- Physical Nature of Biodegradable Polydioxanone Filaments upon Synthetic Conditions
Department of Environmental Engineering, Anyang University, Anyang 14028, Korea
*Department of Chemical Engineering, Hanyang University, Seoul 04763, Korea- 제조조건에 따른 생분해성 PDO 필라멘트의 물리적 특성
안양대학교 환경공학과, *한양대학교 화학공학과
In the medical field,
polydioxanone (PDO) has increasingly attracted scientific interests in both
fundamental research and applications for synthesizing sutures due to its
safety, biodegradability, and mechanical strength. Chemical pathways of the
aforementioned architecture have already been proven via a plethora of
multidisciplinary researches, however, the physical nature of PDO filaments by
each stage of the synthetic condition has yet been solely observed in detail.
The scope of the present study tracks a couple of pre- and post-fiberation to
tailor the success in tunable physical strength with the variance of
purification time and the dosage of a catalyst. We first fabricated PDO
filaments using lauryl alcohol (C12H26O) and stannous
octoate (C16H30O4Sn) as an initiator and a
catalyst, respectively. PDO-3-30 with 3 h of vacuum purification and
30 ppm dosage of a catalyst led to unfavorable thermal properties and
degradability but an increase in physical properties including tensile,
flexural, and Izod impact strengths. From thermal and physical profiles, it was
confirmed that the amount of a catalyst is a major driving factor of
polymerization while the degree of purification could be an additive aid for
more sensitive control of the physical nature of PDO filaments.
의학분야에서
polydioxanone(PDO)는 생체 안전성, 생분해도 및 기계적 강성의 이점으로 인해
기초적 및 실용적 연구 분야에서 큰 관심을 받고 있다. 선행연구 결과에서 PDO의 생화학적 구조는 증명되었으나, 단계별 제조조건 중 촉매와
세척 간 상관관계가 PDO의 물리적 특성에 미치는 영향에 대한 연구는 미미한 실정이다. 따라서 본 연구에서는 PDO의 촉매와 세척의 변화에 따른 물리적
강성 및 생분해도의 변화를 추적하였다. 샘플 중 가장 많은 ppm의
촉매(30 ppm)와 진공세척시간(3시간)을 도입한 PDO-3-30 샘플은 물리적 특성에서 가장 우수한 결과를
도출하였으나, 열역학 및 생분해도에서는 타 샘플대비 상대적으로 낮은 결과를 드러냈다. 모든 샘플의 특성을 비교하였을 때, 촉매의 양은 중합에서 가장 중요한
역할을 한다. 여기에서, 세척등급 또한 보다 세밀한 PDO 필라멘트의 물리적 특성을 제어할 수 있는 보조조건이 될 수 있다.
The resulted profiles from in vitro degradation
showed that PDO with more dosage of catalyst and purification time could be
efficient in case a high level of physical strength with less degradation
profile is required.

Keywords: polydioxanone, suture wire, catalyst, purification, physical strength
Authors thank to KATECH (Korea Automotive Technology Institute) for
technical help with the overall strength measurements.
Sutures and surgery have been tied together throughout the history of
modern medical therapeutics, since the first use of metal wires to set a broken
humerus in 1775.1 One way to classify sutures is with respect to
their synthetic materials, i.e., non-degradable and biodegradable.
Non-degradable sutures have been taking center stage over recent decades using
titanium and its alloys, nitinol, and stainless steels for various medical
applications including orthopedic surgery and sternal closure in cardiac
surgery.2,3 In all cases, non-degradable sutures require a secondary
surgical event for removal regardless of the duration time in the human body.
In this regard, biodegradable sutures may present an appealing alternative to
traditionally used non-degradable sutures. In addition to not necessitate a
secondary operation for removal, the biodegradation offers a slow transfer load
to the healing bone and also has a potential use for drug delivery in
conjunction with medical devices.4,5 With the advent of synthetic
polymers such as polylactic acid, polycaprolactone, and polydioxanone (PDO),
the research and development for medical applications has boosted in recent
years.6 Herein, PDO filaments have received wide attention for
wound-closure sutures as early reported in clinical studies.7,8 The
ideal suture should possess nontoxicity, biodegradability, and mechanical
strength. As a suture, PDO filaments have revealed an attractive safety profile
and complete degradation from previous in vivo studies.9,10
Moreover, a driving motivation for PDO filaments is their usefulness in
high-stressed applications with high crystallinity and mechanical strength.11
It is evident that many sutures were developed by envisaging the trinity
of requirements. Notably, the use of sutures coupled with robotic technology
has enabled teleoperated surgery under harsh conditions for provisional
exploration missions.12 With this content, one may assume that
exhibiting the excellent mechanical strength before the degradation could
influence the range of its potential clinical applications. However,
high-tensile strength sutures could cut through tendon tissue in regard to the
rotator cuff and other tendon repairs.13 Thus, utilizing the physics
in conjunction with contextual chemistry could aid for a professional and
structural design of sutures which may meet all the complexity of mechanical
properties. Based on the material-by-synthesis approach, we observe the physical
nature of PDO filaments by each stage of synthetic condition. PDO is a
semi-crystalline polymer with a glass transition temperature of -10 oC
and a melting temperature of 110 oC. For successive
polymerization, the removal of impurities from the catalyst is one crucial
factor since the impurity may influence the polymer growth and mechanical
strength of the final product. There have been a plethora of attempts to bridge
the gap between mechanical properties and suitable chemical aids, however, the
influence of purification is hampered by the lack of experimental studies.14,15
To the best of our knowledge, this is the sole report that fully observes the
influence of purification over the physical nature of PDO filaments. In the
present study, we synthesized PDO filaments by varying the purification and
chemical dosage. Thereafter, mechanical and degradation properties of prepared
PDO filaments were analyzed using the in vitro degradation with an
emphasis on the effects of purification and polymerization characteristics.
Throughout our experiments, we hypothesized that the overall characteristics of
PDO filament depends on the purification as well as the dosage of a catalyst.
We also postulated that this finding could contribute to expanding the range of
medical-grade sutures for further surgical functionality.
Materials
and the Synthesis of PDO Filaments. Polymerization
solutions were prepared by dissolving p-dioxanone as a monomer with lauryl
alcohol (C12H26O) and stannous octoate (C16H30O4Sn)
as an initiator and a catalyst, respectively. All chemicals were purchased from
Sigma-Aldrich and used without further purification unless otherwise specified.
About 200 mL of reagent grade of toluene were added in a three-necked
polymerization flask and heated by means of temperature up to 150 oC.
One cleaned boiling chip was added to control bubbling for preparing toluene
with its boiling temperature of 111 oC. After the reflux for
3 h, stannous octoate was added by syringe for the purification with respect
to the time variance of 1, 2, and 3 h.16 As depicted in Figure
1, purification enables the removal of cumulative groups such as H-C-X and/or
H-C-O. Herein, X represents the single group of electronegative atoms including
F, Cl, and Br. Lastly, toluene was evaporated off under the nitrogen steam and
the residue was dried under vacuum at room temperature. Herein, samples were
namely labeled by the purification time and the concentration (ppm) of a
catalyst; PDO-1-20, PDO-1-30, PDO-2-20, PDO-2-30, PDO-3-20, and PDO-3-30. In
sum then, bulk polymerization was successfully modulated via oil bath.
The typical procedure used to prepare the PDO filaments was as follows.
Polymerization for PDO was performed in bulk at 65 oC for
2 h under the nitrogen steam. With a fixed amount of an initiator (1800
ppm), 30 ppm of the purified catalyst was used for polymerization. The
precipitated product was dried in a vacuum oven for overnight at 45 oC
to achieve a white form of crystallized polymer out of solution. Subsequently,
thus-prepared polymer was tailored using the electrospinning process with 5 and
20 rpm of an extruder and gear pump speed, respectively. The tip-to-collector
distance was 20 cm and filaments were spun under an electric field of
10 kV. Typically, low nozzle and spinning temperature could influence the
crystallinity of electrospun filaments.17 On the contrary, more than
110 oC could melt the polymer chains. During fabrication, an
average temperature of eight holes in the nozzle with 15 bar pressure was
68 oC (Figure 2). After annealing, drawn PDO filaments were
manually stretched until resistance was felt and kept at room temperature in a
vacuum oven until further use.
Methodology. Thermal properties
of PDO were measured with differential scanning calorimeter (DSC 2910, TA
instruments). A flow of nitrogen gas was maintained to eliminate any air oxidation
of samples. From each annealing and degradation condition, 5 mg of each sample
was mounted in aluminum pans, along with an empty pan as the reference. The
analysis was performed at a temperature range of 0~200 oC with
a heating rate of 10 oC min-1. After the
electrospinning, randomly selected PDO filaments from multiple lots of a given
suture type were tested for physical and degradable properties. The strength
properties of each sample were measured with the universal tensile testing
machine (Instron 3343, Instron) and the impact tester (Impact tester, KATECH)
according to the relevant ASTM standards; ASTM D246, ASTM D638, ASTM D790, and
ASTM D792. The measurements of the force at break (N) and clamp-to-clamp
breaking strain were recorded, and samples were tested per sample type for each
condition and experimental repeat. The in vitro degradation
characteristics were examined to compare the degradability of samples by
different synthesis conditions. Prior to the in vitro degradation, 3 cm
of each sample was washed with deionized H2O and placed in a vacuum
for 3 h.18 Thereafter, the samples were immersed in a shaking
incubator at 37 oC with a phosphate-buffered saline solution
after adjusting pH to 7.4. Knot tensile strength and breaking strength retention
were tested under time points of 168, 336, 504, and 672 h, respectively.
The experimental uncertainties were averaged during 5 trials (n=5).
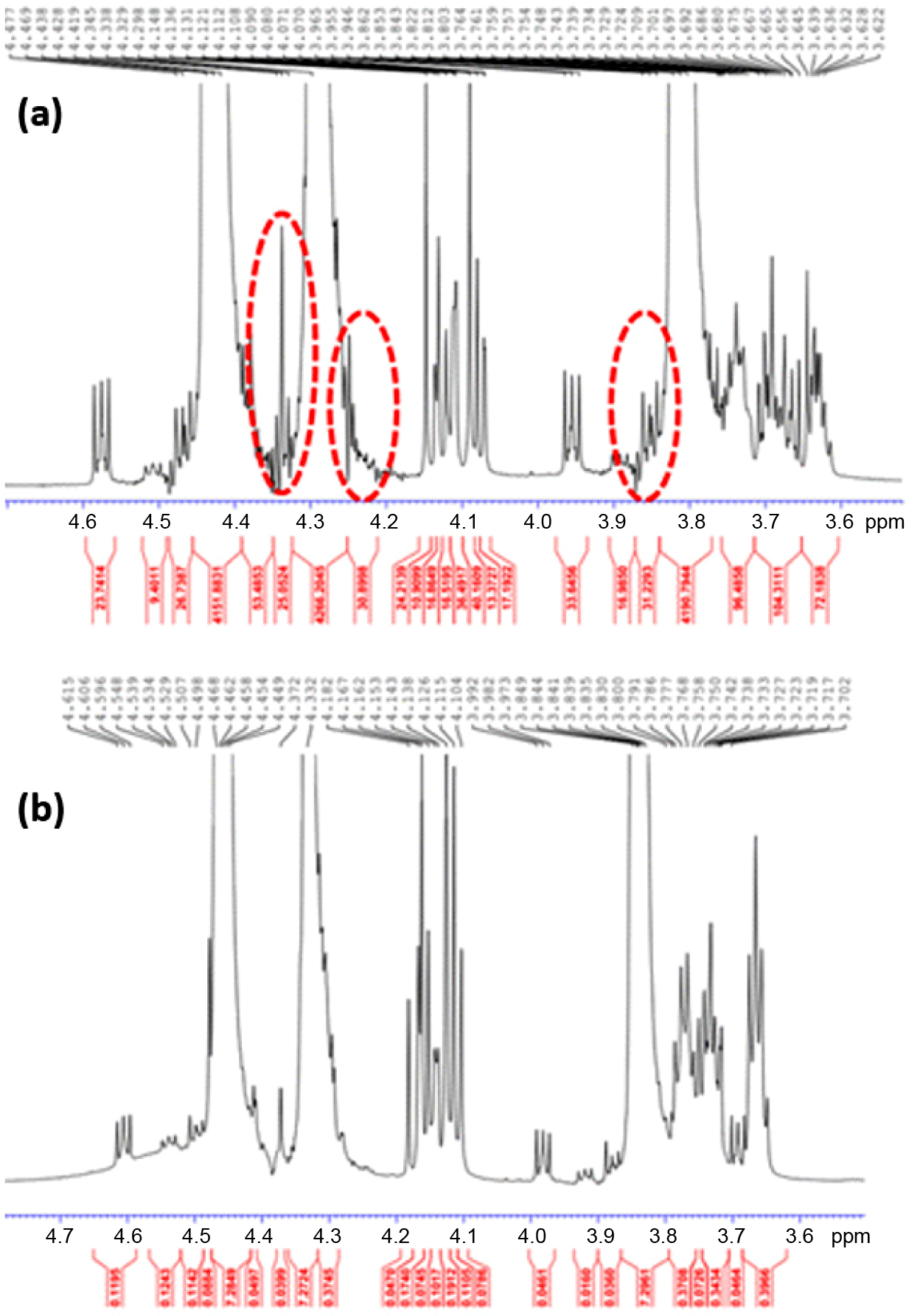
|
Figure 1 Chemical shifts in the 1
H NMR spectra of PDO with
regard to (a) before; (b) after the purification. Note that major shifts
occurred within the range of 3.8–3.9, 4.2–4.3, and 4.3–4.4 ppm,
respectively. |
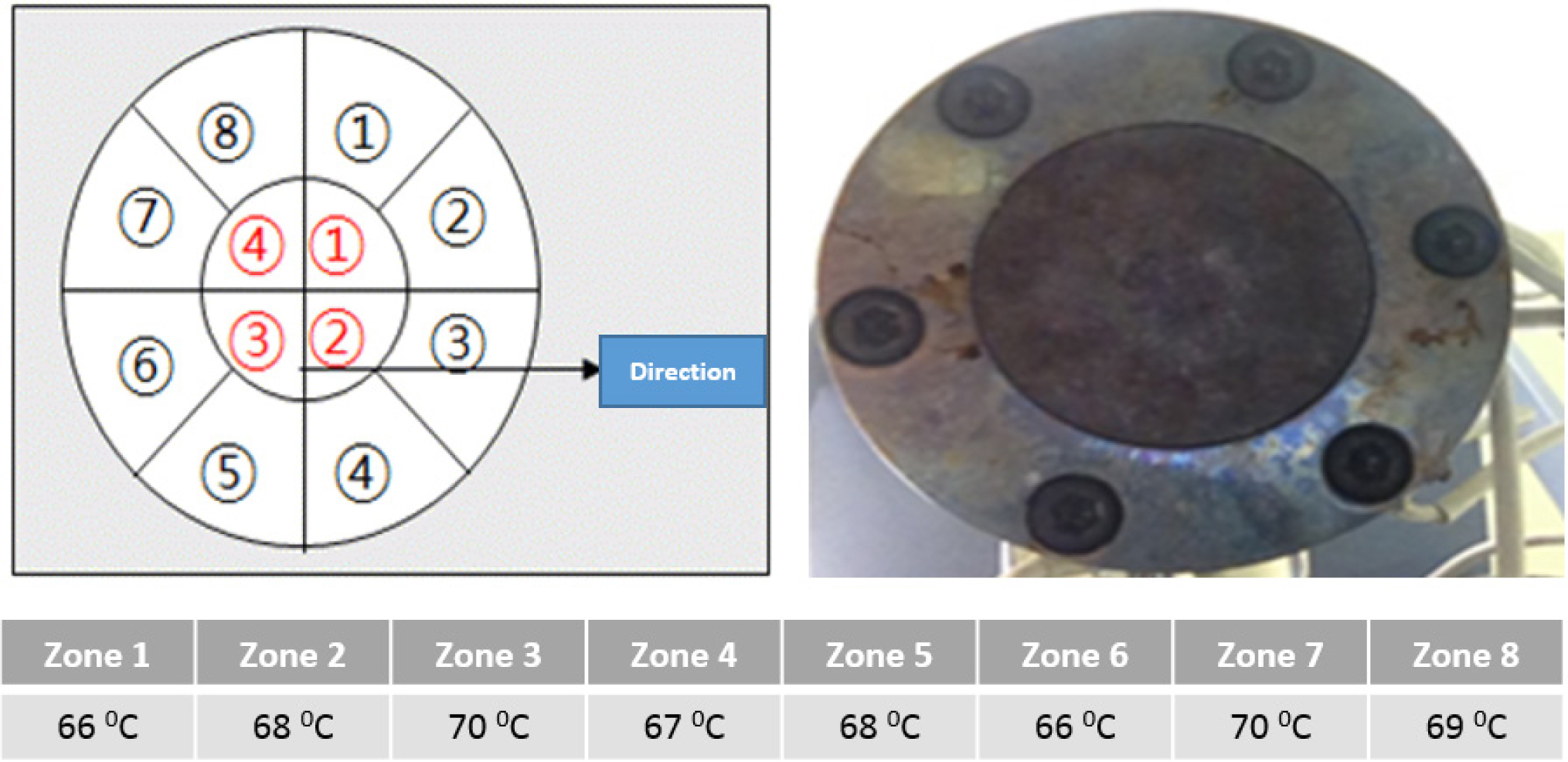
|
Figure 2 An average temperature range of holes during fabrication. |
The Tm test could hint shreds of evidence for the
successive polymerization. In general, Tm increases as the
concentration of a catalyst (ring-opener) increases and may decrease in vice
versa. The results in Figure 3(a) revealed that the values of each sample were
in a linear relation with the purification time and the concentration of a catalyst.
Narrowing the topic down to a catalyst, all samples had the tendency to undergo
increased Tm as the dosage increases; PDO-1-n (5.17%),
PDO-2-n (12.68%), and PDO-3-n (6.95%). Tm
values by the purification time were also in accordance with the concentration
of a catalyst, since impurities (i.e., monomer and/or oligomer) could not
affect the overall quality of polymerization as the purification time
increases. Specifying samples labeled as PDO-n-30, it seems that the
amount of dosage is one driving force for the polymerization compare to the
purification time; PDO-1-30 to PDO-2-30 (1.75% increased) and PDO-2-30 to
PDO-3-30 (1.66% increased). A similar behavior was also observed in the heat of
fusion and melt flow index (Figures 3(a)-(b)). We further tracked PDO-n-30
in a form of filament whether the purification affects their physical
properties, probably as a result of the removal of impurities.
The tensile strength of the samples is plotted in Figure 4(a). An
increase in tensile strength with increasing purification time is generally
observed in all samples. Based on the results, the value from PDO-1-30 to
PDO-2-30 had better tensile strength increment (1.00%) compare to the value
from PDO-2-30 to PDO-3-30 (0.39%). On the contrary, 3 h of purification
time-shifted the values more dramatically in flexural strength and Izod impact
strength than 2 h as depicted in Figures 4(b) and 4(c). Yet, the overall
purification time showed a rising linear plot implying that the purification
has successfully limited some of the imperfect bonding. Samples from PDO-1-30
to PDO-3-30 experienced 2.69 and 5.01% increased flexural and Izod impact
strength, respectively. Herein, PDO-3-30 recorded the highest increment, or
“plateau” value, in Izod impact strength within the overall physical
properties. The aforementioned presumes that PDO-3-30 with the most catalyst
dosage and the longest purification time may have the highest molecular weight
due to the most successive polymerization (Table 1 and Figures 5(a)-(b)). On
the basis of the preceding results, it can be pointed out that while the dosage
of a catalyst leads to the major shift in polymerization, the degree of polymerization
is also non-negligible. We therefore suggest that a decent purification control
could aid in the occurrence of bonding around the polymer interfaces and the
formation of voids, which are known to increase the physical properties.
In vitro degradation profiles of samples are exhibited in Figures
4(d)-(f) and Table 2. The degradation of samples results from the hydrolytic
cleavage of ester bonds, thus the pH environment is closely related to the
degradation profile of PDO.19 However, in vitro does not
correspond to an actual degradation profile in vivo due to the pH
variance environment instead of physiological homeostasis.20 This
method is yet a facile method to observe the effects of additives and synthetic
conditions on the degradation of polymers.21,22 All samples revealed
typical biodegradable properties within 4 weeks of time-period. Tensile
extension, pressure, and BSR retention were in the order of PDO-3-30 (highest)
> PDO-2-30 > PDO-1-30 (lowest), respectively. PDO-1-30 has recorded the
highest BSR retention of 68.60% at the 4th week, redeeming the shortcoming in
tensile, flexural, and Izod impact strengths. Meanwhile, PDO-3-30 showed 3.69%
more BSR retention at 4th week compare to PDO-1-30, indicating the less
degradation behavior. These results are in agreement with the discussion above,
based on the grade of polymerization and the variation of synthetic conditions.
In line with the results from in vitro degradation, PDO-1-30 and/or
PDO-2-30 could be treated in certain medical cases, where decent physical
strength and fast degradation is needed. On the other hand, PDO-3-30 was
found to
be more efficient in case a high level of physical strength with less
degradation profile is required (Figures 5(c)-(f)).
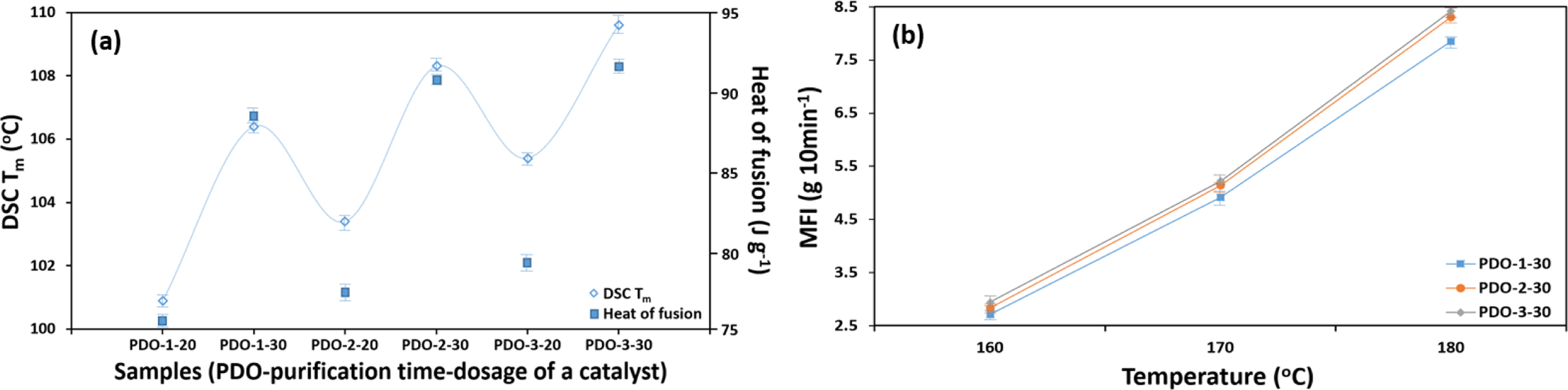
|
Figure 3 Thermal properties of samples by various purification time and the concentration of a catalyst: (a) DSC Tm and heat of fusion; (b)
Melt flow index by various temperature range. |
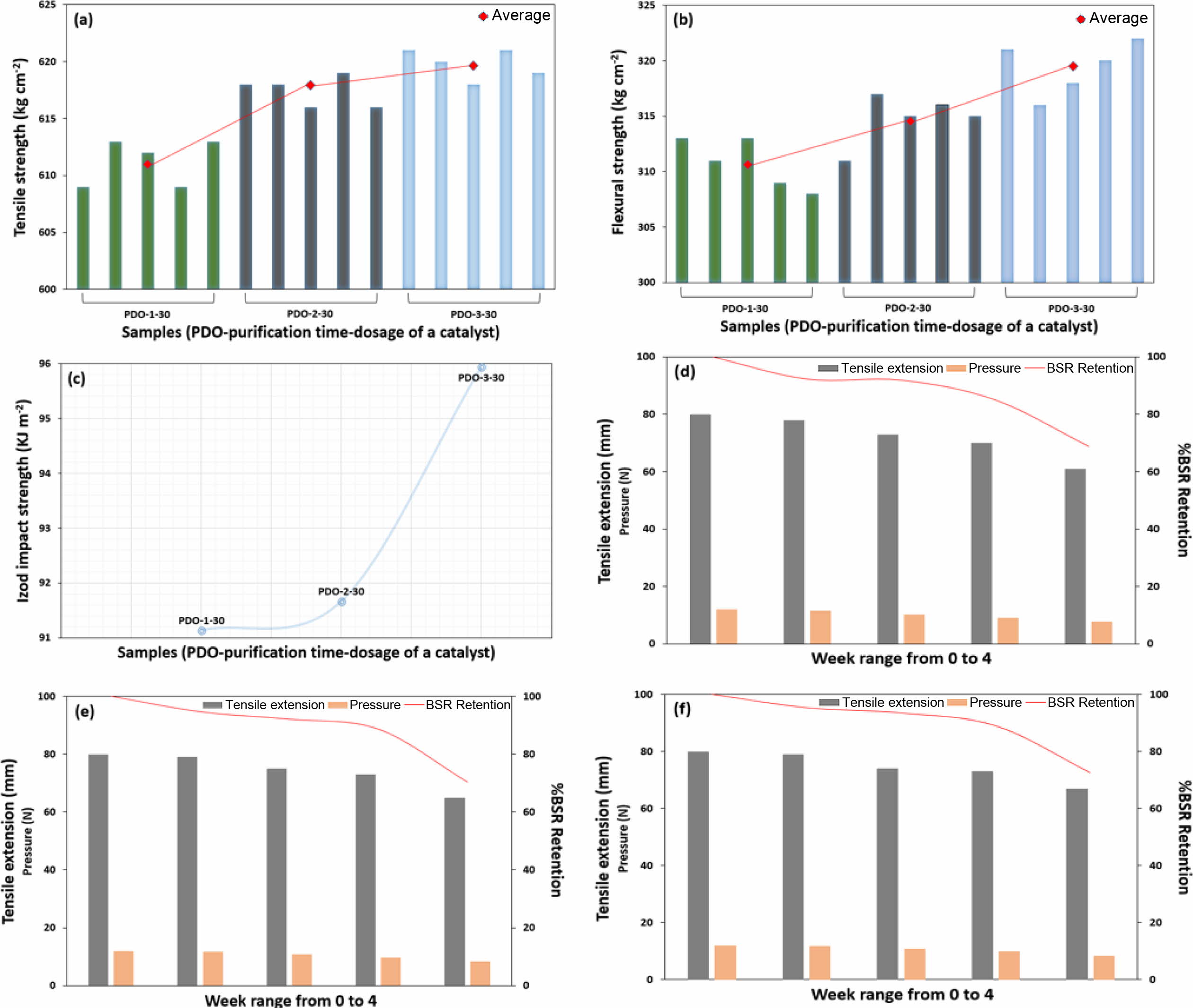
|
Figure 4 Physical properties of samples by various purification time; (a) tensile strength; (b) flexural strength; (c) Izod impact strength.
Degradability of samples by various purification time with a given week range from 0 to 4; (d) PDO-1-30; (e) PDO-2-30; (f) PDO-3-30. |
|
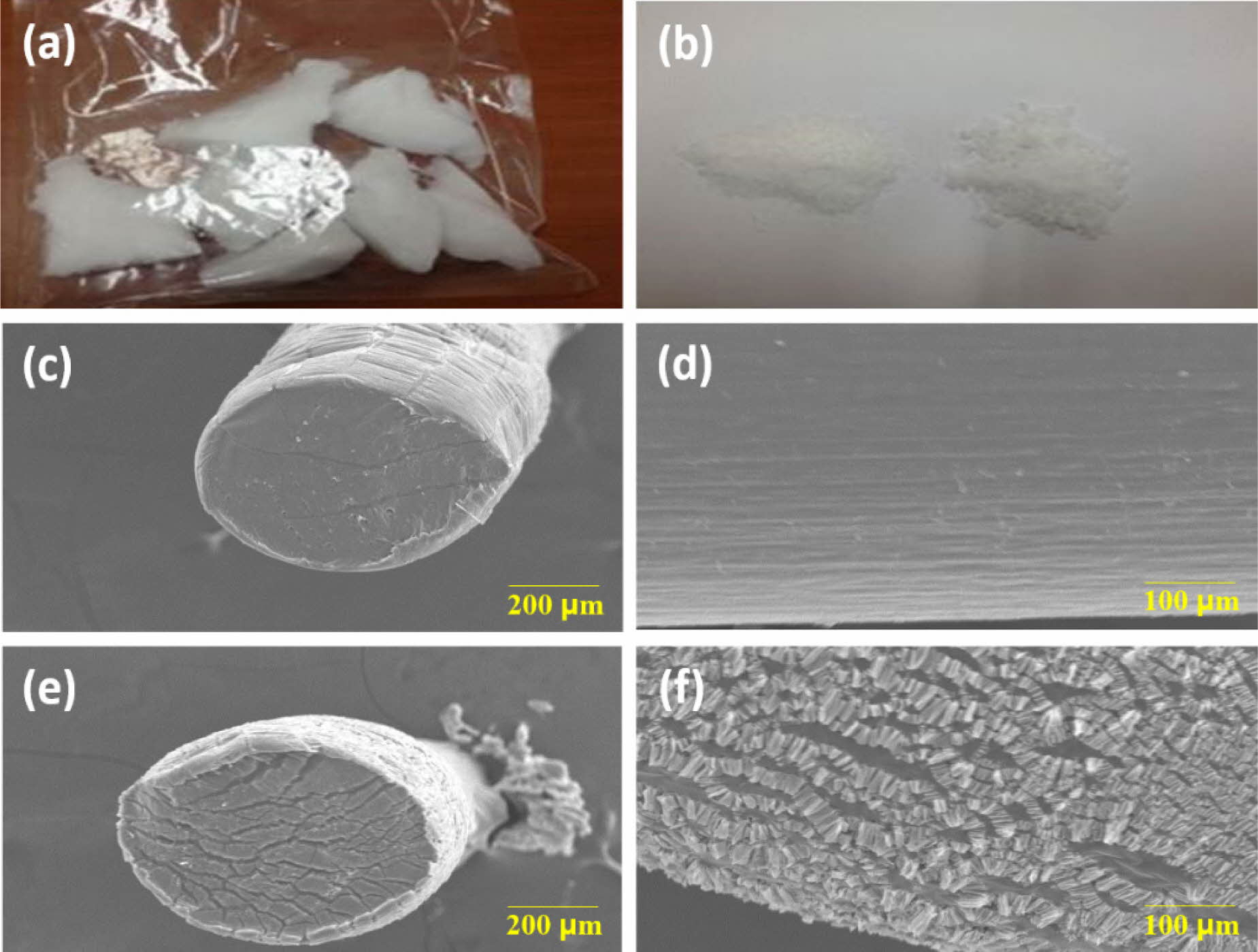
Figure 5 Visualized characteristics of PDO before and after fabrication. (a) After the 1st crush of samples; (b) After the 2nd crush of samples.
Herein, PDO-3-30 (right side) reveal better aggregate grade than PDO-1-30 (left side). Degradation profiles of PDO-3-30 by the 1st and the
4th week are represented in (c)-(d) and (e)-(f), respectively |
|
Table 1 Chip-type Yield Rates by Each Stage of Polymerization
of PDO before Fabrication |

An increased dosage of a catalyst was found to be a major factor for the
successive polymerization as early suggested. For the first time, we
demonstrated the synthetic condition by varying the time of purification. It
led to more sensitive quality control of as-synthesized PDO filament.
Therefore, such an approach could arise the importance and further research
under the topic of sufficient purification. From a mechanical point of view,
there is a consensus that our work could root for further filament-quality
research. Especially, it is crucial to pay attention to purification to achieve
a high-quality polymerization in relevant industries and to promote the low
material consumption from an economic point of view. Our work will further
focus on the degree of polymerization by varying the purification time.
- 1. J. M. Seitz, M. Durisin, J. Goldman, and J. W. Drelich, Adv. Healthcare Mater., 4, 1915 (2015).
-

- 2. M. Asgari, R. Hang, C. Wang, Z. Yu, Z. Li, and Y. Xiao, Metals, 8, 212 (2018).
-

- 3. K. M. Zia, M. Zuber, I. A. Bhatti, M. Barikani, and M. A. Sheikh, Int. J. Biol. Macromol., 44, 18 (2009).
-

- 4. K. A. Athanasiou, C. M. Agrawal, F. A. Barber, and S. S. Burkhart, Arthroscopic, 14, 726 (1998).
-

- 5. J. C. Middleton and A. J. Tipton, Biomaterials, 21, 2335 (2000).
-

- 6. B. J. Magdalena, R. Bedzinski, and A. Kozlowska, Meccanica, 48, 721 (2013).
-

- 7. S. M. Stivaros, L. R. Williams, C. Senger, L. Wilbraham, and H. U. Laasch, Eur. Radio., 20, 1069 (2010).
-

- 8. L. Novotny, M. Crha, P. Rauser, A. Hep, J. Misik, A. Necas, and D. Vondrys, J. Thorac. Cardiovasc. Surg., 143, 437 (2012).
-

- 9. P. A. Mouthuy, N. Zargar, O. Hakimi, E. Lostis, and A. Carr, Biofabrication, 7, 025006 (2015).
-

- 10. N. Goonoo, R. Jeetah, A. Bhaw-Luximon, and D. Jhurry, Eur. J. Pharm. Biopharm., 97, 371 (2015).
- 11. C. E. Wang and P. H. Zhang, Autex. Res. J., 16, 80 (2016).
-

- 12. T. Haidegger, J. Sándor, and Z. Benyó, Surg. Endosc., 25, 681 (2011).
-

- 13. B. D. Owens, J. Algeri, V. Liang, and S. DeFroda, J Shoulder Elbow Surg., 28, 1897 (2019).
-

- 14. D. Yu, L. Sun, W. Xue, and Z. Zeng, CSIJ, 3, 1 (2017).
-

- 15. K. K. Yang, X. L. Wang, Y. Z. Wang, and H. X. Huang, Mater. Chem. Phys., 87, 218 (2004).
-

- 16. E. C. Gryparis, M. Hatziapostolou, E. Papadimitriou, and K. Avgoustakis, Eur. J. Pharm. Biopharm., 67, 1 (2007).
-

- 17. C. W. Kim, D. S. Kim, S. Y. Kang, M. Marquez, and Y. L. Joo, Polymer, 47, 5097 (2006).
-

- 18. R. E. Abhari, P. A. Mouthuy, N. Zargar, C. Brown, and A. Carr, J. Mech. Behav. Biomed. Mater., 67, 127 (2017).
-

- 19. K. K. Yang, X. L. Wang, Y. Z. Wang, and H. X. Huang, Mater. Chem. Phys., 87, 218 (2004).
-

- 20. L. Zhu, K. Liang, and Y. Ji, J. Mech. Behav. Biomed. Mater., 44, 35 (2015).
-

- 21. W. Ji, F. Yang, H. Seyednejad, Z. Chen, W. E. Hennink, J. M. Anderson, and J. A. Jansen, Biomaterials, 33, 6604 (2012).
-

- 22. Z. Yang, S. M. Best, and R. E. Cameron, Adv. Mater., 21, 3900 (2009).
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2020; 44(4): 505-511
Published online Jul 25, 2020
- 10.7317/pk.2020.44.4.505
- Received on Mar 6, 2020
- Revised on Apr 1, 2020
- Accepted on Apr 4, 2020
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Soon Hong Lee
-
Department of Environmental Engineering, Anyang University, Anyang 14028, Korea
- E-mail: leesh@anyang.ac.kr
- ORCID:
0000-0002-9585-3836











 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.