- Influences of Bridge Group on Thermal and Mechanical Properties of Epoxy Resins
Liu Yuan*,**,***, Zhao Jun****, Liu Ai-Qin****, Liu Xiao-Qing***, and Luo Jun*,†

*Engineering Research Center for Materials Protection of Wear and Corrosion of Guizhou Province, Guiyang University, Guiyang 550005 (P. R. China)
**University of Chinese Academy of Sciences, Beijing 100049 (China)
***Key Laboratory of Bio-based Polymeric Materials Technology and Application of Zhejiang Province, Ningbo Institute of Materials Technology and Engineering, Chinese Academy of Sciences, Ningbo 315201 (China)
****Shanghai Space Propulsion Technology Research Institute, No. 3888, Yuanjiang Road, Minhang District, Shanghai 201100 (P. R. China)- 에폭시 수지의 열 및 기계적 특성에 대한 Bridge Group의 영향
In order to obtain
thermosetting epoxy resin, it is the prerequisite condition that the epoxy
precursor must contain at least two epoxy groups. Thus, bridge group is needed
to link the epoxy groups, and naturally, the chemical structure of the bridge
group may also influence the thermomechanical performances of the cured epoxy
resin. However, literature about the effects of bridge group on properties of
cured epoxy is seldom published. To fill the gap, three model epoxy monomers
containing different bridge groups have been synthesized from
4,4'-dihydroxydiphenyl, 1,1-bis(4-hydroxyphenyl)cyclohexane and bisphenol A in
this work. After chemical structure confirmation, all of the monomers are cured
by methylhexahydrophthalic anhydride (HMMPA), and the properties of the
obtained cured network are evaluated by differential scanning calorimetry
(DSC), dynamic thermomechanical analysis (DMA), tensile test and scanning
electron microscope (SEM). The results show that bulky bridge group can
effectively increase the glass transition temperature, enhance the tensile
strength, and enlarge elongation at break of the cured epoxy resin.
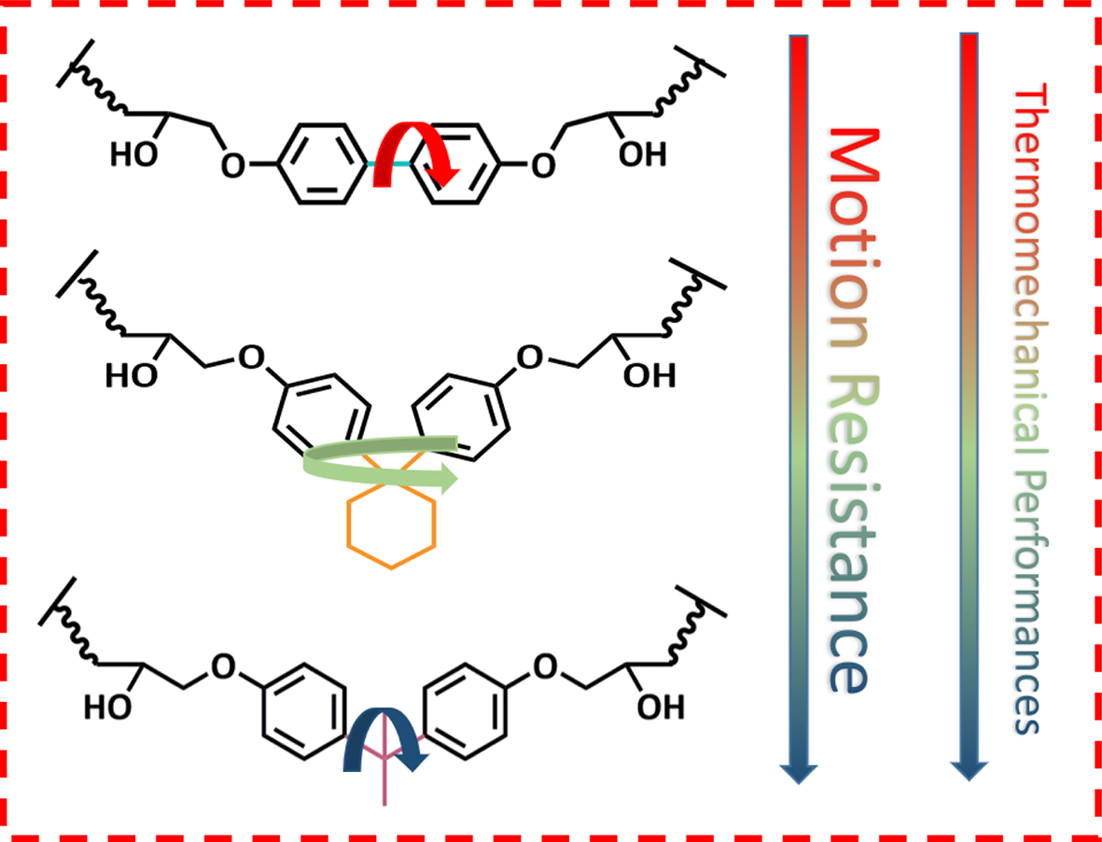
Three model epoxy monomers (DHBPH, DHPCH, and DGEBA) containing different
bridge groups were carefully synthesized. It is found the bulky bridge groups
endow the epoxy resins with admirable Tg and mechanical
properties.
Keywords: epoxy, bridge group, thermomechanical performance
The authors are grateful for the financial support
from Natural Science Foundation of Guizhou Province of China (Grant NO. [2018]
1008), and Discipline & Master's Site Construction Project of Guiyang
University (Grant NO. [HC-2019]).
As one of excellent thermosetting materials, epoxy resin has been widely
used in fields of coating,1,2 adhesive,3 electronic
package,4 3D printing5 and functional composite6
due to its remarkable processability, prominent chemical and corrosion
resistance, and wonderful thermal and mechanical properties.7
However, the epoxy resins are facing more and more challenges in recent decades
because the newly emerged applications request better thermal stability, higher
mechanical performances, and so on.
To meet the new requirements, researchers have persistently directed
toward improving the thermal and mechanical properties of epoxy resins. The
most widely adopted method is adding rigid fillers into epoxy resins. Peng and
coworkers uniformly dispersed rigid sulfonated polyamide in epoxy matrix, and
the tensile strength of modified epoxy was increased by 1.2 fold than that of
the neat epoxy.8 Woo and coworkers used epoxidized soybean oil
modified bisphenol A type epoxy resin to improve its toughness.9 In
addition to physical blending, researchers also justified that increasing the
crosslinking densities is another efficient route to enhance the thermomechanical
properties of epoxy resins as well. Schroeder et al. found the Tg
values of the cured epoxy resins changed linearly with the crosslinking
densities, furthermore, they also claimed that the tensile strength varied with
crosslinking densities.10 Since the epoxy thermosets are obtained by
curing their processable and moldable precursors, and to obtain cured epoxy
resins with admirable crosslinking densities, the precursors must contain at
least two epoxy groups. Naturally, bridge group is needed to connect two or
more epoxy groups, and thus, the nature of the bridge group also plays key role
in determination of the thermomechanical performances of the cured epoxy resin.
For this reason, deeply understanding the effect of the bridge group,
especially the steric hindrance of the bridge group, on thermomechanical
properties of epoxy resin becomes increasingly important and urgent, because it
can provide a guide for design of the epoxy precursors which may demonstrate
attractive thermomechanical properties after curing reaction. Some researchers
had focused on the influences of bridge group on properties of epoxy resin, for
example Lee and coworkers identified that ether bridge group endows the liquid
crystalline epoxy with higher mechanical and thermal properties than the ester
bridge group.11 Moreover, they further proved that when the epoxy
resins were bridged with long mesogenic groups, their storage modulus and glass
transition temperature were can be effectively enhanced.12
Unfortunately, they mainly concerned with the liquid crystalline epoxy,13
and other researchers mainly focus on the influences of the bridge group in
curing agent rather than in epoxy precursor itself.14 Li et al.
used oligomers to disclose valuable information about the effects of bridge
group in epoxy on curing behaviors of epoxy resins,15 however,
instead of the pure monomers, they used oligomers which may cripple the
relationships between the bridge group and properties of obtained epoxy resin
because of the structure ambiguities of the oligomers. Thus, to deeply
understand the influences of bridge group on thermomechanical properties of
epoxy resin, the prerequisite matter is ruling out the uncorrelated factors,
and the most feasible approach is designing the model epoxy monomers. However,
using the model epoxy monomers to inspect the influences of steric hindrance of
the bridge group on curing behaviors of the monomer and the thermomechanical
properties, to the best of our knowledge, there has no literature been
published.
In this contribution, three model epoxy monomers (Scheme 1) with
different volume of bridge groups, including carbon-carbon single bond,16,17
cyclohexyl group and isopropyl bridge group 18 were synthesized
again to investigate the effects, especially the steric hindrance, of bridge
groups on thermomechanical properties of epoxy resins, and we hope that the results
obtained in this study could shed light on the influences of bridge groups on
performances of epoxy resins.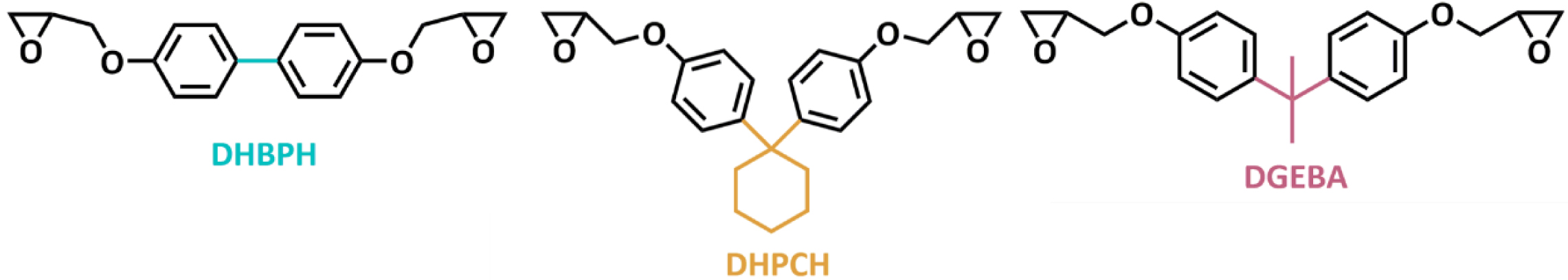
Scheme 1. Chemical structure of three model epoxy monomers.
Materials.
4,4'-dihydroxydiphenyl (96%), 1,1-bis(4-hydroxy-phenyl)cyclohexane (98%),
bisphenol A (99%), methylhexahydrophthalic anhydride (HMMPA, 95%),
tetrabutylammonium bromide (99%) and epichlorohydrin (99%) were purchased
from Aladdin Chemical Reagent Co. (Shanghai, China). Sodium hydroxide (A.R.
grade), chloroform (A.R. grade) and petroleum ether (A.R. grade) were purchased
from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). All of reagents
were used as received.
Synthesis
of 4,4'-bis(oxiran-2-ylmethoxy)-1,1'-biphenyl (DHBPH). 18.6 g
(100 mmol) of 4,4'-dihydroxydiphenyl, 148.0 g (1600 mmol) of
epichlorohydrin and 0.93 g of tetrabutylammonium bromide were charged into a
500 mL round three-necked flask equipped with a nitrogen inlet, a
condenser, a thermometer and a magnetic stirrer. After the reactor was stirred
2 h at 90 °C, the system was cooled down to room temperature, and the
thermometer was replaced by a separatory funnel, 30.0 g (40 wt%) of
aqueous sodium hydroxide solution were added into the reactor dropwise. The
system was maintained at room temperature for another 24 h after the aqueous
sodium hydroxide solution was completely added into the reactor. After that,
the system was first diluted by around 300 mL of chloroform, and then
washed by deionized water for several times to remove the excess sodium
hydroxide. A fine white powder was obtained after being precipitated in
petroleum ether and dried at 45 °C under vacuum (yield: 27%).
1H NMR (400 MHz, DMSO-d6) δ 7.59-7.50 (m, 4H), 7.08-6.97 (m, 4H),
4.35 (dd, J = 11.4, 2.6 Hz, 2H), 3.86 (dd, J = 11.4, 6.6 Hz, 2H),
3.36 (dd, J = 4.2, 2.6 Hz, 2H), 2.85 (t, J = 4.7 Hz, 2H), 2.72
(dd, J = 5.1, 2.6 Hz, 2H).
13C NMR (101 MHz, DMSO-d6) δ 157.85, 133.01, 127.73, 115.37, 69.46,
50.20, 44.23.
FTIR (KBr), cm-1: 3006 cm-1 (benzene ring C–H),
2927 cm-1 (oxirane ring C–H), 1240 and 811 cm-1
(oxirane group C–O-C), 910 cm-1 (oxirane
ring).
Synthesis of 2,2'-(((cyclohexane-1,1-diylbis(4,1-phenylene)) bis(oxy)) bis(methylene)) bis(oxirane) (DHPCH). 26.8 g (100 mmol) of
1,1-bis(4-hydroxyphenyl)cyclohexane, 148.0 g (1600 mmol) of epichlorohydrin and
0.93 g of tetrabutylammonium bromide were charged into a 500 mL round
three-necked flask equipped with a nitrogen inlet, a condenser, a thermometer
and a magnetic stirrer. After the reactor was stirred 2 h at 90 °C, the
system was cooled down to room temperature, and the thermometer was replaced by
a separatory funnel, 30.0 g (40 wt%) of aqueous sodium hydroxide solution
were added into the reactor dropwise. The system was maintained at room
temperature for another 24 h after the aqueous sodium hydroxide solution was
completely added into the reactor. After that, the system was first diluted by
around 300 mL of chloroform, and then washed by deionized water for several
times to remove the excess sodium hydroxide. Then, the mixture was separated
into two layers in a separatory funnel, and the organic layer was collected and
dried by anhydrous MgSO4. A viscous and light yellow liquid was
obtained after removing the solvent at 50 °C under vacuum (yield: 85%).
1H NMR (400 MHz, chloroform-d) δ 7.25 – 7.12 (m, 4H), 6.93 – 6.76
(m, 4H), 4.18 (dd, J = 11.0, 3.3 Hz, 2H), 3.95 (dd, J = 11.0, 5.6
Hz, 2H), 3.34 (dq, J = 7.4, 3.1 Hz, 2H), 2.90 (t, J = 4.5 Hz,
2H), 2.80 – 2.70 (m, 2H), 2.24 (t, J = 5.4 Hz, 4H), 1.66 – 1.42 (m, 6H).
13C NMR (101 MHz, Chloroform-d) δ 156.09, 141.57, 128.15, 114.22,
68.71, 50.19, 45.10, 44.77, 37.39, 26.42, 22.92.
FTIR (KBr), cm-1: 3000 cm-1 (benzene ring C–H),
2930 and 2856 cm-1 (cyclohexyl and oxirane ring C-H),
1239 and 821 cm-1 (oxirane group C–O-C),
912 cm-1 (oxirane ring).
Synthesis of 2,2'-(((propane-2,2-diylbis(4,1-phenylene)) bis(oxy)) bis(methylene)) bis(oxirane) (DGEBA). 22.8 g
(100 mmol) of bisphenol A, 148.0 g (1600 mmol) of epichlorohydrin and
0.93 g of tetrabutylammonium bromide were charged into a 500 mL round
three-necked flask equipped with a nitrogen inlet, a condenser, a thermometer
and a magnetic stirrer. After the reactor was stirred 2 h at 90 °C, the
system was cooled down to room temperature, and the thermometer was replaced by
a separatory funnel, 30.0 g (40 wt%) of aqueous sodium hydroxide
solution were added into the reactor dropwise. The system was maintained at
room temperature for another 24 h after the aqueous sodium hydroxide solution
was completely added into the reactor. After that, the system was first diluted
by around 300 mL of chloroform, and then washed by deionized water for several
times to remove the excess sodium hydroxide. Then, the mixture was separated
into two layers in a separatory funnel, and the organic layer was collected and
dried by anhydrous MgSO4. A viscous and transparent liquid was
obtained after removing the solvent at 50 °C under vacuum (yield: 85%).
1H NMR (400 MHz, DMSO-d6) δ 7.26 – 7.00 (m, 4H), 6.94 – 6.75 (m,
4H), 4.26 (dd, J = 11.3, 2.7 Hz, 2H), 3.80 (dd, J = 11.3, 6.5 Hz,
2H), 3.30 (tt, J = 6.5, 2.7 Hz, 2H), 2.91 – 2.76 (m, 2H), 2.69 (dd, J
= 5.1, 2.6 Hz, 2H), 1.58 (s, 6H).
13C NMR (101 MHz, DMSO-d6) δ 156.48, 143.37, 127.90, 114.33, 69.31,
50.21, 44.21, 41.63, 31.14.
FTIR (KBr), cm-1: 3000 cm-1 (benzene ring C–H),
2930 and 2856 cm-1 (methyl and oxirane ring C-H), 1227
and 826 cm-1 (oxirane group C–O-C),
913 cm-1 (oxirane ring).
Preparation
of the Cured Epoxy Resins. Each of the epoxy
monomer was mixed with HMMPA at a fixed molar ratio of 1:1, and the mixture was
dissolved in a small amount of chloroform or dimethyl sulfoxide at room
temperature to get a light yellow liquid. Then the mixture was transferred to a
steel mold and degassed for 30 min, followed by step-by-step heating at 120,
140, 160 and 180 °C for 2 h, respectively, and the obtained products were
marked as DHBPH-HMMPA, DHPCH-HMMPA and DGEBA-HMMPA.
Characterizations. Nuclear magnetic
resonance (NMR) spectra were performed on a Bruker AVANCE III 400 MHz at
room temperature using chloroform-d or DMSO-d6 as the solvent.
Fourier transform infrared (FTIR) spectra were obtained on a Thermo Scientific
Nicolet iS30. Differential scanning calorimetry (DSC) was recorded on a NETZSCH
DSC 214 under protection of high purity nitrogen gas, and the heating rate was
10 °C min-1. Thermogravimetric analysis (TGA) was conducted on
a Mettler-Toledo TGA/DSC1 thermogravimetric analyzer under pure nitrogen and
pure air, and flow rate was fixed at 50 mL min-1. Around 5 mg of
cured epoxy sample was charged into an 80 μL corundum crucible and heated from
50 to 800 °C with a fixed heating rate of 20 °C min-1.
Dynamic thermomechanical analysis (DMA) was performed on a TA Instruments Q800
under tensile mode with the amplitude of 2 μm, and the data were collected
from 0 to 250 °C at rate of 3 °C min-1. Tensile tests were
carried out on an Instron 5567 machine with a stretching rate of 2 mm·min-1
at room temperature. The samples were prepared as rectangular shape, and the
dimensions were 25×5×2 mm (length×width×thickness). For each sample, three
specimens were tested, and the average value is taken as the tensile strength.
The tensile sections of the samples were observed on a ZEISS EVO18 scanning
electron microscope (SEM), and the sections of samples were sprayed gold for 12
min before testing.
Design,
Synthesis and Characterization of DHPCH, DHBPH and DGEBA. In this work,
three model epoxy monomers were synthesized by a commonly reported route,19,20
that
is a general glycidylation reaction of phenolic hydroxyl with excess
epichlorohydrin in the presence of sodium hydroxide and tetrabutylammonium
bromide (Scheme 2). The chemical structure of obtained epoxy monomer is firstly
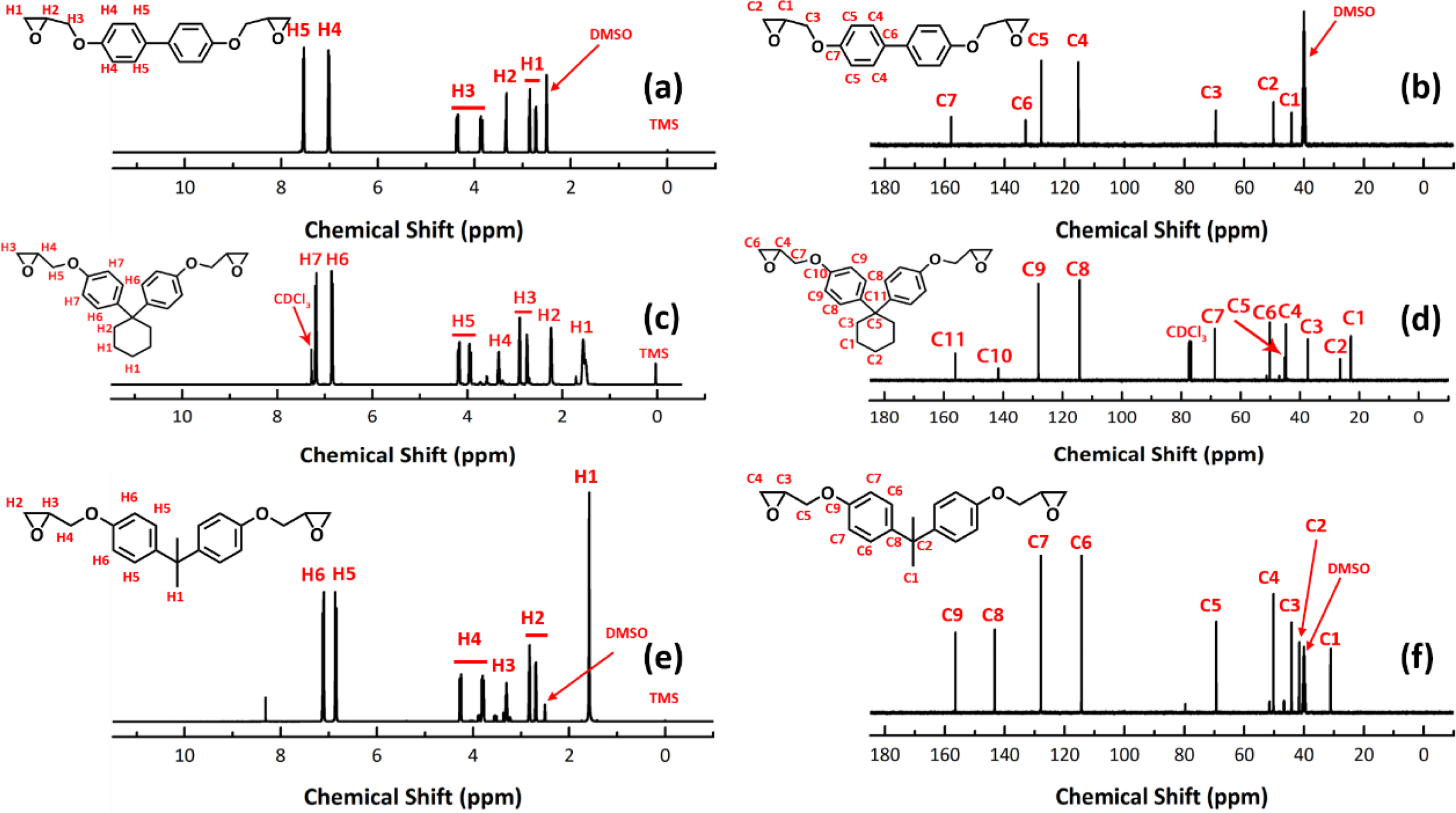
characterized by means of 1H NMR and 13C NMR (Figure 1).
Note that, depending on the solubility, NMR of DHPCH was recorded in
chloroform-d, whereas the NMR spectra of DHBPH and DGEBA were obtained
in DMSO-d6. In the 1H NMR, the three peaks at around 2.4, 2.6
and 3.2 ppm correspond to the three protons in oxirane groups (Figure 1(a),
1(c) and 1(e)).21 The two peaks at about 6.7 and 7.2 ppm belong to
the protons of benzene. Two additional peaks at 1.5 and 2.2 ppm (marked as H1
and H2) are attributed to the protons in cyclohexyl group of DHPCH (Figure
1(c)), and a large peak appears at 1.6 ppm (marked as H1) in Figure 1(e) is
ascribed to protons in methyl group of DGEBA. The peaks appear at 4.3 and 4.8
ppm in Figure 1(a), 1(c) and 1(e) are attributed to the protons of -CH2-
groups. The 13C NMR spectra of DHBPH, DHPCH and DGEBA are
exhibited in Figure 1(b), 1(d) and 1(f). As it is shown, the peaks at 43.5 and
50.1 ppm are attributed to the carbons in oxirane group, and signal at around
68.7 ppm corresponds to the carbons of -CH2- group
that connected to oxirane group.22 Moreover, all other peaks are
assigned to corresponding carbons accordingly.
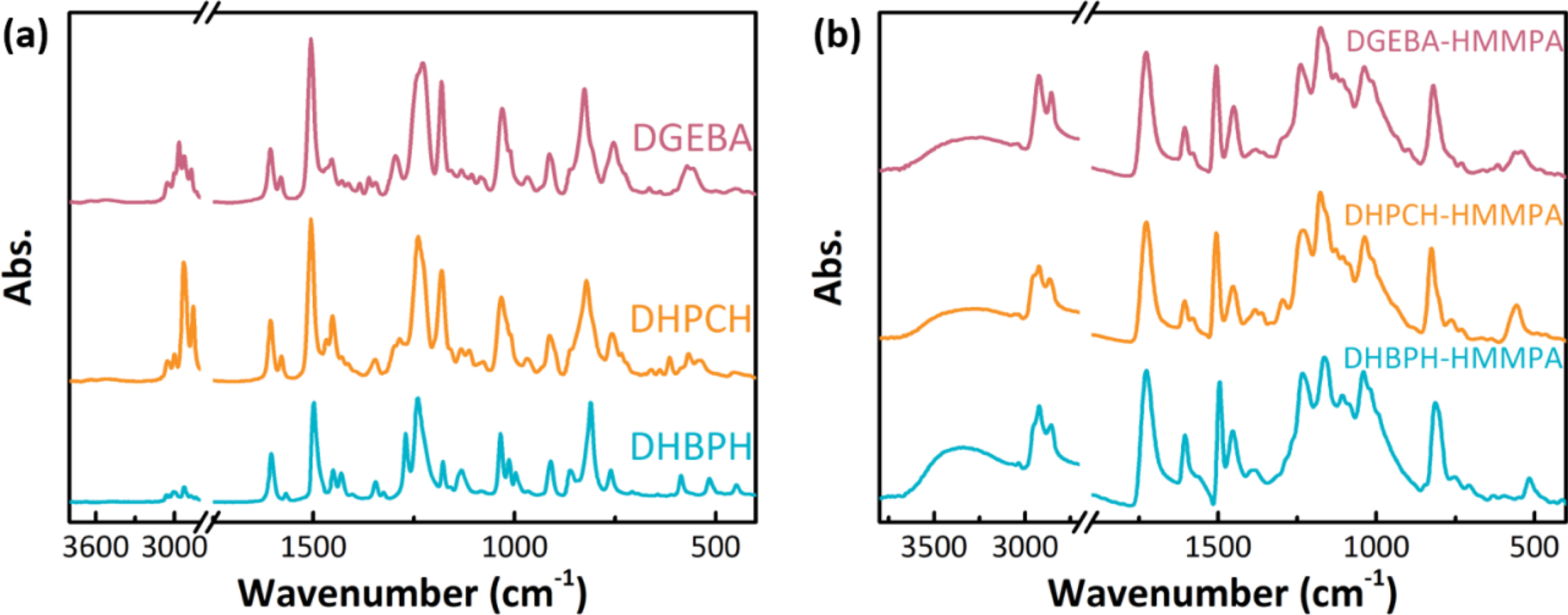
In addition to the NMR, the FTIR spectroscopy was also employed to
further inspect the chemical structures both of monomers and cured resins
(Figure 2). The characteristic peaks of the oxirane group has appeared at
around 1240, 910 and 810 cm-1, which correspond to the vibration
of C-O-C ring.23,24 The absorption bands display
at 1573 cm-1 corresponds to the skeleton vibration in the benzene
ring, and the signals at about 1503 cm-1 are ascribed to the C-H in
plane bending vibration in the benzene ring (Figure 2(a)).25 It is
worth nothing that the bands correspond to asymmetric and symmetric stretching
vibration of C-H display at about 2970 and 2964 cm-1,25
and the two bands are more obvious in FTIR spectra of DHPCH and DGEBA than that
of DHBPH. This is due to the presence of extra alkyl group in DHPCH (cyclohexyl
group) and DGEBA (methyl group). After curing reaction, the characteristic
bands of oxirane ring have disappeared in all FTIR spectra (Figure 2(b)),
indicating the complete curing of epoxy monomers. Moreover, new signals appear
at about 1700 cm-1 are attributed the stretching vibration of C=O
in curing agent. The broad bands centered at 3300 cm-1 are due to
the presence of hydroxyl groups which may further form hydrogen bonds in cured
epoxy resins. With testing results of NMR and FTIR, it is concluded that the
target epoxy monomers in high purity has been successfully synthesized and
fully cured by HMMPA.
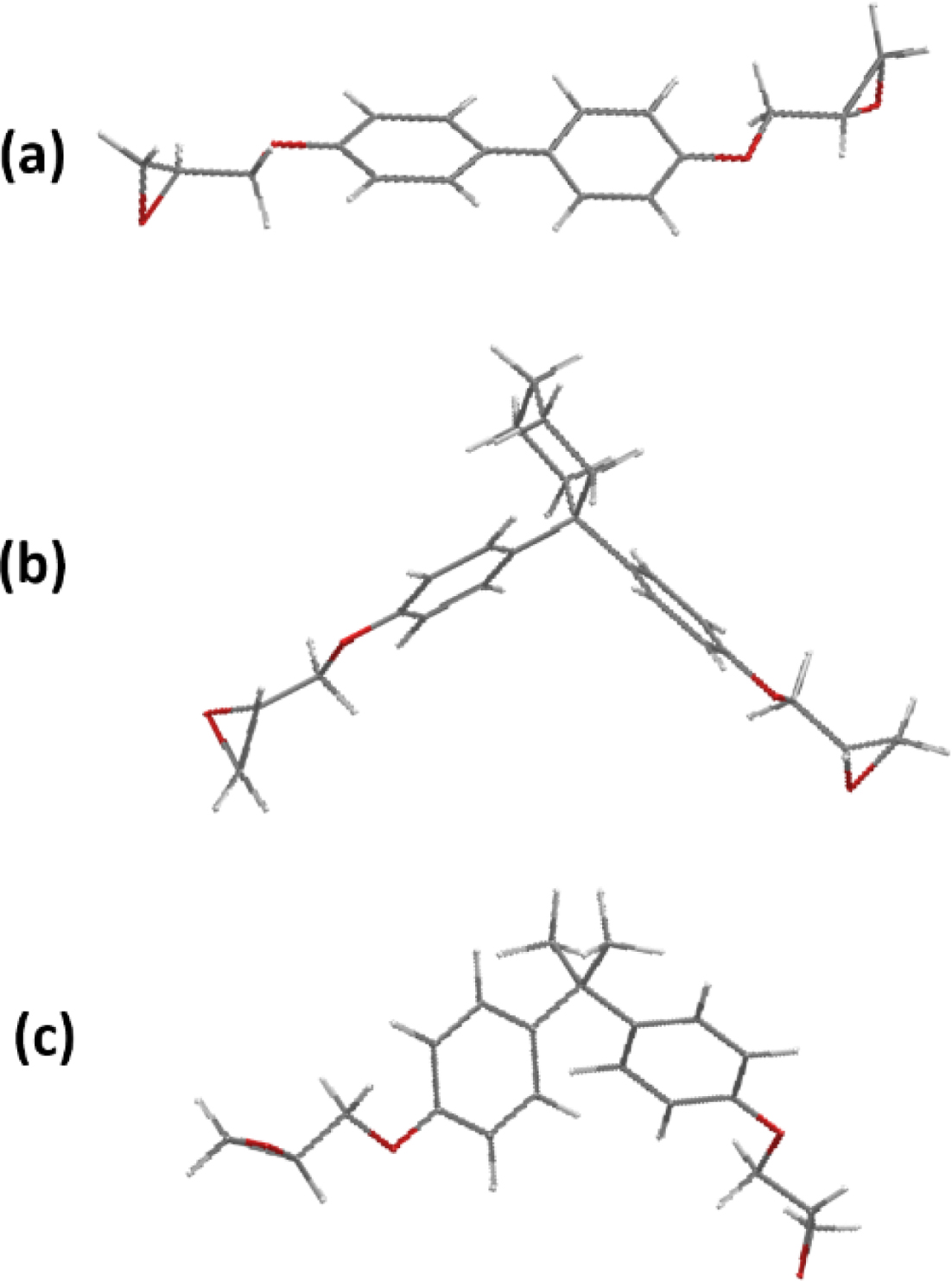
Moreover, the 3D chemical structures of DHBPH, DHPCH and DGEBA are
presented in Figure 3. With the visual geometry of the three model epoxy
monomers, it is easy to find that the DHBPH is the most stretched and linear
one among the three model epoxy monomers, furthermore, the DHBPH almost shows
almost no distortion, indicating that its bridge group (C-C
single bond) does not bring any discernible steric hindrance to DHBPH. In
contrast to the DHBPH, the other two epoxy monomers are twisted out of the
molecular plane due to the presence of the different bridge groups (Figure 3).
It should be noted that the DGEBA displays the most twisted configuration among
them, due to the steric hindrance resulted from its bridge group.11
Thus, it is easy to find that the bridge groups of the three model epoxy
monomer can impose significantly varied steric hindrance on corresponding
DHBPH, DHPCH and DGEBA, which can be further approved by testing the
thermomechanical properties of corresponding cured epoxy resins.
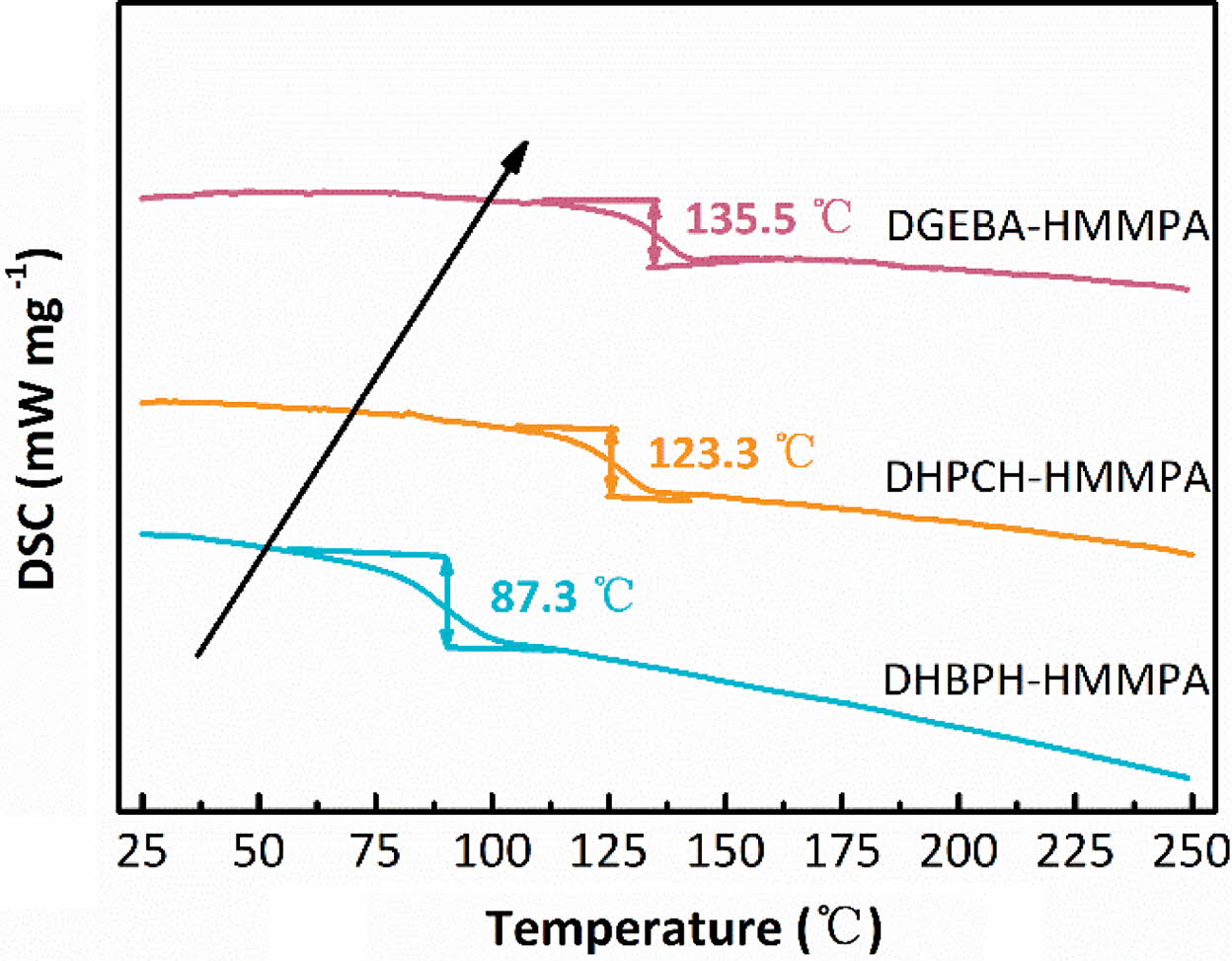
DSC Analysis of Different Cured Epoxy Resins. In order to have a
better understanding of the thermal performances of the cured resins, the
thermal properties of DHBPH-HMMPA, DHPCH-HMMPA and DGEBA-HMMPA were monitored
and assessed using a DSC and a TGA. For evaluation of the Tg,
typical DSC curves of cured epoxy resins are obtained after eliminating the
heat history and heating in a dynamic process at heating rate of 10 K min-1
under protection of high purity nitrogen gas (Figure 4).
Obviously, except for a parallel movement of the baseline toward the
endothermic direction, neither exothermic peak nor endothermic peak has been
observed in all DSC curves (Figure 4), suggesting the completeness of the
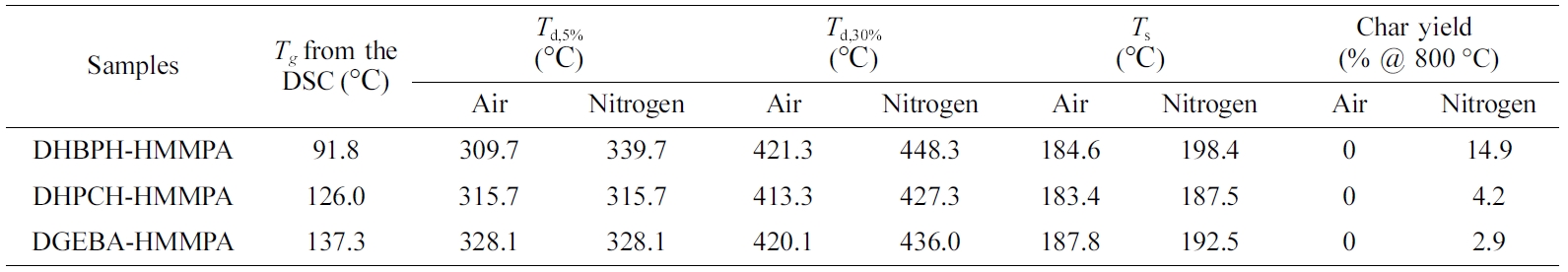
curing reaction. Moreover, the Tg values are 87.3, 123.3 and 135.5 °C
for DHBPH-HMMPA, DHPCH-HMMPA and DGEBA-HMMPA, respectively (Table 1). As it is
shown, the DHBPH-HMMPA shows the lowest value of Tg
(87.3 °C), and this is probably due to the biphenyl groups can rotate
freely around the single bond. By contrast, the presence of the cyclohexyl
group and isopropyl group in DHPCH and DGEBA lay significant steric hindrance
on cured epoxy resins, which further severely restricts motion of the chain
segments, and thus the Tg values of DHPCH-HMMPA and
DGEBA-HMMPA are increased apparently. The phenomenon is also reported by other
researchers, for example Balizer and coworkers used hindered and unhindered
cure agent to cure DGEBA, and they found that using methyl group to substitute
a hydrogen atom would apparently enhance the Tg of cured
epoxy resin, and Moreover, other research group also reported similar results.26,27
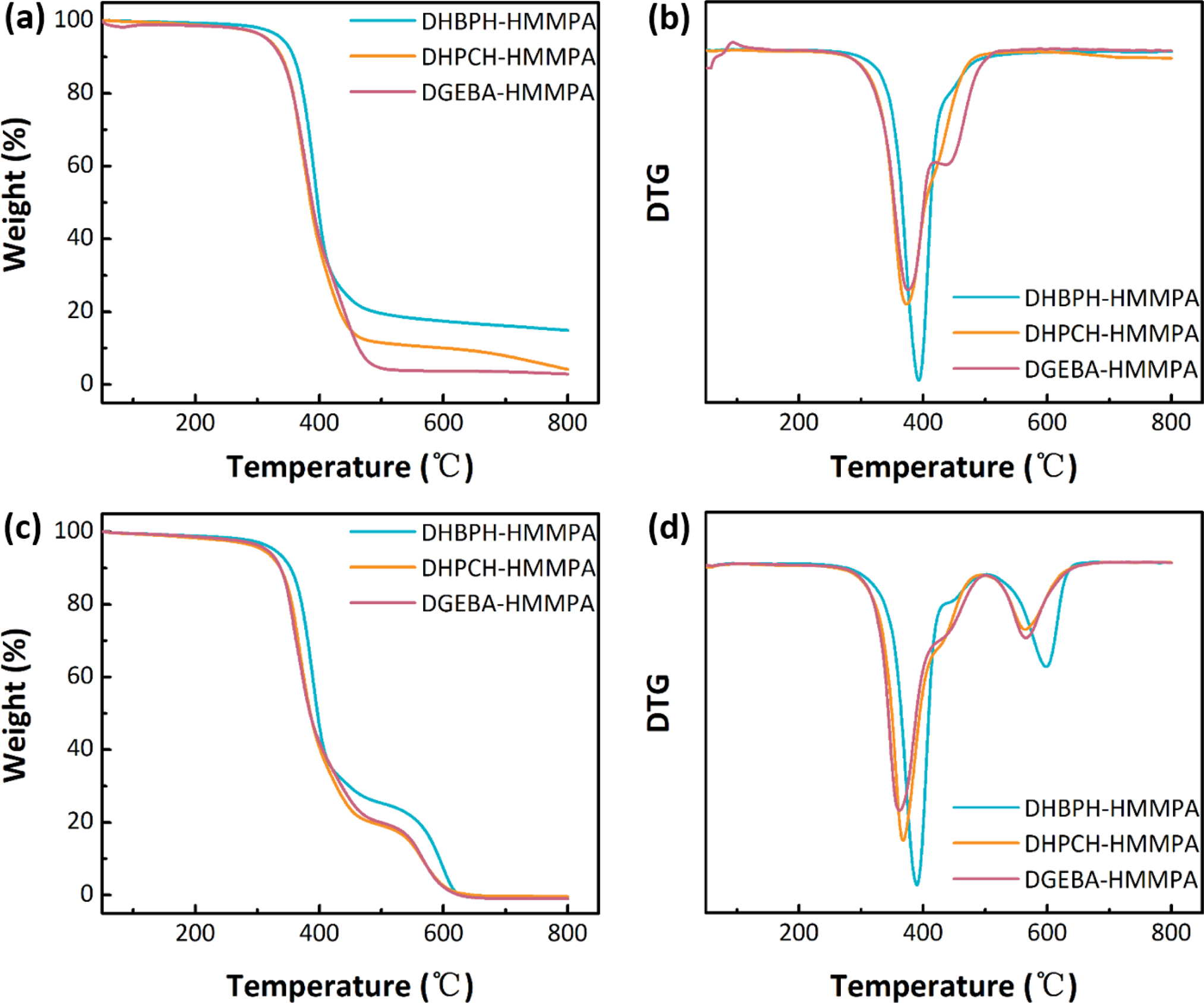
Thermal degradation processes of three cured epoxy resins both in air and
nitrogen atmospheres were inspected by TGA (Figure 5). The Td,5%
is taken as the characteristic temperature for evaluating the thermal stability
of each cured epoxy resin, which has been listed in Table 1. Interestingly,
although the DHBPH-HMMPA gives the highest Td,5% value,
it seems that introduction of large bridge group can reduce the influences of
the atmosphere on thermal stability of cured epoxy resin because compared with
the DHBPH-HMMPA, the Td,5% values of DHPCH-HMMPA and
DGEBA-HMMPA show no change both in air and nitrogen, whereas the Td,5%
of DHBPH-HMMPA lower 30 °C when the atmosphere changes from nitrogen to air.
Moreover, due to the high percent of aromatic groups in DHBPH-HMMPA, it
demonstrates the highest percentage of char yield at 800 °C in nitrogen among
three cured epoxy resins.
To make a quantitative comparison of thermal stability, heat-resistant
index temperature (Ts eq. (1))28 is further
employed to evaluate thermal stability of cured epoxy resin.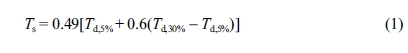
where Ts is the statistic heat-resistant index
temperature, Td,5% and Td,30% are
the temperatures at 5% and 30% weight loss, respectively (Table 1). The
calculated Ts values again demonstrate the best thermal
stability of DHBPH-HMMPA among three cured epoxy resins.
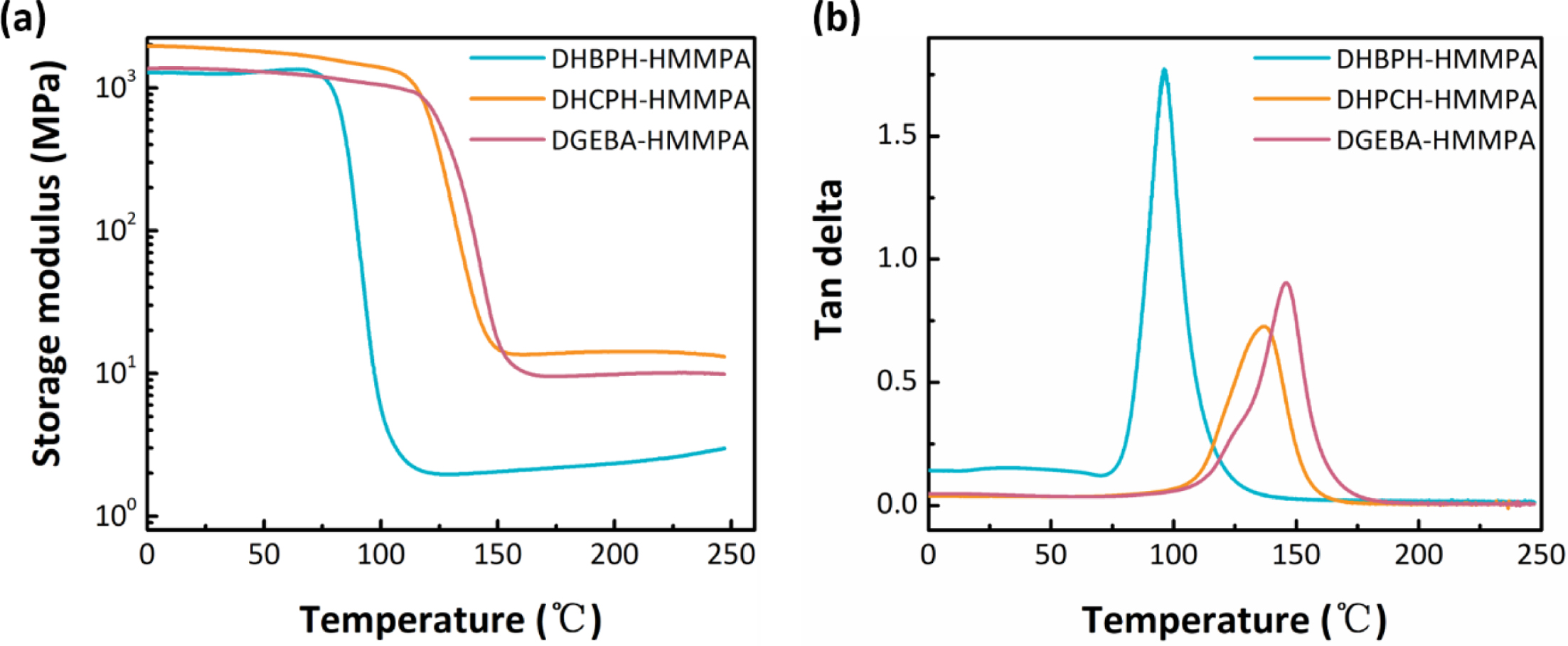
Dynamic
Mechanical Properties of Different Cured Epoxy Networks. Figure 6(a) and 6(b) show the dependence of storage modulus and tan δ of
the cured epoxy resins on temperatures. It is observed one-step decrease of the
storage modulus for all cured epoxy resins (Figure 6(a)). The values of storage
modulus for DHBPH-HMMPA, DHPCH-HMMPA and DGEBA-HMMPA are 1.3, 1.9 and
1.4 GPa at room temperature, indicating that introduction of the bridge group
with large size can evidently enlarge the rigidity of the cured resins.
Moreover, Li et al reported that enlarging the size of the bridge group
would lead to longer inter-segment distance, larger steric hindrance, worse
chain movement and bigger storage modulus value,15 which is
consistent with the results observed in this contribution.
The glass transition temperatures (Tg) for three cured
epoxy networks are determined by the peak temperatures of tan δ as a function
of temperature (Figure 6(b)), which are 95.6, 136.6 and 145.6 oC,
respectively. Obviously, the Tg values obtained from DMA are
in good agreement with the results of DSC test. Again, one may notice that the
cyclohexyl-bridged (DHPCH) and isopropyl-bridged (DGEBA) epoxy exhibited higher
Tg values than that of single carbon-carbon bond bridged
epoxy. This can also be ascribed to the motion resistance imparted by
cyclohexyl and isopropyl groups to the crosslinked networks.
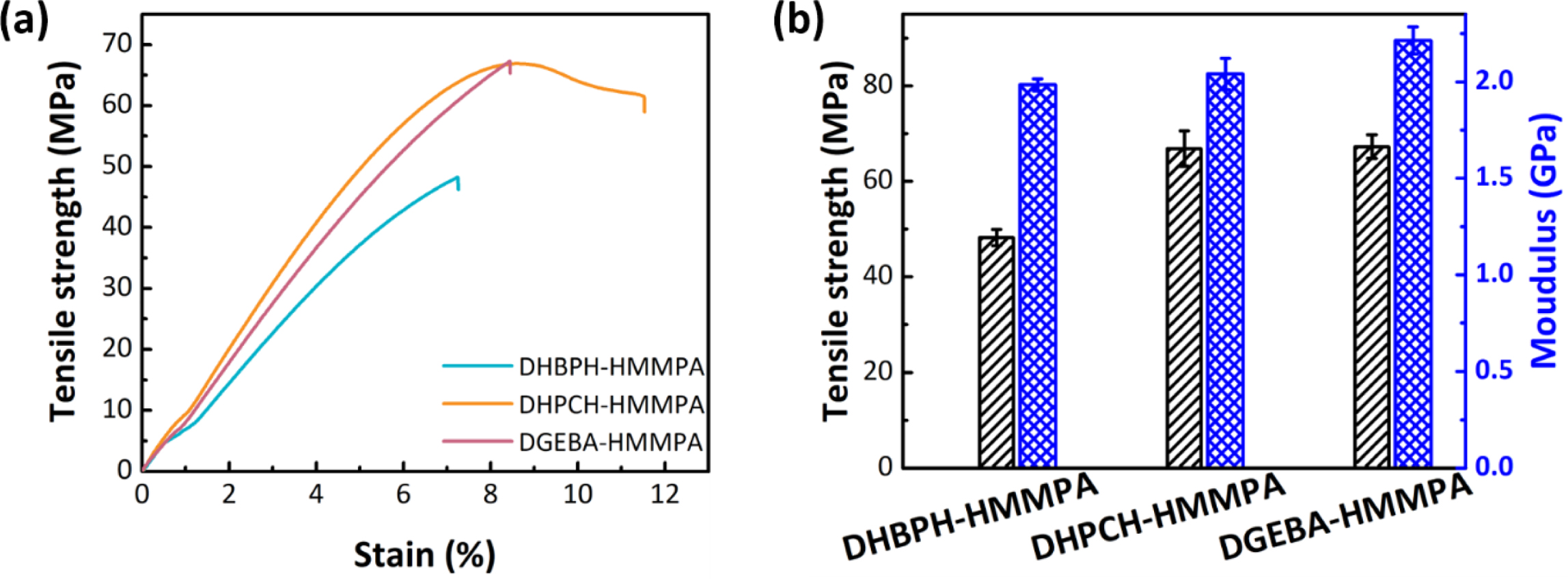
Mechanical
Properties of Different Cured Epoxy Networks. The influences of
the bridge groups on mechanical performances of cured epoxy resins were
appraised by tensile properties of standard specimens (Figure 7).
Typical stress-strain curves for each cured epoxy resins are displayed in
Figure 7(a), as it is shown, the DHPCH-HMMPA and DGEBA-HMMPA show higher
tensile strength and larger elongation at break than those of DHBPH-HMMPA.
Moreover, the elongation at break value of DHPCH-HMMPA is larger than that of
DGEBA-HMMPA, which is probably due to the fact the boat and chair conformation
transformation of cyclohexyl group in DHBPH-HMMPA, and thus endow the
DHBPH-HMMPA with more chain mobility.29 Moreover, the higher tensile
strength values of DHPCH-HMMPA and DGEBA-HMMPA than that of DHBPH-HMMPA is
attributed to the fact that the bulky bridge group can increase the tensile
strength of cured epoxy resin by restricting chain movement. It should be noted
that the tensile strength of DHPCH-HMMPA and DGEBA-HMMPA are quite similar to
each other, indicating the cyclohexyl and isopropyl may have the similar
effects on enhancement of the tensile strength. When refer to the toughness of
the DHPCH-HMMPA and DGEBA-HMMPA, the DHPCH-HMMPA exhibits more admired tensile
toughness than that of DGEBA-HMMPA because of the deformation of the cyclohexyl
ring.30
The tensile strength and Young’s modulus (average value) of three cured
epoxy resins are shown in Figure 7(b). As expected, for epoxy resins containing
bulky bridge groups (DHPCH-HMMPA and DGEBA-HMMPA) exhibit higher tensile
strength and Young’s modulus than those of the DHBPH-HMMPA. It is easy to
conclude from the results of the mechanical tests that introducing bulky bridge
group into the chemical structure of epoxy monomer is an efficient route to
improve the mechanical properties of corresponding cured epoxy resin.
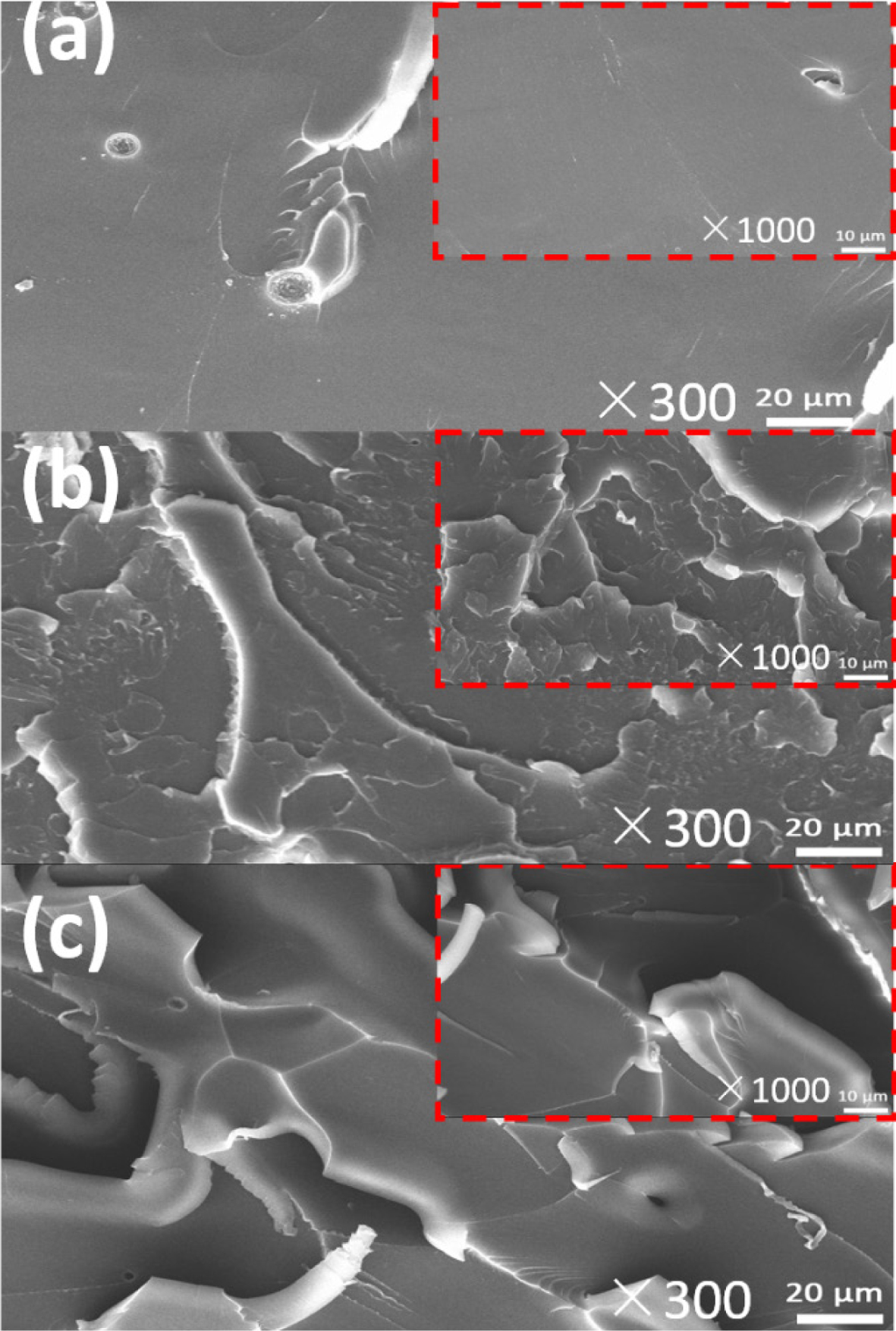
Morphological
Properties of the Different Cured Epoxy Networks. Microscopic
appearances of the fractured surfaces of three cured epoxy resins after the
tensile testing were recorded by a SEM (Figure 8). As it is shown the epoxy
bridged only with a single carbon-carbon bond (DHBPH-HMMPA) exhibits a smooth,
glass-like, and very flat surface, which is the typical character of brittle
fracture.31 Comparatively, both the DHPCH-HMMPA and DGEBA-HMMPA
demonstrate very rough fractured surfaces, suggesting better toughness than
that of DHBPH-HMMPA. Since the chemical structures of three epoxy monomers are
very similar, it is reasonable to deduce that the bulky bridge groups in DHPCH
and DGEBA are responsible for the increased toughness of the DHPCH-HMMPA and
DGEBA-HMMPA. Moreover, the images obtained by SEM are also consistent with the
results for the mechanical properties of the epoxy resins.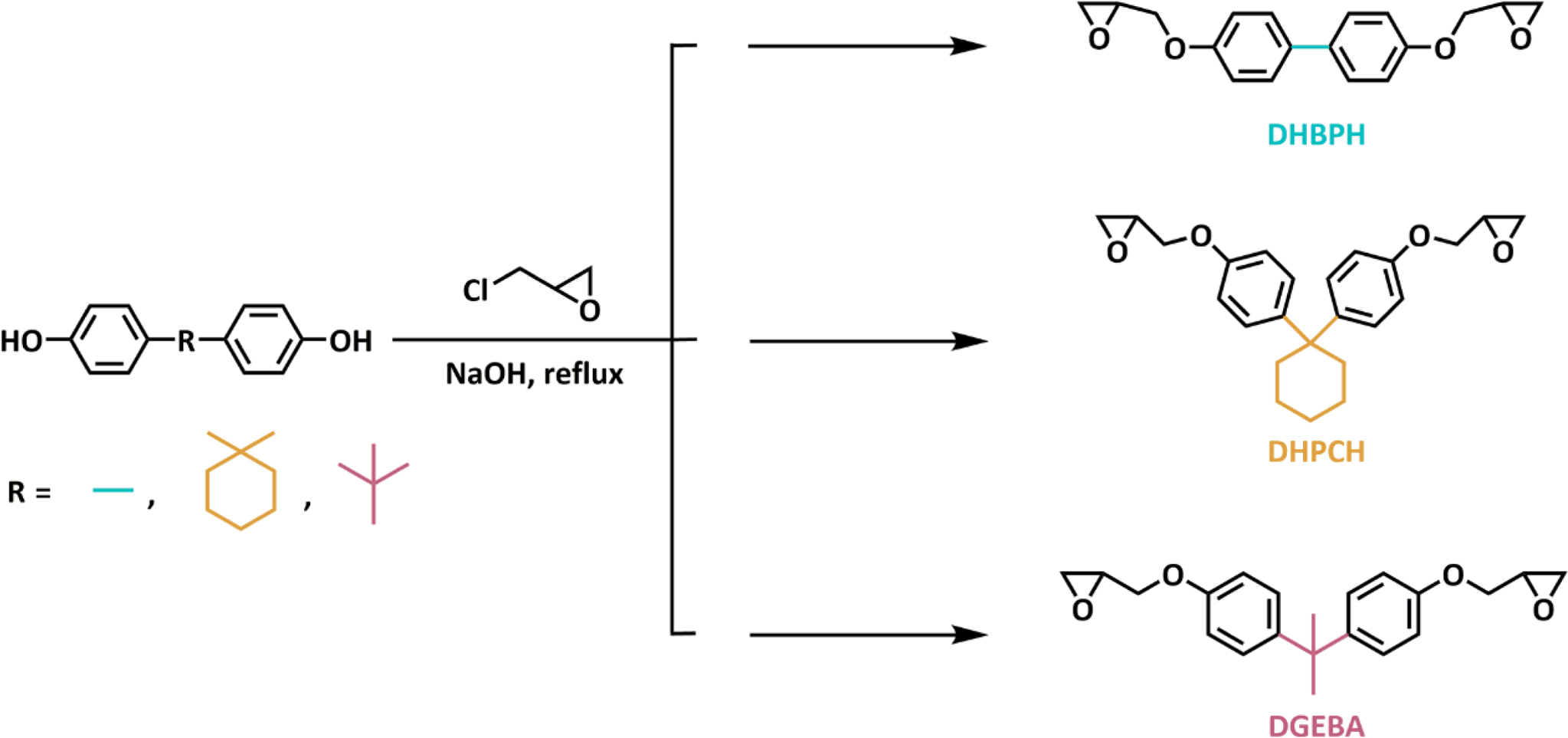
Scheme 2. Synthetic route for DHPCH, DHBPH and DGEBA.
|
Figure 1 NMR spectra of DHBPH, DHPCH and DGEBA: (a) 1H NMR of DHBPH; (b) 13C NMR of DHBPH; (c) 1H NMR of DHPCH;(d) 13C NMR of DHPCH; (e) 1H NMR of DGEBA; (f) 13C NMR of DGEBA. |
|
Figure 2 FTIR spectra of monomers and cured epoxy resins: (a) epoxy monomers; (b) cured epoxy resins. |
|
Figure 3 3D chemical structures of the three model epoxy monomers. |
|
Figure 4 DSC thermogram of cured epoxy resin networks. |
|
Figure 5 TGA and DTG curves obtained both in nitrogen ((a) and (b)); in air ((c) and (d)) for DHBPH-HMMPA, DHPCH-HMMPA and DGEBA-HMMPA. |
|
Figure 6 DMA curves of different cured epoxy resins: (a) storage modulus as a function of temperature; (b) tan δ as a function of temperature. |
|
Figure 7 Tensile properties of three cured epoxy networks: (a) stress-strain curves of one of splines; (b) tensile strength and Young's modulus. |
|
Figure 8 SEM images of tensile fracture of cured epoxy resins: (a) DHBPH-HMMPA; (b) DHPCH-HMMPA; (c) DGEBA-HMMPA. |
|
Table 1 Parameters Obtained from the DSC and TGA Curves of DHBPH-HMMPA, DHPCH-HMMPA and DGEBA-HMMPA |
Three model epoxy monomers (DHBPH, DHPCH and DGEBA) containing different
bridge groups were carefully synthesized to evaluate the effects of bridge
group on properties of cured epoxy resins. It is found the cured epoxy resins
containing bulky bridge groups demonstrate higher Tg than the
one only bridged with a single carbon-carbon bond. Moreover, the DMA and
mechanical measurements demonstrate the bulky bridge groups can also endow the
cured epoxy resins with admirable mechanical performances. The results
presented in this contribution can be a meaningful guidance for establishing the
relationship between the bridge group and the thermomechanical properties of
corresponding cured epoxy resin.
- 1. B. Ramezanzadeh, S. Niroumandrad, A. Ahmadi, M. Mahdavian, and M. H. M. Moghadam, Corros. Sci., 103, 283 (2016).
-

- 2. Y. Hong, S. Jung, and J. Choi, Polym. Korea, 31, 526 (2007).
- 3. L. Zhai, G. Ling, J. Li, and Y. Wang, Mater. Lett., 60, 3031 (2006).
-

- 4. S. Rimdusit and H. Ishida, Polymer, 41, 7941 (2000).
-

- 5. Q. Shi, K. Yu, X. Kuang, X. Mu, C. K. Dunn, M. L. Dunn, T. Wang, and H. J Qi, Mater. Horiz., 4, 598 (2017).
-

- 6. J. Liang, Y. Wang, Y. Huang, Y. Ma, Z. Liu, J. Cai, C. Zhang, H. Gao, and Y. Chen, Carbon, 47, 922 (2009).
-

- 7. S. Kumar, S. K. Samal, S. Mohanty, and S. K. Nayak, Polym-Plast. Technol., 57, 133 (2018).
-

- 8. G. Zhou, W. Wang, and M. Peng, Polymer, 163, 20 (2019).
-

- 9. Y. J. Woo and D. S. Kim, Polym. Korea, 43, 359 (2019).
-

- 10. J. A. Schroeder, P. A. Madsen, and R. T. Foister, Polymer, 28, 929 (1987).
-

- 11. J. Y. Lee, J. Jang, S. M. Hong, S. S. Hwang, and K. U. Kim, Polymer, 40, 3197 (1999).
-

- 12. J. Y. Lee and J. Jang, Polymer, 47, 3036 (2006).
-

- 13. J. Y. Lee, J. Jang, S. S. Hwang, S. M. Hong, and K. U. Kim, Polymer, 39, 6121 (1998).
-

- 14. P. Jain, V. Choudhary, and I. K. Varma, Eur. Polym. J., 39, 181 (2003).
-

- 15. G. Pan, Z. Du, C. Zhang, C. Li, X. Yang, and H. Li, Polym. J., 39, 478 (2007).
-

- 16. Y. Li, P. Badrinarayanan, and M. R. Kessler, Polymer, 54, 3017 (2013).
-

- 17. S. Han, W. G. Kim, H. G. Yoon, and T. J. Moon, J. Polym. Sci., Part A: Polym. Chem., 36, 773 (1998).
-

- 18. W. Erich and M. J. Bodnar, J. Appl. Polym. Sci., 3, 296 (1960).
-

- 19. M. Shibata and T. Ohkita, Eur. Polym. J., 92, 165 (2017).
-

- 20. F.-L. Jin, H.-C. Liu, B. Yang, and S.-J. Park, J. Ind. Eng. Chem., 24, 20 (2015).
-

- 21. L. Kong, Y. Cheng, Y. Jin, T. Qi, and F. Xiao, J. Appl. Polym. Sci., 133, 43456 (2016).
-

- 22. J. Cao, H. Fan, B.-G. Li, and S. Zhu, Polymer, 124, 157 (2017).
-

- 23. M. Sanchezsoto, P. Pages, T. Lacorte, K. Briceno, and F. Carrasco, Compos. Sci. Technol., 67, 1974 (2007).
-

- 24. R.-H. Lin, J. Polym. Sci., Part A: Polym. Chem., 38, 2934 (2000).
-

- 25. T. Wu, Y. Li, Q. Wu, L. Song, and G. Wu, Eur. Polym. J., 41, 2216 (2005).
-

- 26. E. Balizer and J. V. Duffy, Polymer, 33, 2114 (1992).
-

- 27. J. V. Duffy and G. F. Lee, J. Appl. Polym. Sci., 35, 1367 (1988).
-

- 28. J. Gu, X. Yang, Z. Lv, N. Li, C. Liang, and Q. Zhang, Int. J. Heat Mass Transfer, 92, 15 (2016).
-

- 29. W. S. Johnson, V. J. Bauer, J. L. Margrave, M. A. Frisch, L. H. Dreger, and W. N. Hubbard, J. Am. Chem. Soc., 83, 606 (1961).
-

- 30. A. Chandramohan and M. Alagar, Int. J. Polym. Anal. Charact., 18, 73 (2013).
-

- 31. M. R. Ayatollahi, S. Shadlou, and M. M. Shokrieh, Eng. Fract. Mech., 78, 2620 (2011).
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2020; 44(4): 415-424
Published online Jul 25, 2020
- 10.7317/pk.2020.44.4.415
- Received on Nov 26, 2019
- Revised on Mar 20, 2020
- Accepted on Mar 25, 2020
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Luo Jun
-
Engineering Research Center for Materials Protection of Wear and Corrosion of Guizhou Province, Guiyang University, Guiyang 550005 (P. R. China)
- E-mail: luojun_gyu@sina.com
- ORCID:
0000-0002-1444-576X









 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.