- Drug Delivery Behavior of PCL and PCL/PEG Microcapsules Prepared by High-speed Agitator and Syringe Pump
Hyunju Lim, Ji-Yeon Shin, Deuk Yong Lee†
 , Bae-Yeon Kim*, and Yo-Seung Song**
, Bae-Yeon Kim*, and Yo-Seung Song**Department of Biomedical Engineering, Daelim University, Anyang 13916, Korea
*Department of Materials Science and Engineering, Incheon National University, Incheon 22012, Korea
**Department of Materials Engineering, Korea Aerospace University, Goyang 10540, Korea- 고속교반기와 주사기 펌프로 제조한 PCL과 PCL/PEG 마이크로캡슐의 약물방출거동
대림대학교 의공융합과, *인천대학교 신소재공학과, **한국항공대학교 재료공학과
Nifedipine (NF)-loaded PCL and
PCL/PEG microcapsules with diameters ranging from 1 to 5 µm are
synthesized by the oil-in-water emulsion-solvent evaporation technique to
evaluate the influence of stirring speed, PCL concentration, and PCL/PEG weight
ratio on morphology and drug delivery behavior of the capsules. The size of PCL
capsules decreased as the PCL concentration decreased from 8 to 2 wt% and
the stirring speed increased from 3000 to 5000 rpm. The diameter of
PCL/PEG capsules increased by varying the PCL/PEG ratio from 10/0 to 6/4.
Higher amount of released NF is observed for smaller PCL capsules and larger
PCL/PEG capsules, respectively. FTIR results revealed that the NF-loaded PCL
and PCL/PEG capsules were formed due to hydrogen bonding between PCL and NF.
The capsules showed no evidence of cytotoxicity, suggesting that the capsules
were safe.
1~5 μm 직경을 가진 니페디핀(NF)을 함유하는 폴리카프로락톤(PCL)과 PCL/폴리에틸렌 글리콜(PEG) 마이크로캡슐을 고속교반기를 이용하여
액중건조법으로 제조하여 교반속도, PCL 농도, PCL/PEG 중량비에
따른 캡슐의 형상과 약물전달거동을 조사하였다. PCL 농도가 8에서 2 wt%로 감소하고, 교반속도가 3000에서 5000 rpm로 증가함에 따라 캡슐크기는 감소하였다. 반면에, PCL/PEG 캡슐의 크기는 PCL/PEG 비가 10/0에서
6/4로 변화함에 따라 증가하였다. 직경이 작은 PCL 캡슐과
큰 PCL/PEG 캡슐에서 많은 양의 NF가 관찰되었다. FTIR 관찰결과, NF의 카보닐기와 아민기에 의해 PCL과 NF가 성공적으로 결합되어 있었다. 세포독성 실험결과, PCL과
PCL/PEG 캡슐은 세포 용해나 독성이 없어 안전하였다.
Nifedipine(NF)-loaded PCL and PCL/PEG microcapsules with
diameters of 1 to 5 ¥ìm
are synthesized by the oil-in-water emulsion-solvent evaporation technique
using a high-speed agitator and a syringe pump. The NF-loaded microcapsules,
exhibiting no sign of causing cytotoxicity, are successfully formed via
hydrogen bond between PCL and NF.
Keywords: poly(e-caprolactone) (PCL), poly(ethylene glycol) (PEG), nifedipine, microcapsule, drug release
This work was supported by the Materials &
Components Technology Development Program (Project No. 20003560), funded by the
Ministry of Trade, Industry & Energy (MOTIE, Korea).
Microcapsules consist of two parts: core and shell material. They have a
structure in which solid or liquid or gas material (core) is encapsulated with
a membranous material (shell). Core material containing an active ingredient
can be extracted by breaking the shell to provide controlled release of drug
with various means such as pressure, heat, light, acid, or chemical reaction.1-7
The desired location can be reached at the therapeutic level, making it a very
useful carrier because the shell material shields the core material from the
external environment and allows good release characteristics.2-7
Lung-targeted drug delivery systems (LTDDSs) are designed to deliver
drugs to the disease site without causing adverse effects in other tissues and
improve the therapeutic efficiency of the lung diseases such as asthma,
tuberculosis, pneumonia, and lung cancer.8 Drug is likely to be
delivered either via pulmonary inhalation or intravenous injection. The inhalation
method is a non-invasive route that provides efficient lung targeting due to
large surface area for drug absorption without the first-pass metabolism and
the side effects. Particle size of drug or drug carriers play an important part
in the particle stability in lung. Particles smaller than 1 µm and larger
than 5 µm are mostly removed by exhalation and mucociliary, respectively.
On the other hand, the particles with diameters ranging from 1 to 5 mm are found to be the optimal size due to easy deposition
in alveolar regions and fast drug delivery.8 It usually takes
20 h to reach the plateau regime of nifedipine (NF) drug release for the
PCL/PVP and PCL/PEG capsules in the size range of 150 to 250 µm.5 In
the present study, a high-speed agitator and a syringe pump are employed to
obtain a tailored particle size of 1 to 5 µm and fast drug delivery
behavior. Bashir et al. also reported that increased concentration of
polymer and larger capsule size are ascribed to delay in drug release, implying
that the polymer/drug ratio is likely to be adjusted to achieve the controlled
drug release rate.9
Poly(e-caprolactone)
(PCL) is widely used as a shell material for DDS due to its biodegradability,
chief cost, elasticity, and excellent biocompatibility.5,10 PCL and
poly(ethylene glycol) (PEG) have been used as shell materials in microcapsules
with proper composition ratio. NF, as a core material, is a medication used for
angina, hypertension, Raynaud’s phenomenon, and premature labor.1,5-7
It has low cost as well as a short body half-life, causing low drug
bioavailability as a result of fast release.6 The NF-loaded PCL and
PCL/PEG microcapsules can be the practical approach to gain its therapeutic
efficacy through achieving the sustained drug-release rate and improving the
drug bioavailability.5-7 Uniform microcapsules with diameters in the
range of 1 to 5 µm are synthesized by the oil-in-water (O/W)
emulsion-solvent evaporation (ESE) technique using a high-speed agitator and a
syringe pump. The water-insoluble oil phase is formed by mixing of PCL, PEG, NF
and dichloromethane (DCM) and then dispersed in polyvinyl alcohol (PVA)
emulsifier solution to obtain the microcapsule having the controlled drug
delivery.2,5 In the present study, NF is microencapsulated and then
the capsule size, the NF release rate, and the cytotoxicity of the PCL and
PCL/PEG microcapsules are then investigated.
Materials. PCL (Mn=80000),
PVA (Mw=85000~124000), and NF (≥98%) are purchased from
Sigma-Aldrich. PEG (Mw=2000) is purchased from Yakuri Pure
Chemicals (Japan). NF is
1,4-dihydro-2,6-dimethyl-4-(2-nitrophenyl)-3,5-pyridinedicarboxylic acid
dimethyl ester, C17H18N2O5. DCM
(99.5%, Samchun Pure Chemical, Korea) is purchased as a solvent and used as
received without further purification.
Microcapsule
Synthesis. The 2~8 wt% PCL solutions dissolved in DCM are
prepared by stirring for 24 h at a speed of 500 rpm and then mixed with
10 mg NF to obtain a homogeneous solution. PCL and PCL/PEG solutions
containing weight ratios in the range of 10/0 to 6/4 are prepared. PVA dissolved
in distilled water is refluxed at 500 rpm and 80 oC to
obtain a viscous homogeneous solution.5 The as-prepared PCL and
PCL/PEG solutions are placed in a 20 mL B-D luer-lok syringe attached to the
syringe pump (KDS-200, Stoelting Co., USA) and are fed into the 22-gage
blunt-end metal needle at a flow rate of 0.25 mL/min.11-14 They are
injected into PVA aqueous solution (1.0 wt%) and stirred with a high-speed
agitator (HD-312, HsiangTai, China) for 4 h at speed in the range of 3000 to
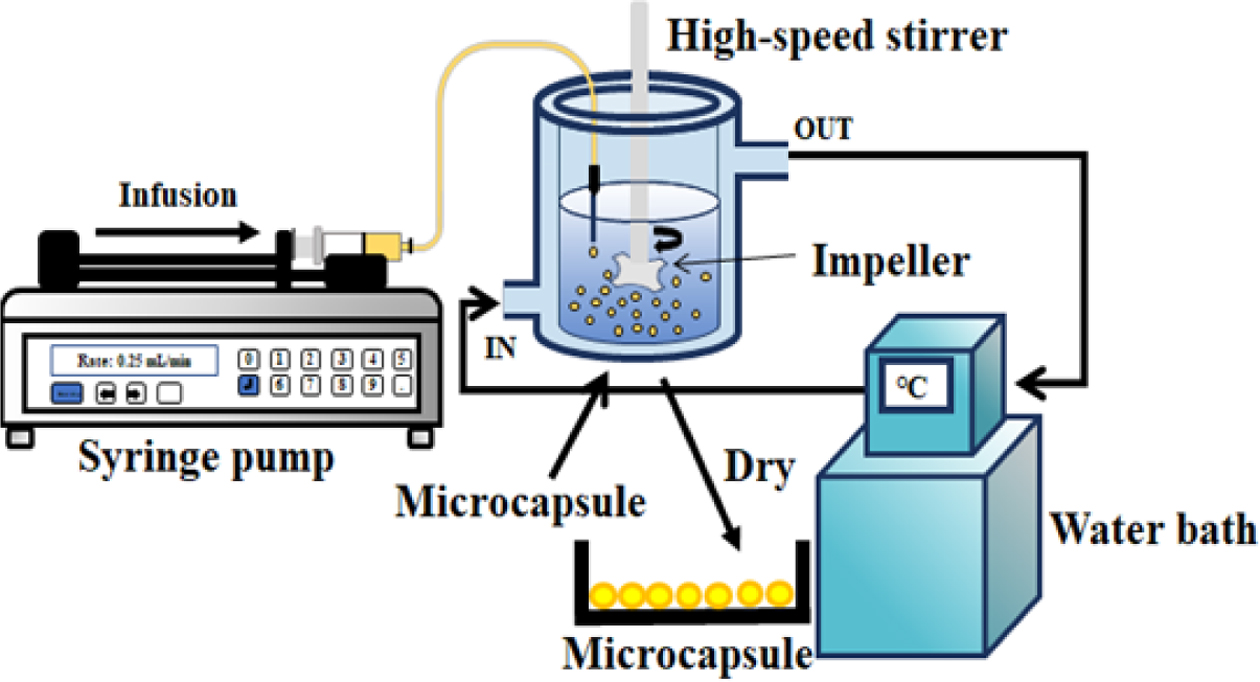
5000 rpm to obtain the desired microcapsules, as shown in Figure 1. The
microcapsules are screened through a 25 µm sieve. The capsules are then
cleaned in ethanol solution for 0.5 h and subsequently in distilled water for
0.5 h to remove the remnant solvent. The microcapsules are dried overnight
in a vacuum oven. Prior to use, their storage and handling are conducted
cautiously without light due to photosensitivity of NF.5-7 The
as-dried microcapsules are examined by using a SEM (S-3000H, Hitachi, Japan)
and an optical microscope (SV-55, Sometech, Korea) equipped with iSolution
Lite image software to investigate the microcapsule size and morphology.
For the SEM observation, the microcapsules are sputtered with Au/Pd to ensure
higher conductivity.11-19
Characterization. NF (10 mg) and dried
microcapsules (0.1 g) are dissolved in 20 mL ethanol by stirring in a 50
mL conical tube at 100 rpm and 37 oC. The samples (4 mL) are
taken regularly throughout the experiment from the supernatant of the dissolved
solution and then filtered through a 0.45 µm Millipore filter to remove
the impurities. The adsorption at 238 nm is monitored to identify the variation
in NF concentration using an UV-vis spectrophotometer (Jasco V-670, Japan). The
amount of drug release is examined to evaluate the drug release rate as a
function of time.5 The chemical properties of the capsules are analyzed by
using the Fourier trans- form infrared spectroscopy (FTIR, Spectrum Two,
PerkinElmer, UK). All experiments are carried out in triplicate. Values in the text are
stated as the means±standard deviation, and p<0.05 is weighed
statistically significant.5
Cytotoxicity. The extract test method is
conducted on the PCL and PCL/PEG microcapsules to evaluate cytotoxicity
quantitatively according to the International Organization for Standardization
(ISO 10993-5).5,13-19 The PCL and PCL/PEG microcapsules are
extracted aseptically in single strength minimum essential medium with serum.5
The ratio of the microcapsules to the extraction vehicle is 0.2 g/mL (ISO
10993-12). The test extracts are placed onto three separate confluent
monolayers of L-929 (NCTC Clone 929, ATCC, USA) mouse fibroblast cells
propagated in 5% CO2. EZ-cytox yields a water-soluble formazan,
which can be provide different absorption spectra of the formed formazans. The
absorbance of the colored solution is quantified by measuring at a wavelength
of 415 nm with the microplate absorbance spectrophotometer (Bio-Rad, USA).
Detailed experimental procedure is described elsewhere.5,13-19
|
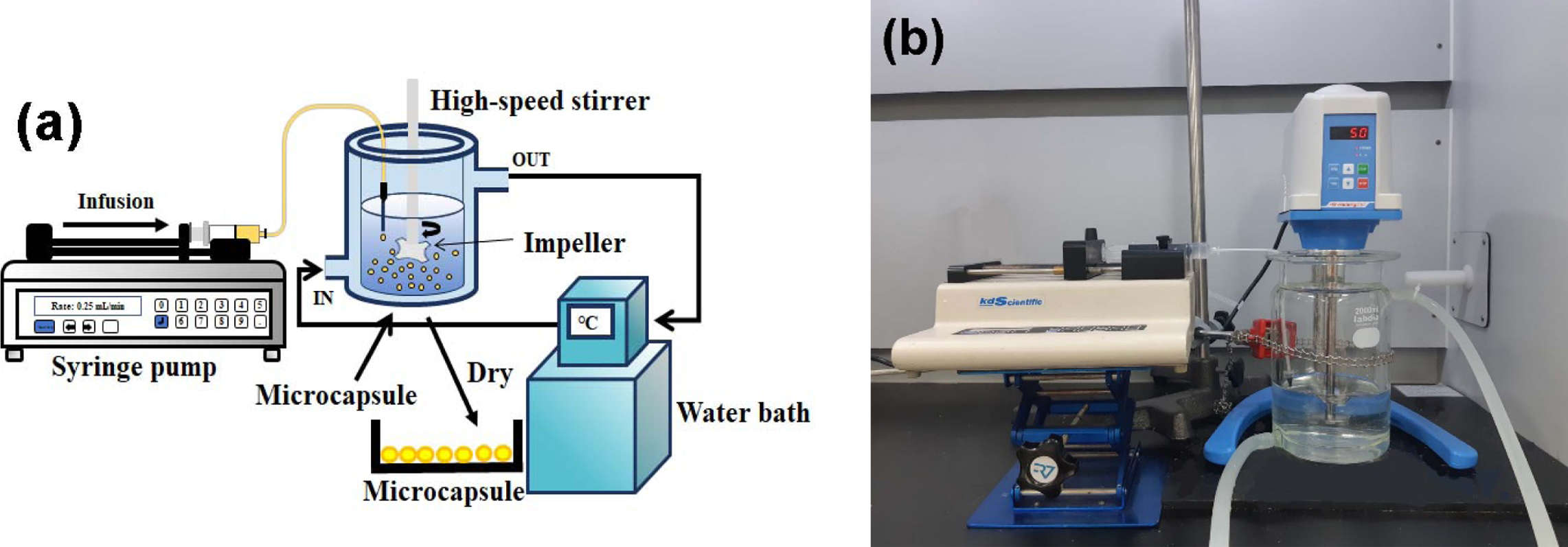
Figure 1 (a) A schematic diagram; (b) photograph of experimental apparatus. |
The effect of PVA emulsifier concentration on the size of the 4 wt%
PCL microcapsule prepared by a magnetic stirrer is previously reported.5
For microcapsules prepared by using PVA emulsifier, uniform and highly stable
spherical PCL, PCL/PVP, and PCL/PEG microcapsules (~150 µm) with a narrow
size distribution are successfully synthesized.5 This may be due to
high affinity of the PVA for the shell materials and the core material.5
The NF release rate of PCL/PVP and PCL/PEG capsules increases with increasing
PVP and PEG concentration due to the increase in capsule size. The 1 wt%
PVA emulsifier is determined to be the best composition for the formation of
uniform microcapsule. In the present study, PCL and PCL/PEG microcapsules using
1 wt% PVA emulsifier are studied by the ESE technique using the high-speed
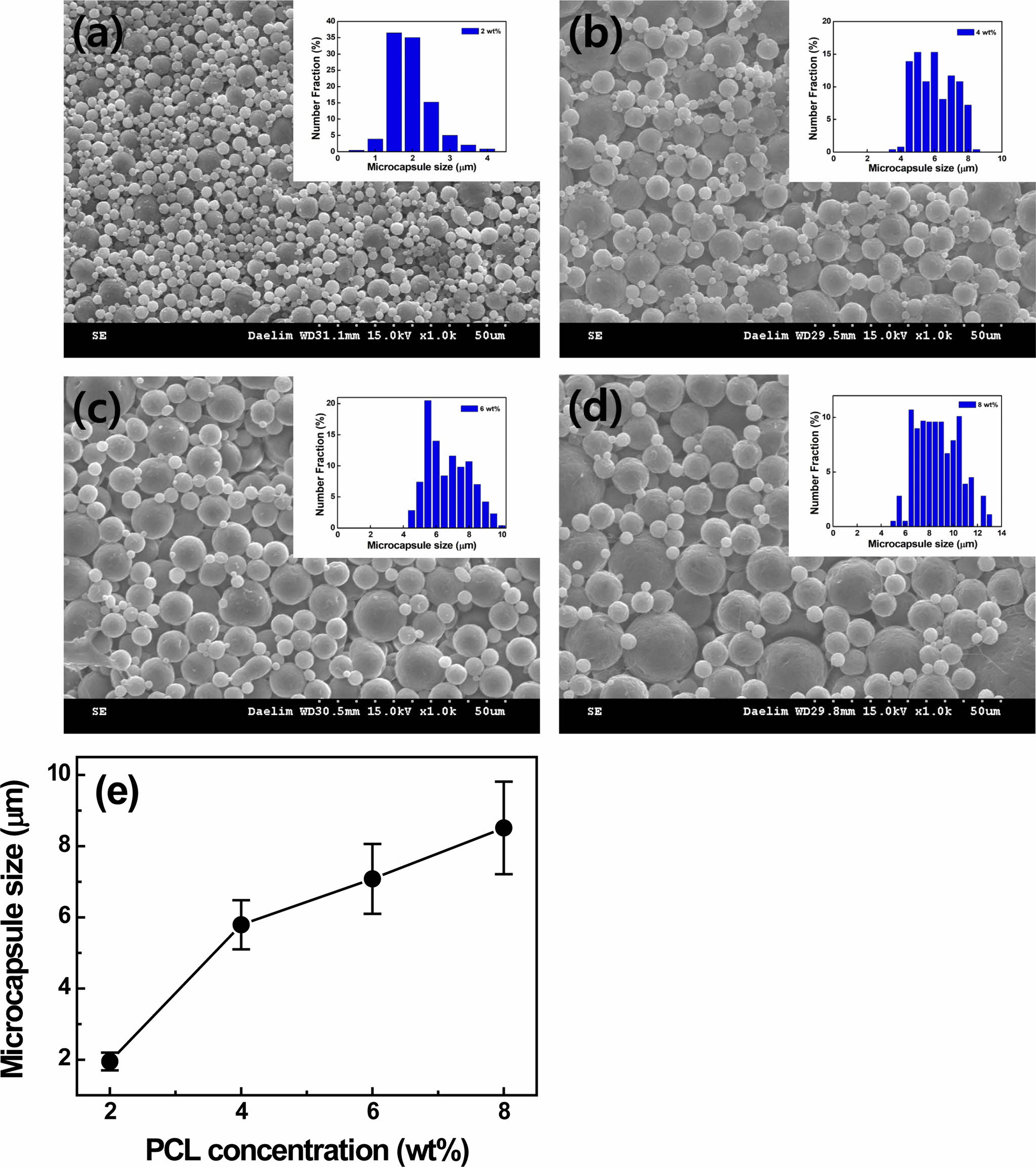
agitator. The PCL capsule size decreases gradually from 5.95±0.85 µm to
1.95±0.25 µm with increasing the stirring speed from 3000 to 5000 rpm. The
microcapsule size is controlled by the balance between the turbulence to break
the microcapsules, which are emulsion particles stabilized by PVA, and the internal
viscosity and interfacial tension to hold the microcapsules. Fast stirring
speed increases the turbulent energy acting on each microcapsule, increasing
the rate at which the capsules becomes smaller, thereby reducing the size of
the microcapsules.4 The capsules prepared at 5000 rpm are selected
in the present study. In addition, the influence of PCL concentration on the
capsule size is investigated. As the PCL concentration rises from 2 to 4, 6,
and 8 wt% at a fixed speed of 5000 rpm, the PCL capsule size increases
from 1.95±0.25 to 5.79±0.69, 7.08±0.98, 8.51±1.37 µm due to higher loading
of polymer,5 as depicted in Figure 2. In the present study, the 2
wt% PCL capsule prepared at a stirring speed of 5000 rpm is chosen due to the
capsule size (~1.95 µm) for LTDDS because carriers with diameters ranging from
1 to 5 µm are known to be responsible for easy deposition in alveolar
regions.8
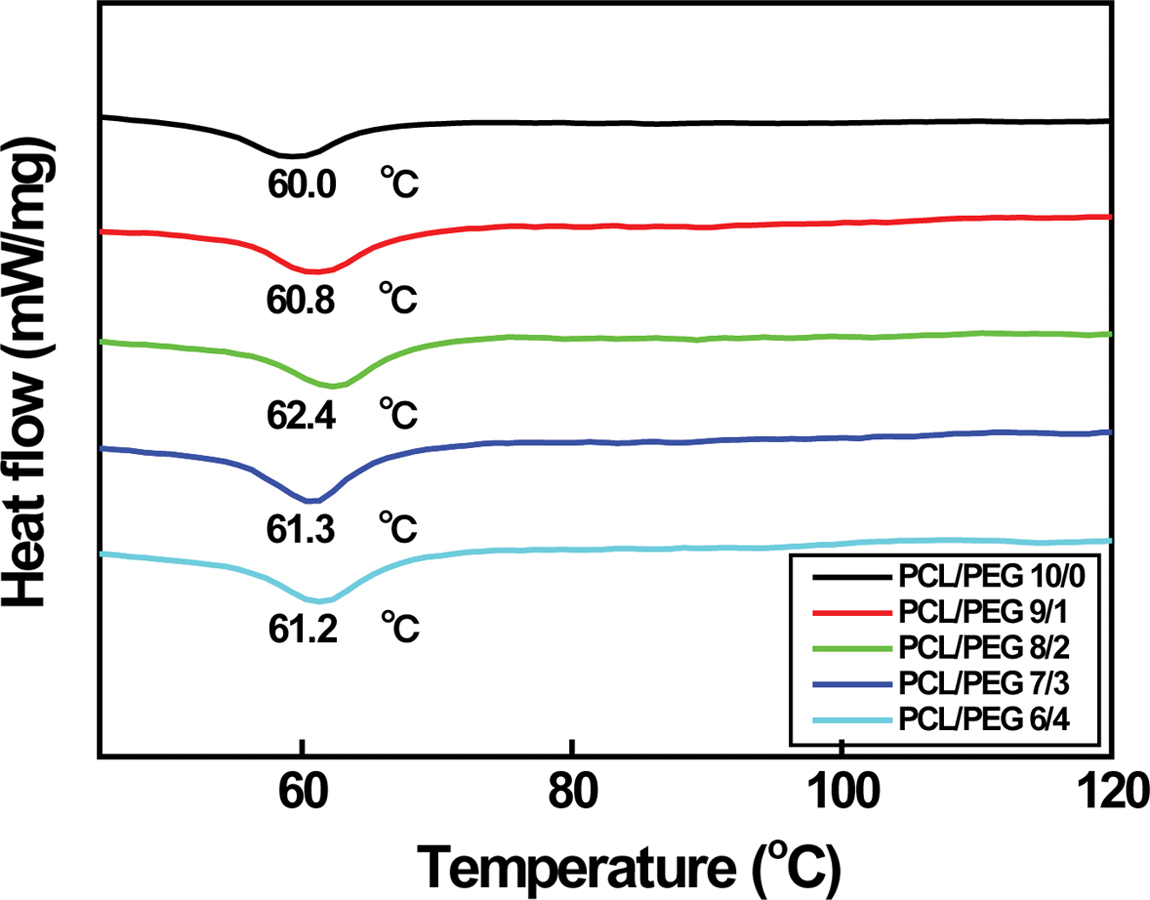
It is reported that melting temperature (Tm) of the
PCL/PEG capsules rises from 65.0 to 65.5 oC with increasing the
PEG content from 0 to 20 wt%.5 However, it decreases sharply
down to ~62.4 oC when PEG is added to PCL more than
30 wt%. It is due to the blending effect of PEG because Tm
of PEG is in the range of 50 to 52 oC. Although Tm
of PCL/PEG capsules in the range of 60.0 to 62.4 oC is lower
than those of the above-mentioned capsules (62.4~65.5 oC)
having a diameter of about 154 µm, similar variation in Tm
is observed as expected. Tm increases from 60.0 to 62.4 oC
with increasing the PEG content from 0 to 20 wt% and then decreases down
to 61.2 oC with further PEG doping, as shown in Figure 3. As
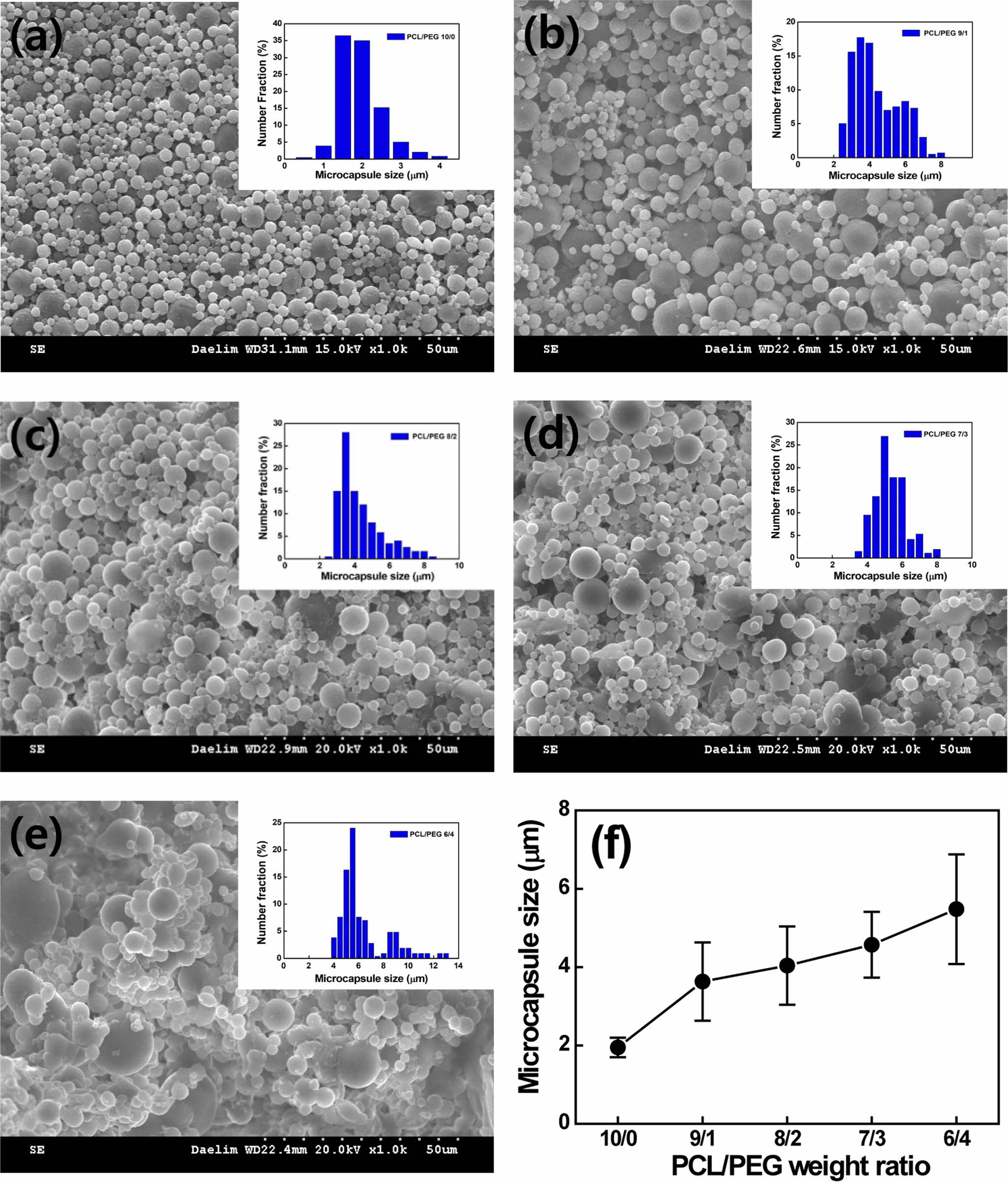
the PCL/PEG weight ratio varies from 10/0 to 6/4, the capsule size increases
gradually from 1.95±0.25 to 5.48±1.6 µm due to the hydrophilic PEG, as
shown in Figure 4. The size of PCL/PEG capsules with a ratio of 10/0 to 8/2 is
between 1 and 5 µm, which is a relevant capsule size for LTDDS.8 The
affinity to water increases with increasing the content of the hydrophilic PEG,
resulting in an increase in capsule size.5 As the PCL/PEG weight ratio
varies from 10/0 to 6/4, the capsule morphology is changed from uniform spheres
to agglomerated particles and airy bread-like distorted shape with a broad size
distribution due to higher porosity.5,20 However, no relationship
between capsule size and Tm of PCL/PEG is found.
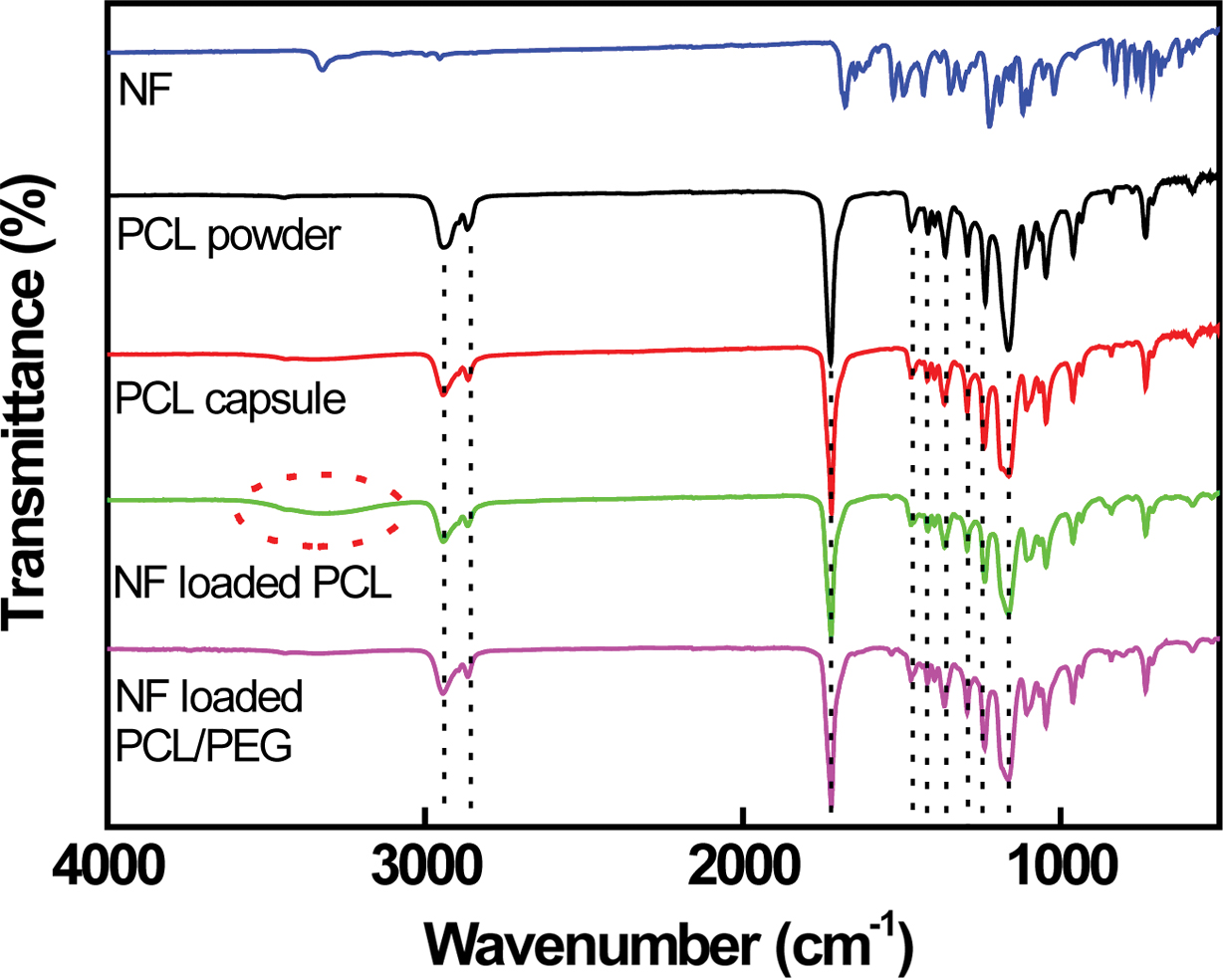
The PCL spectrum exhibits the main peak at 1726 cm-1
corresponding to the carbonyl group (-CO) of the ester group. Peaks at 2937 and
2866 cm-1 corresponding to the asymmetrical and symmetrical
methylene groups (-CH2) are clearly visible.5,21 Peaks
located at 1472, 1412, and 1361 cm-1 correspond to CH2
bending. Bands at 1295, 1230, and 1164 cm-1 related to C-C
stretching, asymmetric and symmetric C-O-C stretching are observed,
respectively.21 NF can potentially act as either proton acceptor
(through the carbonyl groups, -CO) or proton donor (through the amine group,
-NH).5-7,10 The hydrogen bonds (H-bonds) are reported to be formed
via the amine group and the carbonyl groups. The H-bonded NF amine and carbonyl
groups are observed at 3330 cm-1 (-NH) and at 1680 cm-1
(-CO), as depicted in Figure 5. The H-bonds formed among NF molecules are
altered by those formed between NF and PCL. A sharp stretching vibration band
at 1720 cm-1 is clearly observed on the NF-loaded PCL capsule
because of the NF carbonyl group formed H-bonds with PCL. A broad band situated
in 3050 and 3700 cm-1 is visible in the spectra of NF-loaded
PCL capsule, suggesting that the NF amine group associated with PCL via H-bond is established.5-7 The peak intensity
of NF-loaded PCL/PEG capsule located at 1246 cm-1 (C-O-C) increases
slightly compared to that of NF-loaded PCL capsule due to the presence of PEG,
as shown in Figure 5. However, no appreciable change in FTIR peak is detected
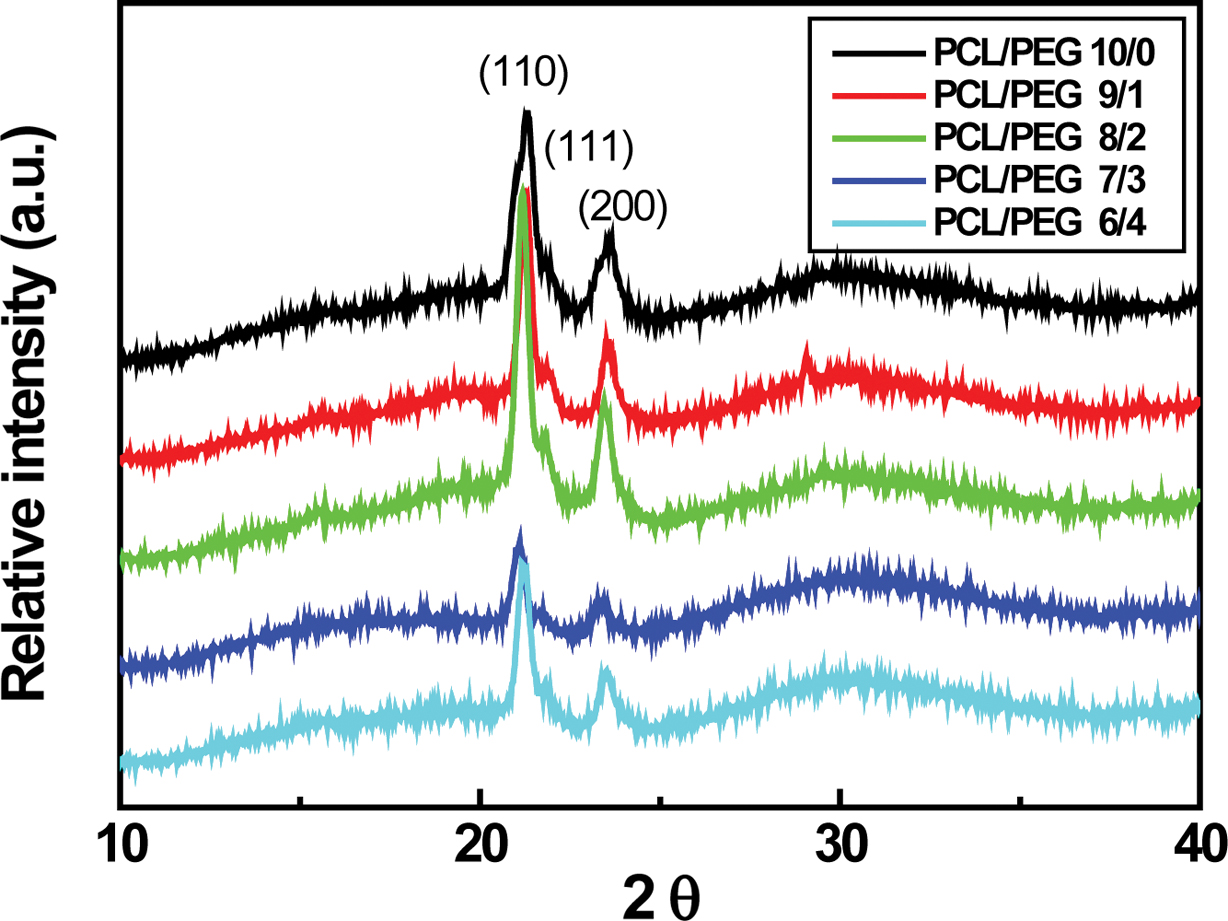
for the PCL/PEG (6/4) capsules. Typical PCL XRD peaks located at 2q = 21.4o, 22o, and 23.7o,
corresponding to the (110), (111), and (200) planes of an orthorhombic crystal,
are clearly visible in Figure 6.5,23 These sharp peaks are
attributed to the crystalline phase of PCL, which originates from the ordering
of polymer side chains due to the intermolecular interaction between PCL chains
through the hydrogen bonding.23 Characteristic PEG peaks at 19.23o
and 23.34o are reported.5,24 The (110) and (200) PCL
peaks are shifted slightly to lower angle. In addition, the (110) PCL peak
intensity starts to decrease with further addition of PCL/PEG ratio more than
7/3 probably due to the enhanced PEG contribution to the PCL/PEG microcapsules,
as demonstrated earlier in Figure 5.5
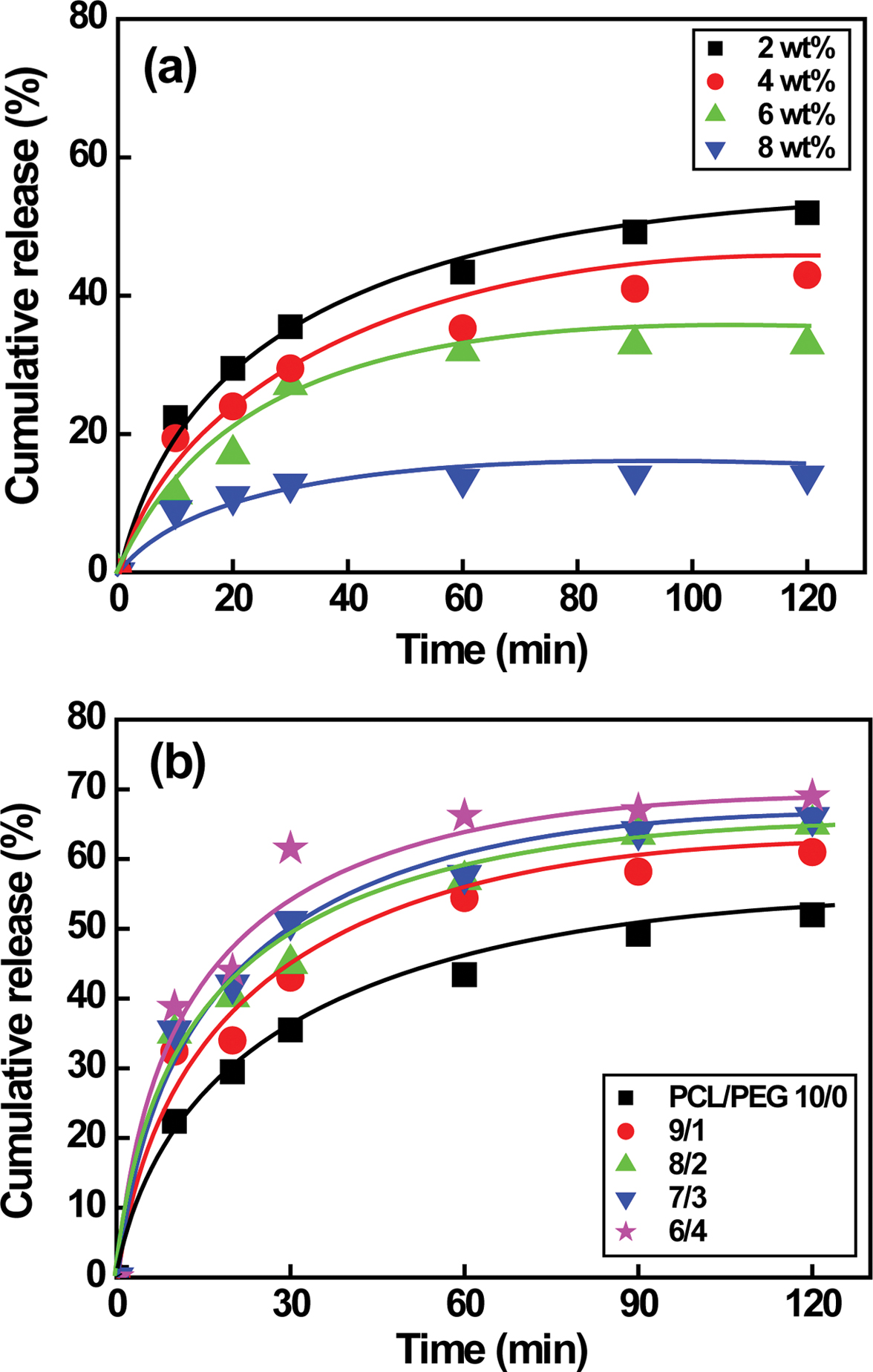
The drug release behavior of NF is shown in Figure 7. Our previous
studies reveal that NF release rate of PCL/PEG capsules prepared by a magnetic
stirrer increases significantly from 0 to 4 h at the beginning and then
reaches the plateau region in 20 h probably due to larger capsule size in
the range of 154 to 248 µm.5 However, the drug release rate of
PCL microcapsule made by a high-speed agitator and a syringe pump initially
increases rapidly from 0 to 10 min in a short time and then reaches the plateau
region in 1 h. The time reaches the plateau regime is 20 times faster than
that of capsules prepared by a magnetic stirrer. The amount of released drug
rises significantly from 14 to 52% with decreasing the PCL concentration from 8
to 2 wt% due to higher specific surface area as a result of the reduced capsule
size from 8.51±1.37 to 1.95±0.25 µm, as shown in Figure 7(a). Unlike PCL
capsules, Figure 7(b) suggests that the drug release rate of PCL/PEG capsules
increases from 52 to 69% as the weight ratio of PCL/PEG varies from 10/0 to 6/4
(1.95±0.25 to 5.48±1.6 µm), implying that larger PCL/PEG capsules are
attributed to higher amount of encapsulated drug.5,20 Although the
capsule size affects the amount of drug, the drug release behavior remains
constant regardless of the capsule size and the PEG concentration. The
2 wt% PCL and PCL/PEG (10/0 to 8/2) microcapsules with diameters ranging
from 1 to 5 µm release the encapsulated NF from 52% to 65%.
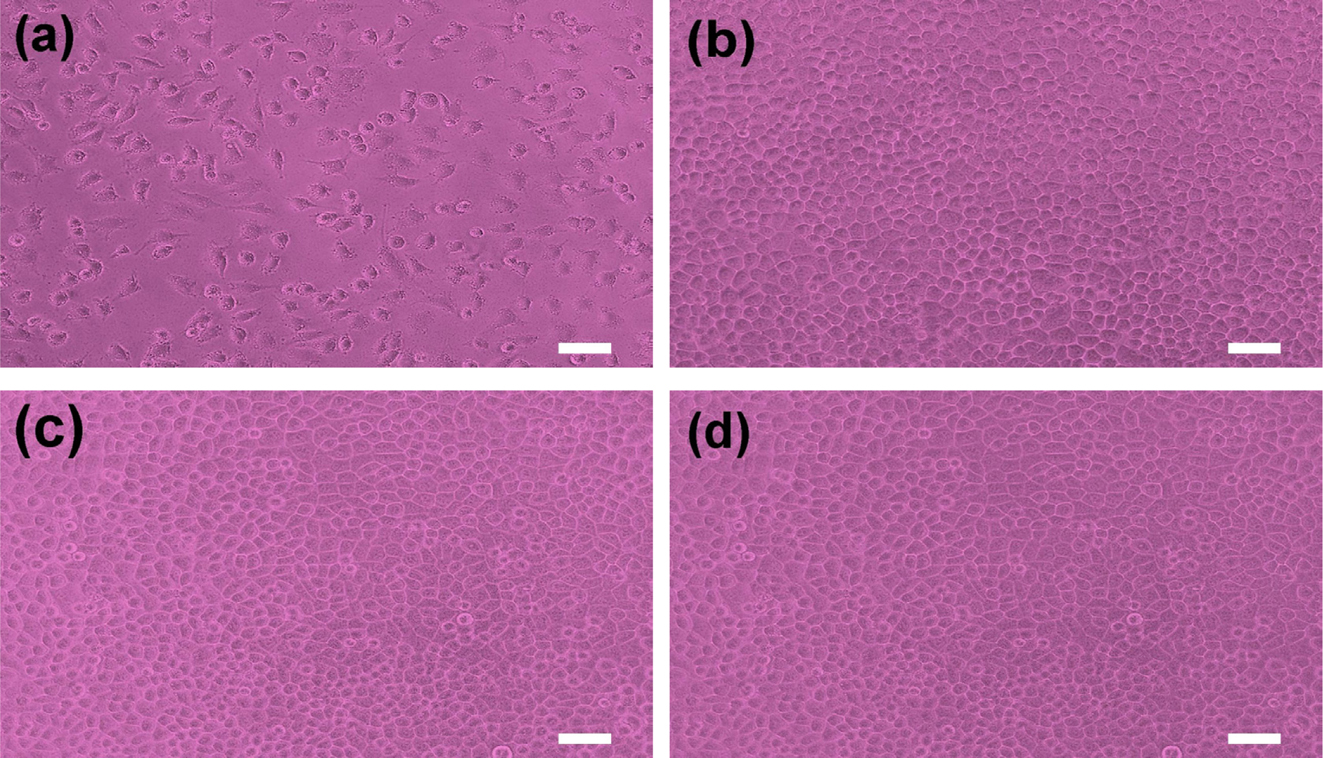
A cytotoxicity test of PCL and PCL/PEG capsules determines whether a
product or compound may have an adverse effect on tissues or cells.13-19
The test extracts with PCL and PCL/PEG capsules with NF show no evidence of
causing cytotoxicity, as depicted in Figure 8. All experiments are performed in
triplicate. The PCL and PCL/PEG (6/4) capsules exhibited quantitative cell
viability of 121% and 133% compared to the negative control, respectively, as
measured at a wavelength of 415 nm by using the iMark microplate absorbance
spectrophotometer. The cell viability of PCL/PEG capsule is slightly higher
than that of PCL capsule due to excellent biocompatibility of PEG.20-22
The qualitative morphological grading of cytotoxicity of PCL and PCL/PEG
capsules is determined to be scale 0.5 Therefore, it is conceivable
that the NF-loaded PCL and PCL/PEG microcapsules have no cytotoxicity
throughout the experiment and are considered to be clinically safe.
|
Figure 2 SEM images of (a) 2 wt%; (b) 4 wt%; (c) 6 wt%; (d) 8 wt% PCL microcapsules; (e) capsule size as a function of PCL concentration, respectively. Note that the microcapsules are prepared by using 1 wt% PVA emulsifier at a speed of 5000 rpm. |
|
Figure 3 DSC curves of various PCL/PEG microcapsules. |
|
Figure 4 SEM images of microcapsules containing various PCL/PEG weight ratios of (a) 10/0; (b) 9/1; (c) 8/2; (d) 7/3; (e) 6/4; (f) capsule size as a function of PCL/PEG weight ratio, respectively. |
|
Figure 5 FTIR spectra of NF, PCL powder, PCL capsule, NFloaded PCL, and PCL/PEG (6/4) capsules, respectively. |
|
Figure 6 XRD patterns of PCL/PEG microcapsules having different concentration. |
|
Figure 7 NF release behavior of (a) PCL; (b) PCL/PEG microcapsules. Note that the capsules are prepared by a high-speed agitator at a speed of 5000 rpm. |
|
Figure 8 Photographs of cell morphologies: (a) positive control; (b) negative control; (c) PCL; (d) PCL/PEG microcapsule with NF, respectively. Scale bar is 50 ㎛. |
The NF-loaded PCL and PCL/PEG microcapsules are prepared by ESE technique
through proper adjustment of the process parameters. The NF release rate of PCL
and PCL/PEG microcapsules increases greatly from 0 to 10 min in a short time
and then reaches the plateau region in 1 h. The amount of released drug rises
drastically from 14 to 52% with decreasing the PCL concentration from 8 wt%
(8.51±1.37 µm) to 2 wt% (1.95±0.25 µm) due to higher specific surface
area. Unlike PCL capsules, the amount of encapsulated drug in PCL/PEG capsules
increases with increasing the capsule size due to the increased hydrophilic PEG
contribution to the PCL/PEG capsules. The PCL and PCL/PEG capsules exhibiting
no evidence of cytotoxicity suggest that the microcapsules are clinically
suitable for DDS.
- 1. M. Jang, C. Choi, W. Kim, Y. Jeong, and J. Nah, Polym. Korea, 28, 291 (2004).
- 2. S. Kang, M. Baginska, S. R. White, and N. R. Sottos, ACS Appl. Mater. Interfaces, 7, 10952 (2015).
-

- 3. N. V. N. Jyothi, P. M. Prasanna, S. N. Sakarkar, K. S. Prabha, P. S. Ramaiah, and G. Y. Srawan, J. Microencapsul., 27, 187 (2010).
-

- 4. Y. A. Kim, S. H. Kim, J. S. Park, D. S. Lee, J. G. Kim, and J. S. Shin, J. Adhes. Interf., 13, 17 (2012).
-

- 5. H. Lee, D. Y. Lee, Y. Song, and B. Kim, J. Biomed. Eng. Res., 40, 7 (2019).
- 6. J. Huang, R. J. Wigent, and J. B. Schwartz, J. Pharm. Sci., 97, 251 (2008).
-

- 7. C. J. Lee, H. J. Ha, S. Y. Kim, J. Y. Park, N. K. Jang, J. E. Song, and G. Khang, Polym. Korea, 39, 739 (2015).
-

- 8. S. Nejati, E. M. Vadeghani, S. Khorshidi, and A. Karkhaneh, Eur. Polym. J., 122, 109353 (2020).
-

- 9. S. Bashir, M. Asad, S. Qamar, F. U. Hassnain, S. Karim, and I. Nazir, Trop. J. Pharm. Res., 13, 505 (2014).
-

- 10. J. Park, J. Kim, and N. Jeong, Appl. Chem. Eng., 26, 341 (2015).
- 11. H. Lee, D.Y. Lee, M. Lee, B. Kim, and Y. Song, J. Electroceram., 42, 124 (2019).
-

- 12. Y. Song, Y. Kim, D. Y. Lee, M. Lee, and B. Kim, J. Nanosci. Nanotechnol., 17, 7943 (2017).
-

- 13. B. Seol, J. Shin, G. Oh, D. Y. Lee, and M. Lee, J. Biomed. Eng. Res., 38, 248 (2017).
-

- 14. G. Oh, J. Rho, D. Y. Lee, M. Lee, and Y. Kim, Macromol. Res., 26, 48 (2018).
-

- 15. J. Kim, D. Y. Lee, E. Kim, J. Jang, and N. Cho, Tissue Eng. Regen. Med., 11, 32 (2014).
-

- 16. H. Jeong, J. Rho, J. Shin, D. Y. Lee, T. Hwang, and K. J. Kim, Biomed. Eng. Lett., 8, 267 (2018).
-

- 17. S. Son, J. Choi, H. Cho, D. Kang, D. Y. Lee, J. Kim, and J. Jang, Polym. Korea, 39, 323 (2015).
-

- 18. J. Shin, H. Jeong, and D. Y. Lee, J. Biomed. Eng. Res., 39, 161 (2018).
-

- 19. S. Kim, J. Shin, Y. Yun, D. Y. Lee, D. Yang, B. Kim, and Y. Song, Polym. Korea, 43, 764 (2019).
-

- 20. Y. Li, C. Zhu, D. Fan, R. Fu, P. Ma, Z. Duan, X. Li, H. Lei, and L. Chi, Macromol. Biosci., 19, e1800424 (2019).
-

- 21. E. Bolaina-Lorenzo, C. Martinez-Ramos, M. Monleon-Pradas, W. Herrera-Kao, J. V. Cauich-Rodriguez, and J. M. Cervantes-Uc, Biomed. Mater., 12, 015008 (2017).
-

- 22. N. S. V. Capanema, A. A. P. Mansur, A. C. de Jesus, S. M. Carvalho, L. C. de Oliveira, and H. S. Mansur, Intl. J. Biol. Macromol., 106, 1218 (2018).
-

- 23. M. Ravi, S. Song, J. Wang, X. Tang, and Z. Zhang, Ionics, 22, 661 (2016).
-

- 24. C. Wang, L. Feng, H. Yang, G. Xin, W. Li, J. Zheng, W. Tian, and X. Li, Phys. Chem. Chem. Phys., 14, 13233 (2012)
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2020; 44(4): 487-494
Published online Jul 25, 2020
- 10.7317/pk.2020.44.4.487
- Received on Feb 27, 2020
- Revised on Apr 4, 2020
- Accepted on Apr 13, 2020
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Deuk Yong Lee
-
Department of Biomedical Engineering, Daelim University, Anyang 13916, Korea
- E-mail: duke1208@gmail.com
- ORCID:
0000-0003-1674-412X









 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.