solvents for PAN and PVA,
respectively) prior to suspending them in each solvent. The so-obtained
suspensions were gently sonicated for 30 min in the sonic bath, followed by the
dissolution of PAN and PVA at 80 oC. The resulting PAN and PVA
concentrations were 15 and 12 wt%, respectively. The spinnable PAN/MWNTs
solutions were extruded into a coagulant bath containing distilled water at
room temperature through a syringe needle (diameter = 0.65 mm).
The PVA/MWNTs solutions were cast on glass plates at room temperature.
Measurement
of the Physical Properties. After the mixing
process of MWNTs with sugar syrup was completed, aliquots of the samples were
collected to evaluate the dispersion status by using a optical microscope
(Olympus BH-2) at 400 times magnification.
The viscosity of the MWNT/sugar syrup mixtures was measured at 30 oC
in dynamic mode through an advanced rheometric expansion system (ARES;
Rheometric Scienctific Inc.). Parallel plate geometry in a diameter of
25 mm was employed, with a plate gap and a strain level of 1 mm and
5%, respectively.
The fractured surfaces of the prepared composites in liquid nitrogen were
observed via field emission scanning electron microscopy (FESEM; Philips
JSM6330F) to examine the quality of nanotube dispersion in the polymer
matrices.
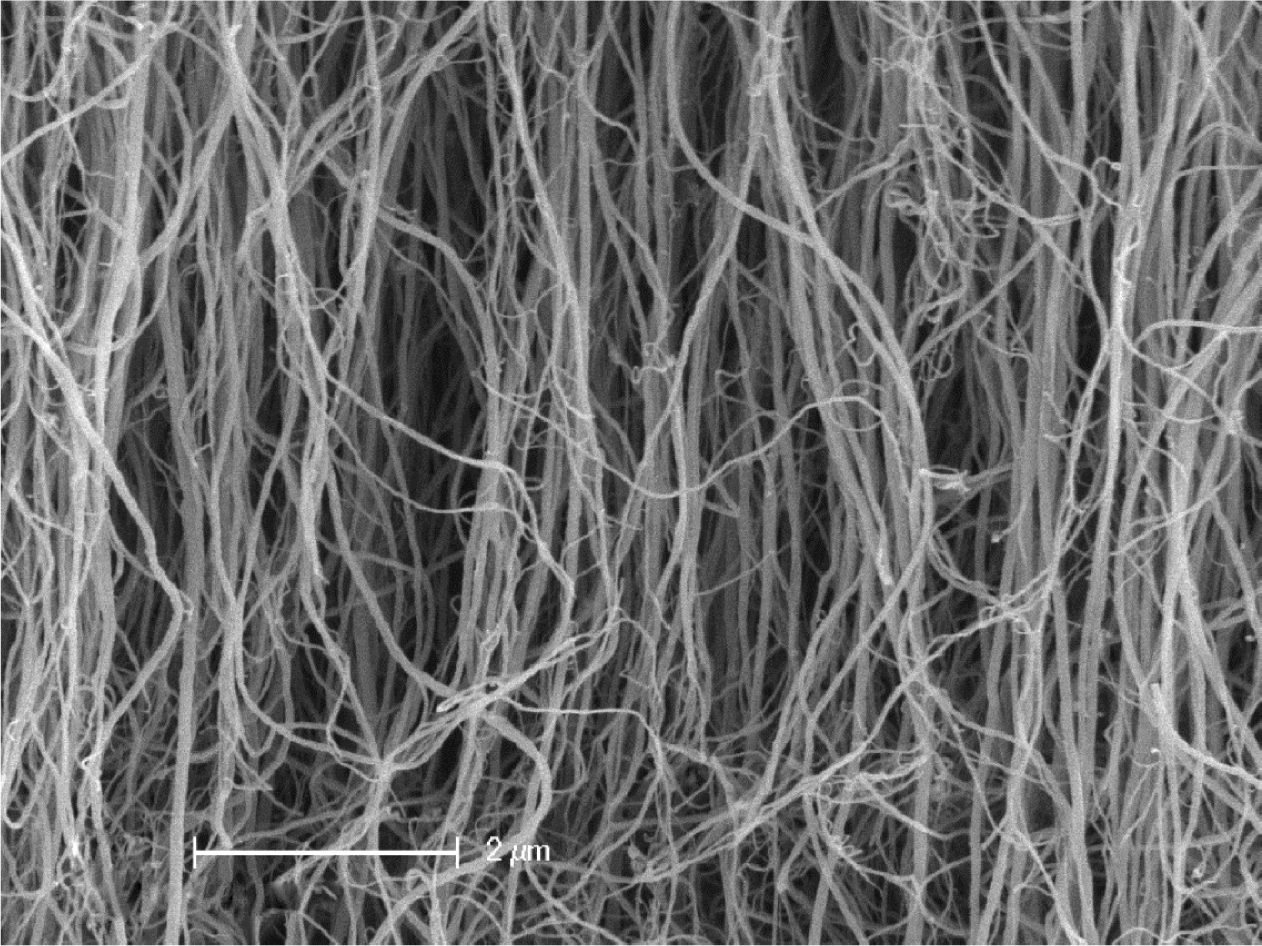
As shown in Figure 1, the significant entanglement and bundling of CNTs
in the bulk state, typical pattern of nanotubes when produced by CVD, gives
rise to the extreme difficulty in their separation. The proper mixing with a
sticky material is considered as one of the ways to debundle these CNTs. In
addition, such a sticky medium should be removed with little difficulty for the
successive usage of the as-debundled nanotubes. Thus, a sugar syrup prepared by
mixing equal proportions of glucose, fructose, and sucrose with distilled water
was chosen as the viscous medium. Using these three types of sugars in the same
amount prevents crystallization, which is a crucial factor for a viscous medium
since a crystallized sugar syrup could not wet the nanotubes effectively. In
addition, the viscosity of sugar syrup can be easily controlled by regulating
the water content. This enables viscosity optimization in relation to the mixing
conditions.
Since the sugar syrup is highly viscous and should be added little by
little over the mixing process, typical commercial thermoplastic fabrication
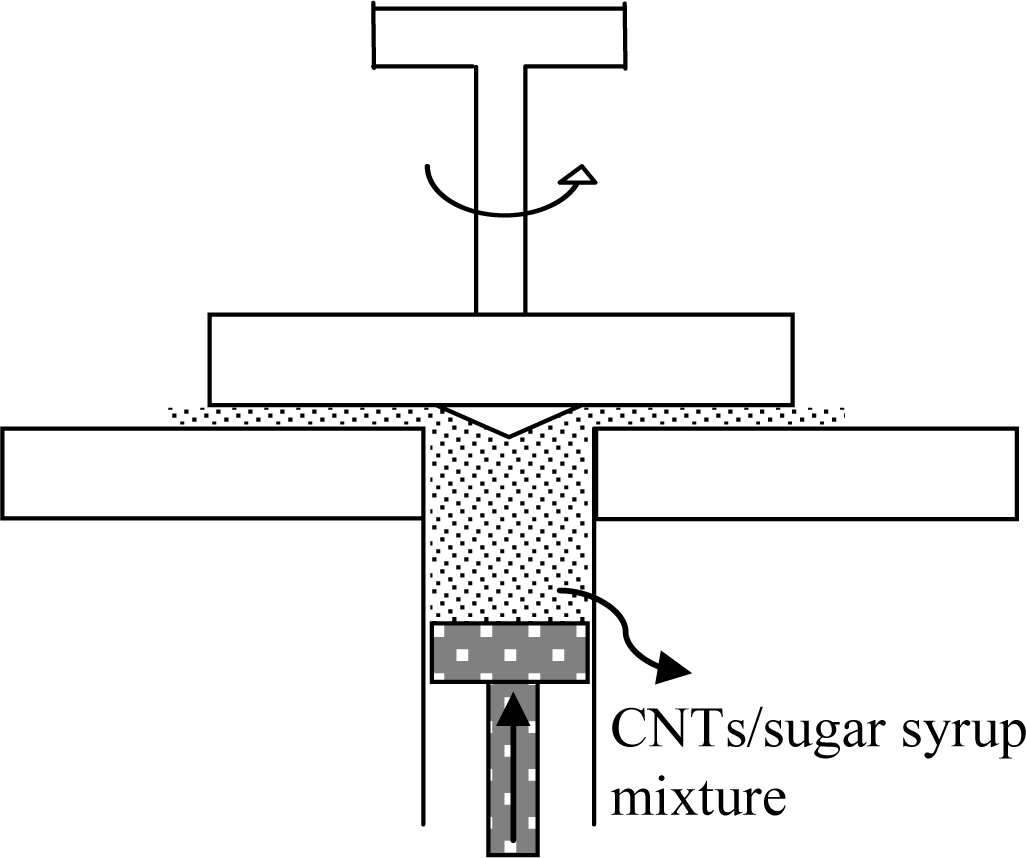
equipment is not suitable for this procedure. Thus, a disk-type mixer was
specially designed for a reproducible mixing, as shown in Figure 2. This mixer
enables the shearing level to be adjustable via varying the gap distance and is
very effective in treating even small sample quantities. Due to the low
flowability of the MWNT/sugar syrup mixtures and the consequent frequent
occurrence of blocking between feeding zone and top plate, the cone-shaped
attachment is placed on the top plate, facilitating its movement without
retention.
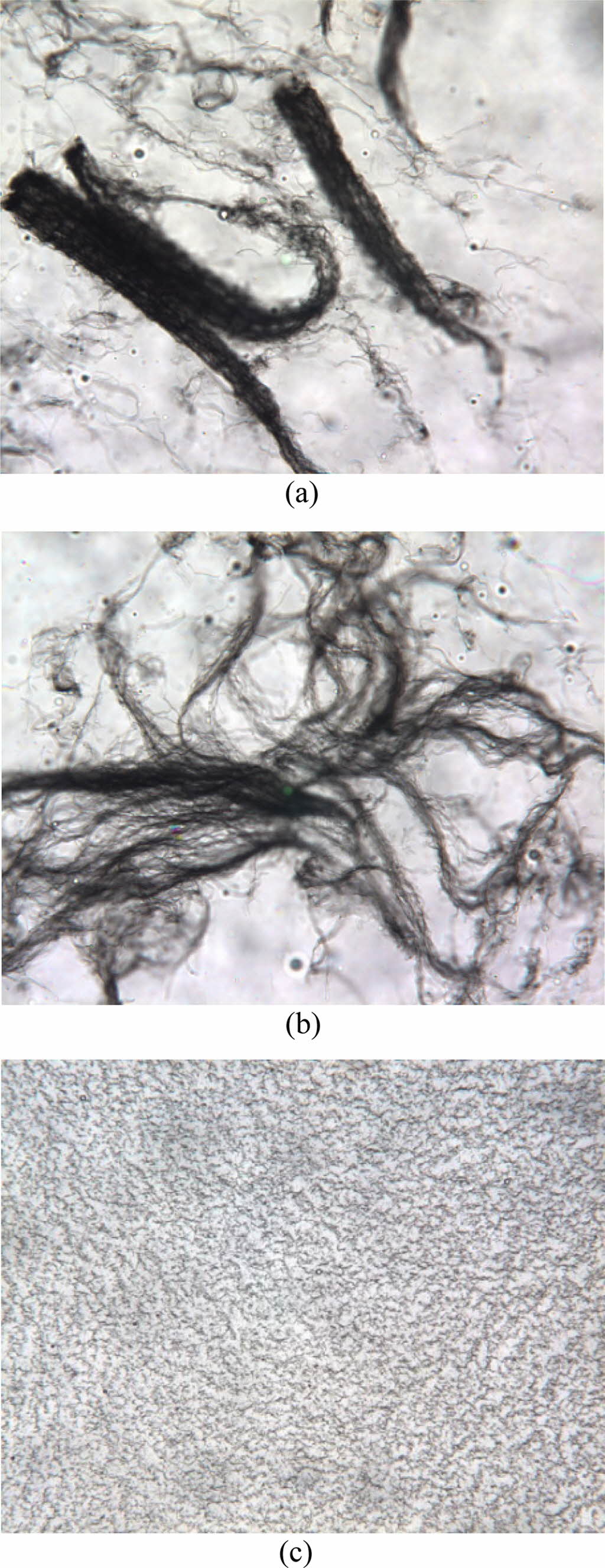
Figure 3 illustrates the disentanglement process of the MWNTs in the sugar
syrup during the mixing, showing how the nanotube bundles disappear in the
dispersing medium with increasing the mixing time. At the initial stage, the
high-viscosity syrup creates an appropriate interface among MWNTs bundles by
drawing and opening the nanotube side-walls. Then, the further added syrup
penetrates this interface, resulting in a single, disentangled nanotube. This
phenomenon can be explained as follows. Shearing forces propagate via the
viscous medium and pass through the nanotube bundles. In consequence, the
shearing force not only diffuses between the nanotube bundles but also peels
off the nanotubes located at their outer part, finally resulting in the
exfoliation of individualized nanotubes. At this point, the sugar syrup also
plays a role in preventing these dispersed nanotubes from returning to the
initial bundle state by locking them with a segregated network. That is, the
further added syrup fills the space between single nanotubes, leading to a
fully debundled state.
When mixing nanotubes with sugar syrup, the dispersion quality is
significantly affected by how the viscous medium is added. When kneading flour
with water, a gradual addition rather than all at once is considered more
effective for the preparation of well-mixed pastes. Therefore, adding sugar
syrup divisionally over the mixing process is proposed to prove the superiority
of step addition. Three different MWNT/sugar syrup mixtures are prepared by
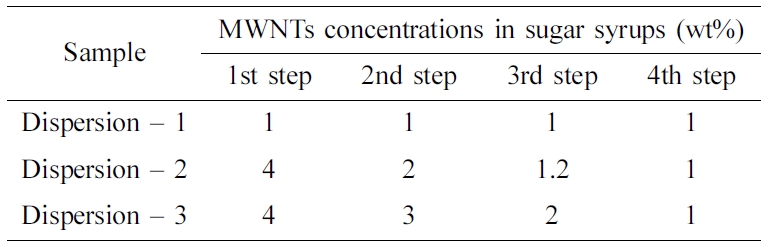
varying their ratio at each mixing step, as summarized in Table 1. The dispersion
method entails a sequence of addition of sugar syrup in specified amounts and
mixing with MWNTs. The Dispersion-1 sample is prepared by adding the sugar
syrup all at once in the first step, while the Dispersion-2 and Dispersion-3
samples are obtained by gradual additions at each step; the final MWNT
concentration is identical (1 wt%) in the three samples.
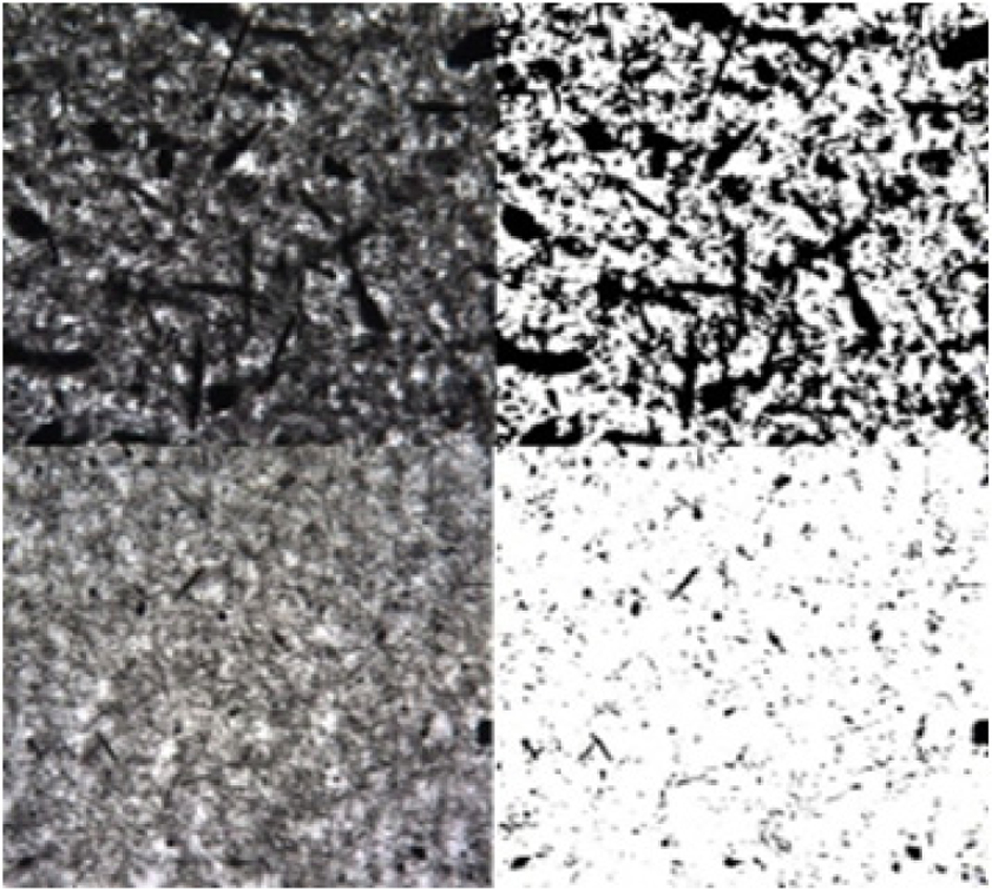
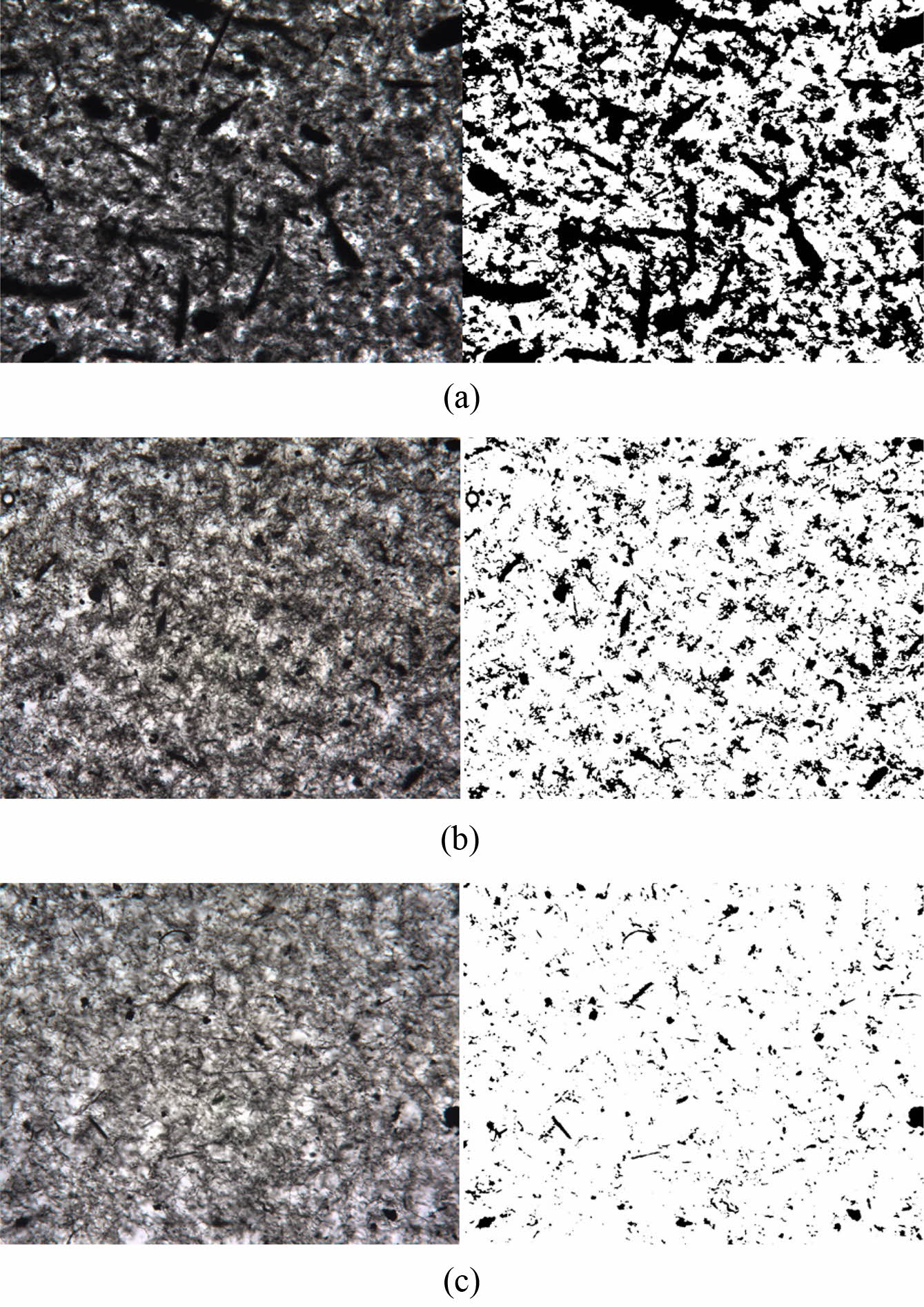
Figure 4 shows the original optical micrographs and their binary images
of the MWNTs mixed with sugar syrup. Digital image processing by using Matlab
software is conducted to assess quantitatively the dispersion degree of
nanotubes. The original microscope images are binarized by empirically setting
the threshold to distinguish the black and white pixels. The ratio of black
area to the entire area gives the value of 45, 17, and 5% for dispersion-1,
dispersion-2 and dispersion-3, respectively. An increase in the ratio
represents poor dispersion of nanotubes, still existing in such a large bundle
state in the media. The step addition of sugar syrup gives rise to the
effective disentanglement of nanotubes. Bigger bundles are frequently observed
when adding more sugar at the initial step and the final degree of dispersion
seems influenced by the initial dispersion state. When two immiscible
components are mixed, the viscosity gap between them is an important criterion
to determine the extent of mixing. That is the lower difference in viscosity
the more effective mixing at the corresponding final concentration. The large
viscosity mismatch between MWNTs and sugar syrup can lead to incompatibility
and partial distribution of the minor phase in the major one. In addition,
since the viscosity of solid MWNTs is extremely high, a relatively
high-viscosity sugar syrup should be selected as the dispersing medium. At the
first stage, the addition of the least amount of sugar syrup (i.e., only as
much as the wetting nanotubes) results in the least viscosity mismatch. Both
the increased stresses generated by the high viscosity of sugar syrup and the
maximized distribution of mixing stresses due to viscosity matching of
two components would give rise to the extensive break-up of the bundled phases.
The Dispersion-3 sample exhibits better dispersion than the Dispersion-2 one,
indicating that the MWNT disentanglement is maximized when adding the sugar
syrup little by little over the mixing process. In other words, since the MWNTs
are not completely disentangled in the medium at the first stage, the second
stage of mixing still plays a significant role in determining the final degree
of disentanglement.
The rheological properties of a mixture are directly correlated with the
spatial and orientational distribution status of the nanotubes in the matrix.17
These can be used as a physical signal to monitor the overall quality of
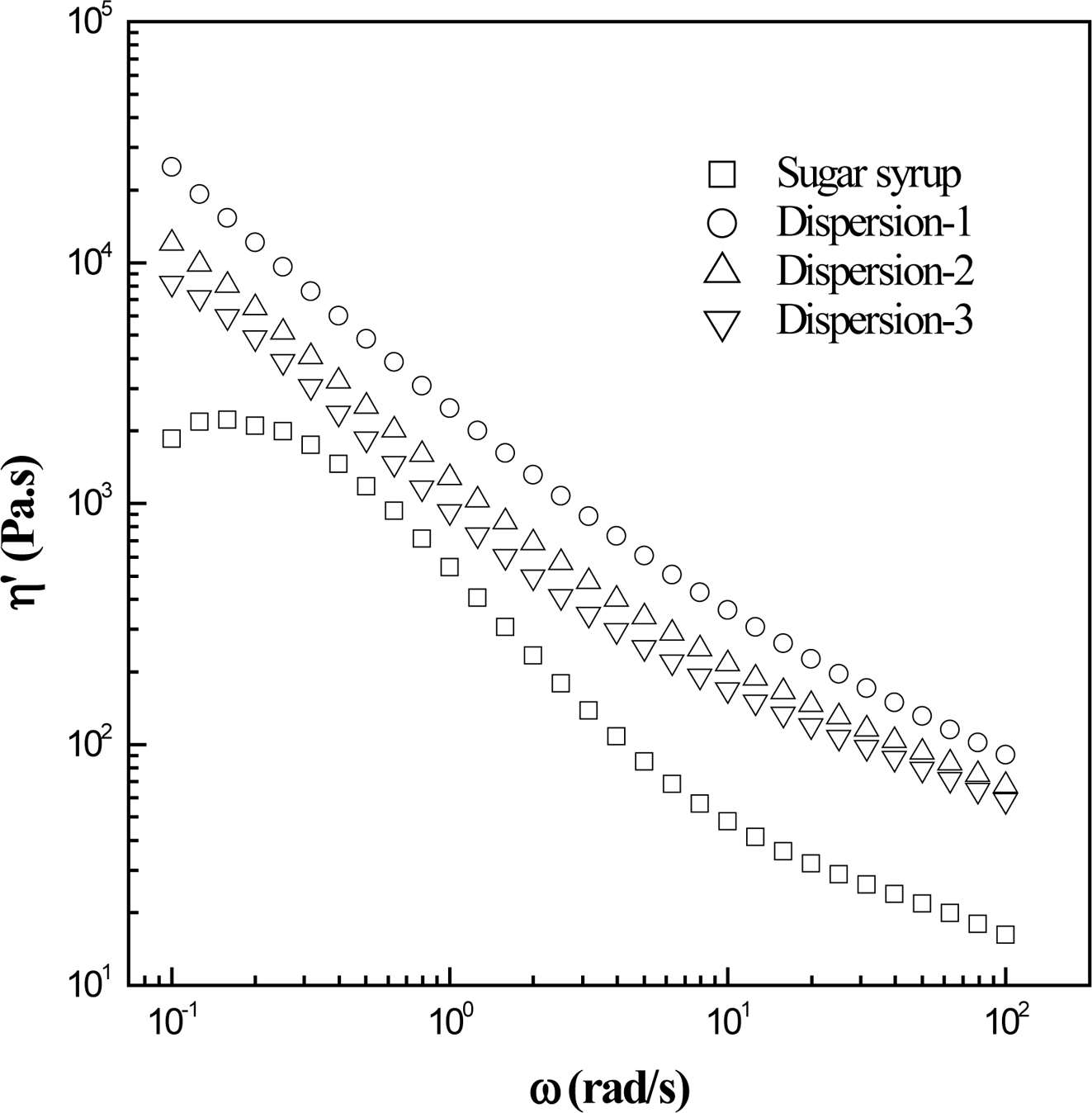
dispersion. Figure 5 shows the dynamic viscosity (η') curves of MWNT/sugar
syrup mixtures at 30 oC. The pure sugar syrup sample exhibits
Newtonian behavior in the low frequency range, followed by shear thinning; this
is the typical pattern of pseudoplastic materials. However, the incorporation
of MWNTs increases the η' of sugar syrup and produces notably yield behavior
with the lower Newtonian flow region disappearing. This increase of η' suggests
that there are a large number of contacts between MWNTs and the molecule of
sugar syrup leading to restriction in its mobility. For MWNT/sugar syrup
mixtures, the viscosity is inversely proportional to the degree of dispersion,17,18
therefore resulting in the following viscosity order in the tested frequency
range: Dispersion-1 > Dispersion-2 > Dispersion-3. The alignment of the
nanotubes is expected to occur in the medium by shearing, favored by the degree
of disentanglement. The individualized nanotubes react more easily to external
stresses than the bundled ones, resulting in their alignment. This reduces the
number of tube–tube contacts and, hence, the matrix motion was less hindered.
Thus, the viscosity decreases with increasing the alignment of nanotubes.19
In addition, since the poorly dispersed suspensions contain many nanotubes in a
bundled or semi-debundled state, some of the dispersing medium becomes
immobilized within these aggregates. Therefore, the apparent volume of nanotube
aggregates is larger than the true volume occupied by the sum of individualized
nanotubes, resulting in increased viscosity. At the frequency of about 0.5–1
rad/s, the viscosity difference between the nanotube mixture and the pure sugar
syrup decreases because of the aligned nanotubes or break-up of agglomerate
under moderate shear. However, as the frequency further increases, the
viscosity gap increases as well, suggesting that a higher shearing is not
always more effective in terms of uniform dispersion since it may lead to
rerandomization or reaggregation of the nanotubes.
To use the proposed method for the preparation of polymer composites, the
disentangled state of the MWNTs in sugar syrup is required to be maintained in
the desired solvent for the polymer matrix after the syrup removal. To verify
this possibility, PAN and PVA, whose composites with nanotubes have been deeply
studied because of their potential applications, were selected as the polymer
matrices.20-27 DMF and water, which have definitely different
suspending power for nanotubes, are used for PAN and PVA, respectively. The
sugar syrup is removed by repeated washing with water and then the filtered
nanotubes are immediately transferred to each solvent to prevent
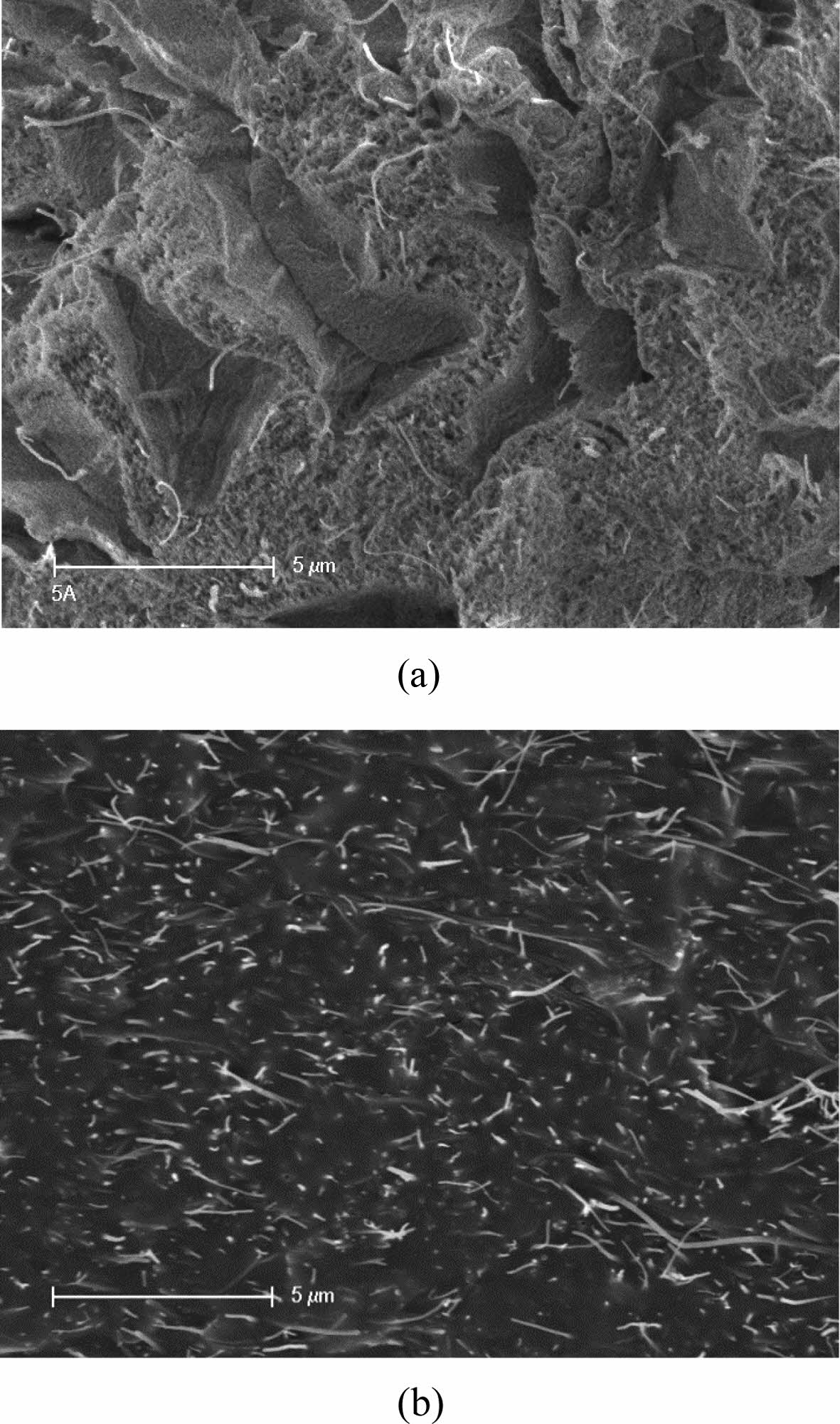
reagglomeration, prior to the polymer dissolution. Figure 6 shows the FESEM
images of freeze-fractured interfaces for both the 5 wt% PAN and PVA
composites. The nanotubes are homogeneously dispersed with little agglomerates.
This proves that the predispersed state is retained over the whole fabrication
process of the composites. That is, once the MWNTs are debundled by the sugar
syrup, the solvent media also appear to have the ability to suspend the
individualized nanotubes slightly depending on their suspending power. In
addition, the dispersed state is maintained even during the fiber formation and
the film casting processes, where the solvents are removed. This indicates that
the polymer molecules replace the space previously occupied by the solvent
ones, preventing the return to the entangled state. This method could be used
not only for the fabrication of various polymer composites but also in
CNT-matrix applications such as bucky papers and CNT fibers when considering
that nanotube suspensions fully dispersed in various media are possibly
prepared.
|
Figure 1 Field emission scanning electron microscopy image of
highly entangled multi-walled carbon nanotubes produced by chemical vapor deposition. |
|
Figure 2 Schematic diagram of the automatic mixer designed for
mixing multi-walled carbon nanotubes with the sugar syrup. |
|
Figure 3 Optical micrographs showing the disentanglement of
multi-walled carbon nanotube bundles in the sugar syrup during the
mixing process: (a) bundle; (b) semi-disentangled; (c) fully disentangled states. |
|
Figure 4 Optical micrographs (left column) and their binary images
(right column) of multi-walled carbon nanotube bundles mixed with
sugar syrup in different conditions (see Table 1): (a) Dispersion-1;
(b) Dispersion-2; (c) Dispersion-3 samples. |
|
Figure 5 Dynamic viscosity (η') curve of multi-walled carbon
nanotube/sugar syrup mixtures prepared with different mixing conditions. |
|
Figure 6 Field emission scanning electron microscopy images of
the fractured surfaces of (a) a polyacrylonitrile fiber; (b) a poly(vinyl
alcohol) film, both containing 5 wt% multi-walled carbon nanotube. |
|
Table 1 Different Concentrations of Multi-walled Carbon
Nanotubes in the Sugar Syrup at Each Mixing Step |
To the best of our knowledge, we explored for the first time the
disentanglement of MWNTs by mixing with sugar syrup without chemical
modification or additive incorporation, which is not expected to change their
inherent properties. Sticky sugar syrup with crystal-inhibiting characteristics
was used, allowing the disentanglement of the nanotubes. The disentanglement
states of MWNTs in the medium affected by the type of mixing process are
quantitatively evaluated by digital image processing. The key factor to
effectively mix the nanotubes with this viscous medium is its divisional
addition in a sense of viscosity matching method. At the initial mixing stage,
the MWNTs should be debundled to some degree suitable for being further
individualized by successive syrup addition at the next stage. Thus, we concluded
that the initial mixing condition is crucial in determining the final
dispersion state. Then, both PAN and PVA composites with uniformly dispersed
nanotubes were successfully prepared, demonstrating that the disentangled state
of the MWNTs resulting from the sugar syrup treatment was retained even in the
successive fabrication processes. This versatile and environmentally benign
method presents a little limitation in the preparation of polymer/CNTs
composites. In addition, CNT-matrix related applications such as CNT fibers and
membranes could be easily accessed through the preparation of stable and
dispersed CNT suspensions.
- 1. C. H. Liu, H. Huang, Y. Wu, and S. S. Fan, Appl. Phys. Lett., 84, 4248 (2004).
-

- 2. S. Subramoney, Adv. Mater., 10, 1157 (1998).
-

- 3. F. Du, R. C. Scogna, W. Zhou, S. Brand, J. E. Fischer, and K. I. Winey, Macromolecules, 37, 9048 (2004).
-

- 4. F. Beguin and P. Ehrburger, Carbon, 40, 1619 (2002).
-

- 5. Y. Breton, G. Desarmot, J. P. Salvetat, S. Delpeux, C. Sinturel, F. Beguin, and S. Bonnamy, Carbon, 42, 1027 (2004).
-

- 6. J. Jyoti, S. Basu, B. P. Singh, and S. R. Dhakate, Composites Part B, 83, 58 (2015).
-

- 7. Y. Yang, E. A. Grulke, Z. G. Zhang, and G. Wu, J. Appl. Phys., 99, 114307-1 (2006).
-

- 8. D. Qian, E. C. Dickey, R. Andrews, and T. Rantell, Appl. Phys. Lett., 76, 2868 (2000).
-

- 9. S. Kumar, T. D. Dang, F. E. Arnold, A. R. Bhattacharyya, B. G. Min, and X. Zhang, Macromolecules, 35, 9039 (2002).
-

- 10. B. Vigolo, P. Poulin, M. Lucas, P. Launois, and P. Bernier, Appl. Phys. Lett., 81, 1210 (2002).
-

- 11. J. Zhu, J. Kim, H. Peng, J. L. Margrave, V. N. Khabashesku, and E. V. Barrera, Nano Lett., 3, 1107 (2003).
-

- 12. H. Xia and M. Song, Soft Matter, 1, 386 (2005).
-

- 13. N. Grossiord, J. Loos, O. Regev, and C. E. Koning, Chem. Mater., 18, 1089 (2006).
-

- 14. V. C. Moore, M. S. Strano, E. H. Haroz, R. H. Hange, R. E. Smalley, J. Schmidt, and Y. Talmon, Nano Lett., 3, 1379 (2003).
-

- 15. D. Tasis, N. Tagmatarchis, V. Georgakilas, and M. Prato, Chem. Eur. J., 9, 4000 (2003).
-

- 16. C. Richard, F. Balavoine, P. Schultz, T. W. Ebbesen, and C. Mioskowski, Science, 300, 775 (2003).
-

- 17. P. Ma, N. A. Siddiqui, G. Marom, and J. Kim, Composites Part A, 41, 1345 (2010).
-

- 18. Y. Song and J. Youn, Carbon, 43, 1378 (2005).
-

- 19. F. Du, R. C. Scogna, W. Zhou, S. Brand, J. E. Fischer, and K. I. Winey, Macromolecules, 37, 9048, (2004).
-

- 20. L. Liu, A. H. Barber, S. Nuriel, and H. D. Wagner, Adv. Funct. Mater., 15, 975 (2005).
-

- 21. S. Bhattacharyya, J. Salvetat, and M. Saboungi, Appl. Phys. Lett., 88, 233119 (2006).
-

- 22. C. Pirlot, I. Willems, A. Fonseca, J. B. Nagy, and J. Delhalle, Adv. Eng. Mater., 4, 109 (2002).
-

- 23. A. Koganemaru, Y. Bin, Y. Agari, and M. Matsuo, Adv. Funct. Mater., 14, 842 (2004).
-

- 24. J. Lin, Z. Lin, Y. Pan, C. Hsieh, C. Huang, and C. Lou, J. Appl. Polym. Sci., 133, 43474 (2016).
-

- 25. M. J. Yee, N. M. Mubarak, M. Khalid, E. C. Abdullah, and P. Jagadish, Sci. Rep., 8, 17295 (2018).
-

- 26. H. Zhang, L. Quan, A. Gao, Y. Tong, F. Shi, and L. Xu, Polymers, 11, 422 (2019).
-

- 27. S. Palade, A. Pantazi, S. Vulpe, C. Berbecaru, V. Tucureanu, O. Oprea, R. F. Negrea, and D. Dragoman, Polym. Compos., 38, 1741 (2017).
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2020; 44(4): 512-518
Published online Jul 25, 2020
- 10.7317/pk.2020.44.4.512
- Received on Mar 13, 2020
- Revised on Apr 14, 2020
- Accepted on Apr 17, 2020
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Dong Wook Chae
-
Department of Textile Engineering, Kyungpook National University, Sangju 37224, Korea
- E-mail: dwchae@knu.ac.kr
- ORCID:
0000-0002-2701-5208









 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.