- Standard Energy Computations of Cr(VI) Adsorption via Dissociated Products of Carbonic Acid
Soon Hong Lee, Joon Hyuk Lee*, Joo Young You, Woo Jeong Lee, Jeong Min Kwon, and Sang Sun Choi†

Department of Environmental Engineering, Anyang University, Anyang 14028, Korea
*Department of Chemical Engineering, Hanyang University, Seoul 04763, Korea- 탄산의 해리에 따른 Cr(VI) 흡착거동 및 표준 에너지 변화연구
안양대학교 환경공학과, *한양대학교 화학공학과
The selective utilization of
dissociated products of carbonic acid to induce either pH control or adsorption
of heavy metal ions on the surface of an adsorbent could be generally achieved
by the gas-phased mixture of CO2 and H2O (CHM). Given
that the reduction of chromium (Cr(VI)) has occupied a growing niche within
industrial sectors, we present a set of computation data to observe standard
energies and the adsorption behavior via CHM under the aqueous solution.
Herein, activated carbon fibers (ACF) were adapted as a skeleton absorbent.
With a cross-validation on physicochemical characteristics, thus-produced
sample (CACF) revealed a decrement in the specific surface by 9.58%, but also
experienced an increment of 33.40% in surface functional moieties compared to
ACF. In terms of adsorption capacity, CACF showed 6.36% more Cr(VI) adsorption
capacity than ACF with 200 mg of initial adsorbent amount at 323 K. Based on our calculations, the Langmuir isotherm
was favored by all samples. Adsorption behavior was found to be spontaneous and
feasible in nature over certain temperature. Lastly, thermodynamic parameters
successfully underpinned that the physio-sorption was found to be dominant
compared to the chemisorption,
증기 상태에서의 CO2와 H2O 혼합물(CHM)은 일시적으로 생성되는 탄산해리의
부산물로 pH를 조정하거나 중금속 이온의 표면흡착에 유용화될 수 있다.
산업계에서 Cr(VI)에 대한 제거수요가 증대됨을 인지하였을 때, 본 연구에서는 CHM과
Cr(VI) 간 발생하는 표준 에너지 및 흡착 동역학을 고찰하였다. 여기에서, 활성탄소섬유(ACF)가 흡착제로 사용되었다. 물리화학적 거동에 대한 상호분석 결과, CHM이 적용된 ACF인 CACF는 기존 ACF 대비 9.58%의 비표면적 감소 및 33.40%의 산소 관능기 증대를 가져왔다. 특히, Cr(VI)에 대한 흡착을
200 mg의 흡착제와 323 K 조건 하에서 수행하였을 때 CACF는 기존 대비 6.36% 향상된 흡착능을 보여주었다. 흡착 동역학은 Langmuir isotherm에 부합되었으며 표준
에너지 역시 화학적 흡착보다는 물리적 흡착이 보다 설명에 적합함을 나타내었다.
A mixture of CO2 and H2O
through gas-phase significantly increased the surface functional moieties as
shown in the figure. Herein, as-synthesized sample revealed minimum decrement
in the Brunauer-Emmett-Teller surface area, but increment in acidity.
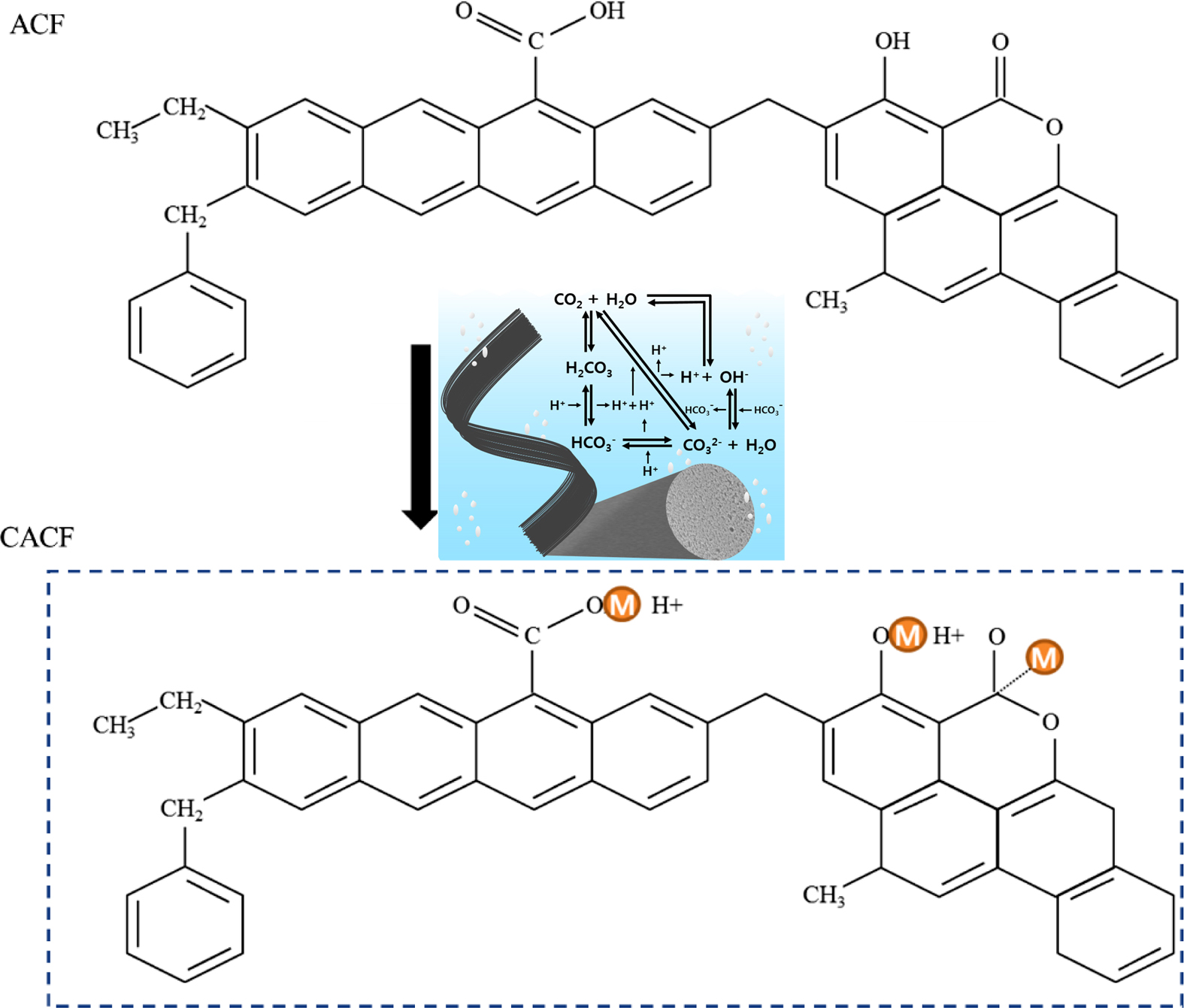
Keywords: hexavalent chromium, activated carbon fibers, kinetics, thermodynamics, physio-sorption
This research acknowledges the sabbatical support
given by Anyang University.
Heavy metal contamination of surface water bodies and ground water has
become a serious environmental concern worldwide. At low-level exposure, the
presence of heavy metal contaminants in water could result in undesirable
consequences. In particular, hexavalent form of chromium (Cr(VI)) and its known
compounds are considered to be carcinogenic, mutagenic, and genotoxic on the
prevailing flora and fauna of the aquatic environment.1 Cr(VI) is
also defined as one of the hazardous pollutants by the US Environmental
Protection Agency in 2004.2 Therefore, adequate treatment of aqueous
Cr(VI) prior to discharge is urgent for the reduction of secondary
contamination. Technical approaches on the Cr(VI) treatment have so far been
mostly focused on the adsorption using low cost of adsorbents such as activated
carbon fibers (ACF). However, major drawbacks of raw carbonaceous adsorbents
including low adsorption capacity and large access time limit their practical
applications. Thus, surface modification is essentially required after the
activation process to introduce an ion-exchangeable environment as a supporter.
A widely accepted idea is that an acidic treatment on the carbonaceous
surface using strong acids is efficient for the feasible environment of heavy
metal removal. Whilst most of the work in this area made the use of strong
acid, we previously suggested a green candidate using an air bubble mixture of
CO2 and H2O (CHM) onto a carbonaceous skeleton.3
The notion that carbonic acid has often considered as a weak acid arising out
of (i) the non-negligible account of undissociated carbon dioxide and (ii)
short lifetime in aqueous environment was held for a long time.4 Therefore,
the use of carbonic acid and its dissociated products as a chemical feedstock
for surface modification and ion-interactions was not highly encouraged with
its low acidity (pKa; 6.4).5 Notwithstanding this, recent
studies have updated that carbonic acid may have comparable acidity to formic
acid with its pKa in a range of 3.4~3.8.6,7 With this content, one may assume the possibility of using dissociated
carbonic acid upon interaction with other ions.
Within the scope of this study, we further tracked the adsorption
behavior of Cr(VI) under computed standard energies. For a better understanding,
we also supported physiochemical properties and adsorption kinetics of ACF
before and after loaded with dissociated products of carbonic acid. Based on
the concept of capturing hazardous materials via hazardous materials, we
believe that an environmental-friendly and economic surface modification
technique compared to other strong acids by solely using the major culprit of
global warming could pave a new way to the relevant academia.
All chemicals were of analytical grade and used without any further
purification. Preparation of samples was followed by our early procedure. In
brief, CACF was prepared at a pressure of 10 bar with 373 K of CHM for 1 h through the
gas-phase (0.2 mL min-1) via a compact size of batch with 160 mm in diameter
and 400 mm in height. This procedure is to introduce surface functional
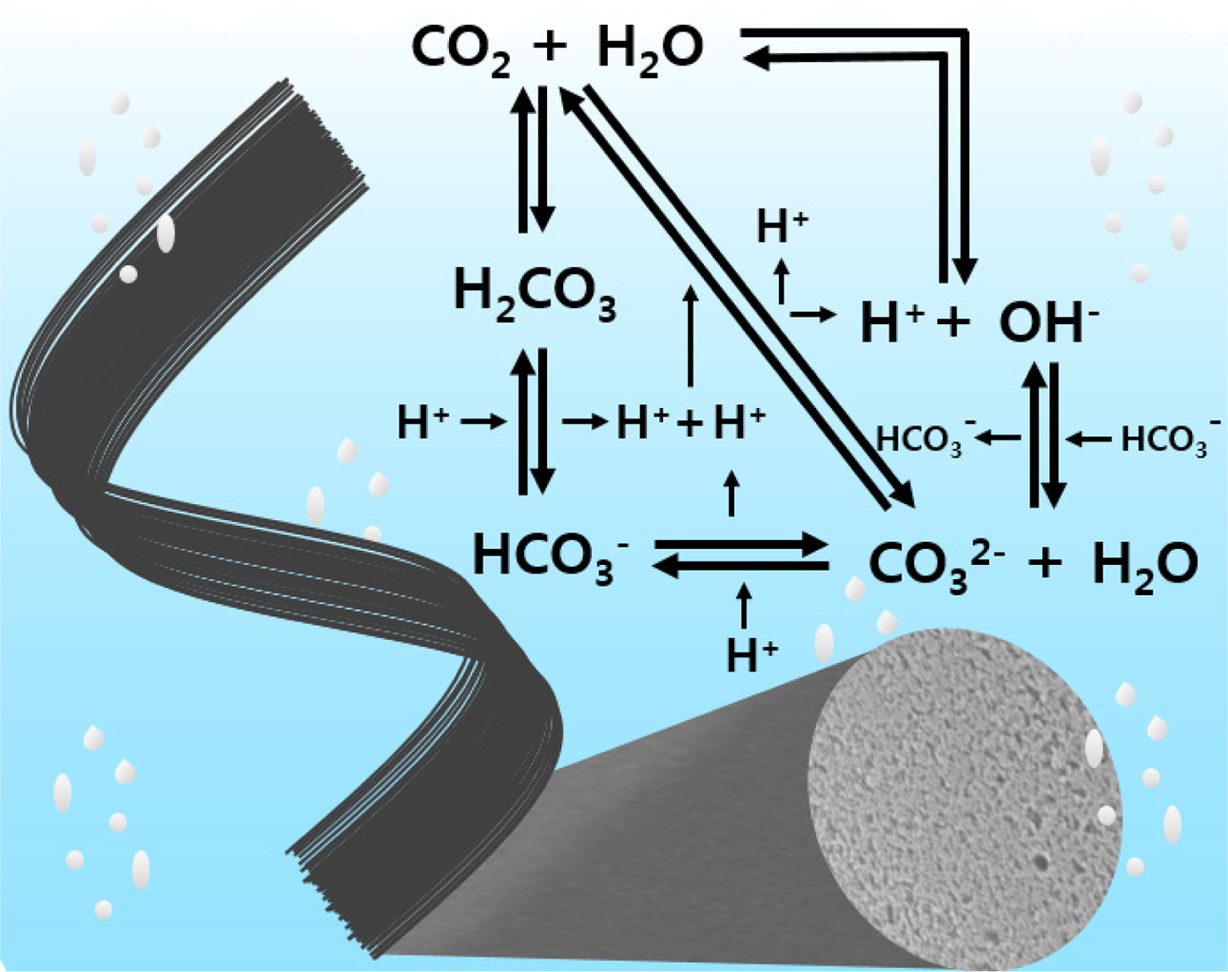
moieties from the dissociated products of carbonic acid (Figure 1). Fixed
temperature of 373 K was introduced to prevent any hot water layer around the
surface of fibers.8 Cr(VI) ion standard solution was diluted by 10
ppm of distilled water. Each sample in a range of 100~200 mg (±1 mg) was doped into
Cr(VI) containing solution after the temperature was adjusted from 303 to 323 K. Surface
characteristics were determined by using ASAP 2460 (Micromeritics). The
pretreatment was performed for 12 h through the heat degradation
at 573 K while maintaining a vaccum state of 1.33×10-3 Pa. The
specific surface area for the prepared ACF was measured by using the BET
equation. The correlation between the surface area (As) and
monolayer (Vm) is shown in the following eq. (1):
As = (Vm/22414)Nas (1)
where, Na is Avogadro number and s is the area
covered by one nitrogen molecule. The generally accepted s value is 0.162 nm2. After the BET
equation for the determination of the total specific surface area, the t-Plot
equation was used to calculate the micropore and external specific surface area
as shown in the following eq. (2):
t = (n/nm)s (2)
t, n, nm refer to the average thickness, total
adsorption amount, and monolayer adsorption amount, respectively. s is the atom size of nitrogen at 298 K. The chemical
properties were analyzed using an element analyzer with a thermal conductivity
detector (EA 1008, FISONS). The Boehm titration was performed to determine the
sum of acidity after a CHM treatment (G20, Mettler Toledo).9
Inductively coupled plasma optical emission spectroscopy (ICP-OES) was adopted
to analyze the adsorption characteristics of Cr(VI) (Optima 5300DV,
PerkinElmer). The adsorption efficiency was calculated by the following eq.
(3)-(4):
Qe = (CO - Ce)V/m (3)
R = (CO - Ce)/CO × 100(%) (4)
where Qe (mg g-1) is the equilibrium, CO
(mg L-1) is the initial concentration, Ce (mg
L-1) is the equilibrium concentration, V (L) is the volume of
aqueous solution, and m (g) is the amount of a sample.
|
Figure 1 A possible mechanism of dissociated products of carbonic acid. |
Physiochemical
Properties of Samples. Observed
physiochemical characteristics of samples are listed in Table 1. CACF
experienced inevitable decreases of 9.58% and 11.64%, respectively, in SBET
and Sm as compared to ACF, indicating the increase of surface
functional moieties which may block the access of N2 molecules onto
the fiber surface. With accordance, CACF showed higher H- and O-contents than
ACF by an increase of H- and O-containing surface functional moieties.10
Throughout the physiochemical properties, it was concluded that dissociated
products of carbonic acid were successfully loaded on the surface of CACF.
Adsorption
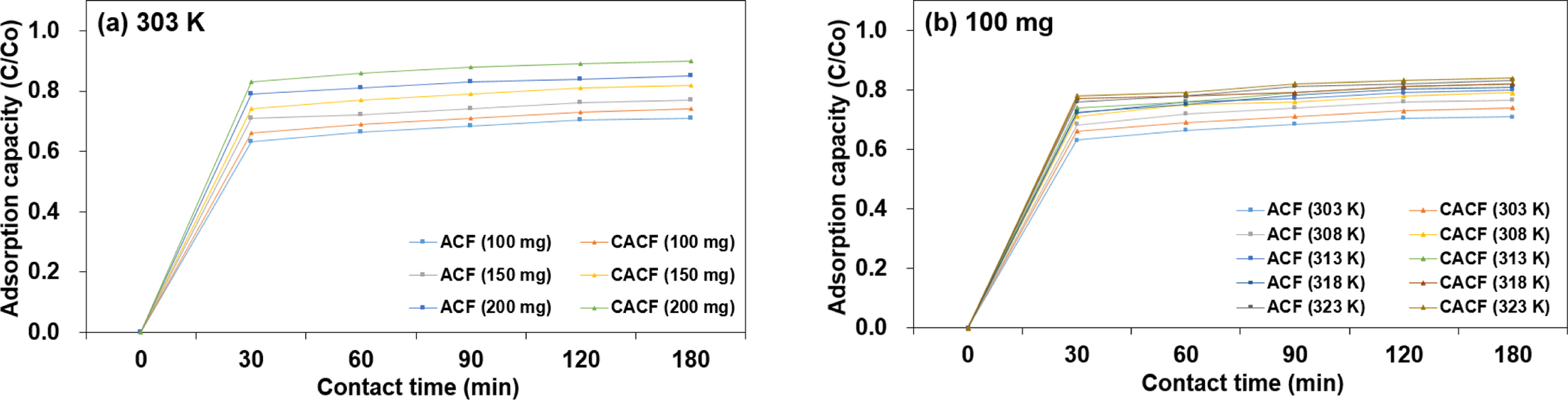
Characteristics. Adsorption plots of Cr(VI) onto samples as a function of
time by initial adsorbent amount and the temperature are depicted in Figure 2.
The direct proportional relationship between initial adsorbent amount and the
adsorption capacity could be attributed to an increased surface area which
allows more binding sites for Cr(VI) ions.11 Herein, 200 mg of
CACF was sufficient to ensure 93.24% Cr(VI) adsorption capacity at room
temperature. With an increase in the temperature from 303 to 323 K, the
maximum adsorption capacity at the equilibrium state increased from 70.10% to
83.03% and from 70.44% to 84.21% in 100 mg of ACF and CACF, respectively. This
indicates that the rate of intra-particle diffusion of Cr(VI) ions into pores
of the adsorbent is more feasible as the temperature rises.12 In all
circumstances, CACF recorded the highest increment, or “plateau” value at 180
min and the status was secured upon the equilibrium state at 240 min.
Especially, more rooms for CHM benefited the overall adsorption efficiency as
can be seen in Figure 2(a). Herein, CACF with improved surface functional
moieties offers strong electrostatic attraction between negatively charged Cr
oxy-anions and protonated sorbent, thus leads to a favorable ion-exchangeable
environment. The pH of the aqueous solution could change the heavy metal ions
into various forms to facilitate adsorption. In case of Cr(VI), HCrO4−,
Cr2O72−, Cr4O132−,
and Cr3O102− are reported to be present as
dominant species in the pH range between 2 and 6.13 Given that the
low pH leads to an increase in Cr(VI) removal, it could be concluded that the
adsorption capacity of Cr(VI) could be benefited by the increased acidity
from the dissociated products of carbonic acid.
Adsorption Kinetics and Thermodynamics. Kinetic parameters were calculated using the
Langmuir isotherm and listed in Table 2. The former was calculated using the
following eq. (5):
Ce/Qe = (Ce/Xm) + 1/(XmKe) (5)
where, Ce (mmol L-1) is the equilibrium
concentration, Qe (mmol g-1) is the equilibrium
constant, Xm (mmol g-1) is the maximum adsorbed
amount of heavy metal, and Ke (L mmol-1) is the adsorption
strength onto the adsorption sites. Major parameters revealed that the Langmuir
isotherm was favored with a high regression coefficient for both ACF and CACF.
The Langmuir monolayer adsorption capacity (qmax) was found
to be increased by the surface modification, and the obtained qmax
values are in accordance with the above adsorption characteristics. In case of
the Freundlich isotherm, it was found to be unfavorable compared to the
Langmuir isotherm (R2 < 0.9900). Thermodynamic
parameters of standard enthalpy (ΔHo), standard entropy (ΔSo)
and standard Gibbs free energy (ΔGo) were calculated for
further computations. The change in ΔHo and ΔSo
was calculated using the following eq. (6):
In KL = ΔSo/R - ΔHo/RT (6)
where, KL is the equilibrium constant, R is the
universal gas constant (8.314 J mol-1 K) and T
represents the temperature in Kelvin. Thereafter, ΔGo could
be obtained by the following eq. (7):
ΔGo = -RT In KL (7)
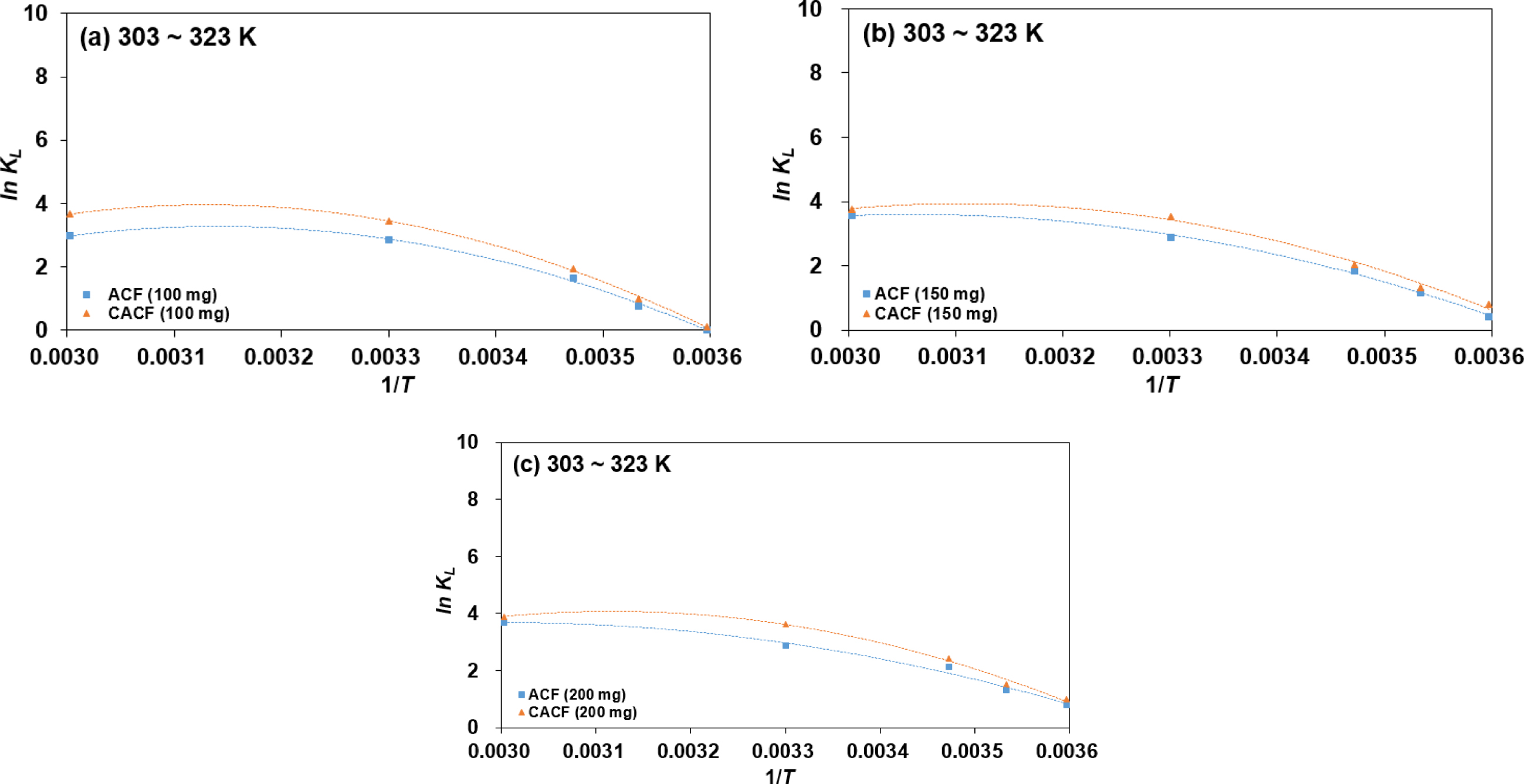
The dependence of In KL on 1/T for the
Cr(VI) adsorption varying initial adsorbent amounts and temperatures are
plotted in Figure 3 and computed thermodynamic parameters are listed in Table
3. Values of ΔHo recorded the lowest at 200 mg of
initial adsorbent amount in both ACF and CACF. Low values of ΔHo
shows that Cr(VI) ions and surface functional moieties are having weak
interactions. In other words, the physio-sorption becomes more favorable than
the chemisorption with more initial adsorbent amounts. The change of ΔGo
for physio-sorption generally occurs in a range of -20~0 kJ mol-1
and chemisorption is between -80 ~ -400 kJ mol-1.14
Values of ΔGo from the samples were in a range of -0.01 ~
-10.78 kJ mol-1, thus the process was feasible and spontaneous. This
implies that a more advanced technique is required to develop evenly dispersed
surface functional moieties, which may cover the majority area of the surface.
Decreased values of ΔGo with the temperature rise suggest
that the adsorption is more preferable at high temperatures. Herein, CACF was
more favorable for Cr(VI) adsorption than ACF in all circumstances. Lastly,
positive values of ΔSo confirm the increased randomness at
the solid-solution interface and the occurrence of ion-replacement reactions
during Cr(VI) adsorption.
|
Figure 2 Adsorption of Cr(VI) onto samples by (a) adsorption capacity; (b) the temperature as a function of time. |
|
Figure 3 Dependence of In KL on 1/T for the Cr(VI) adsorption by (a) 100 mg; (b) 150 mg; (c) 200 mg of initial adsorbent. |
|
Table 1 Physicochemical Characteristics of Samples |
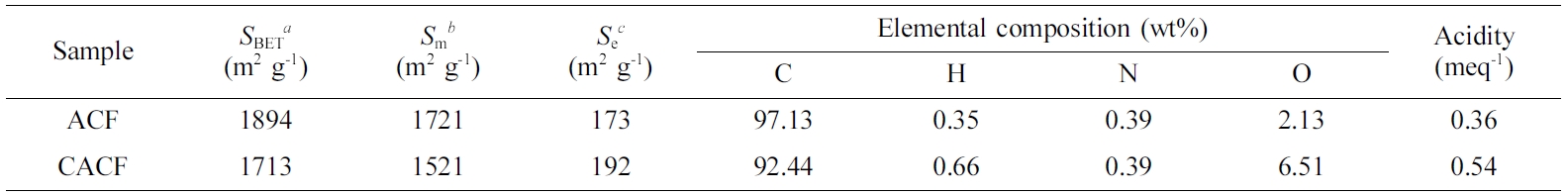
aBrunauer-Emmett-Teller specific surface area. bMicropore area. cExternal surface area. |
|
Table 2 Langmuir Isotherm Parameters for the Cr(VI) Adsorption by Sample Amount |
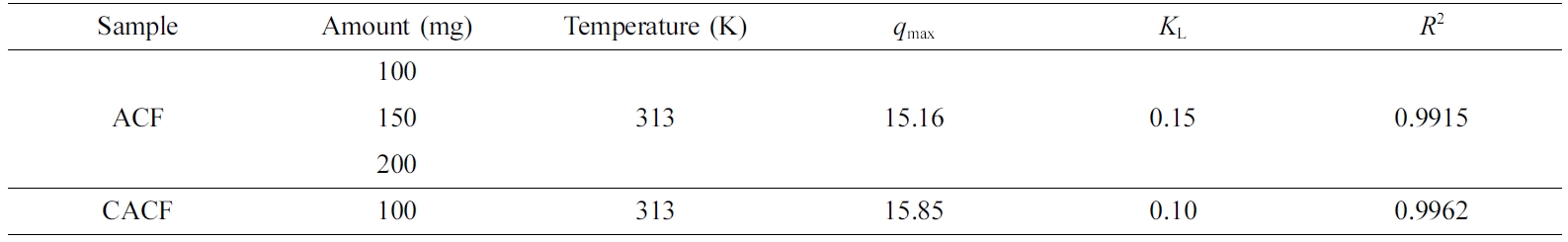
|
Table 3 Computed Thermodynamic Parameters for the Cr(VI) Adsorption by Sample Amount |
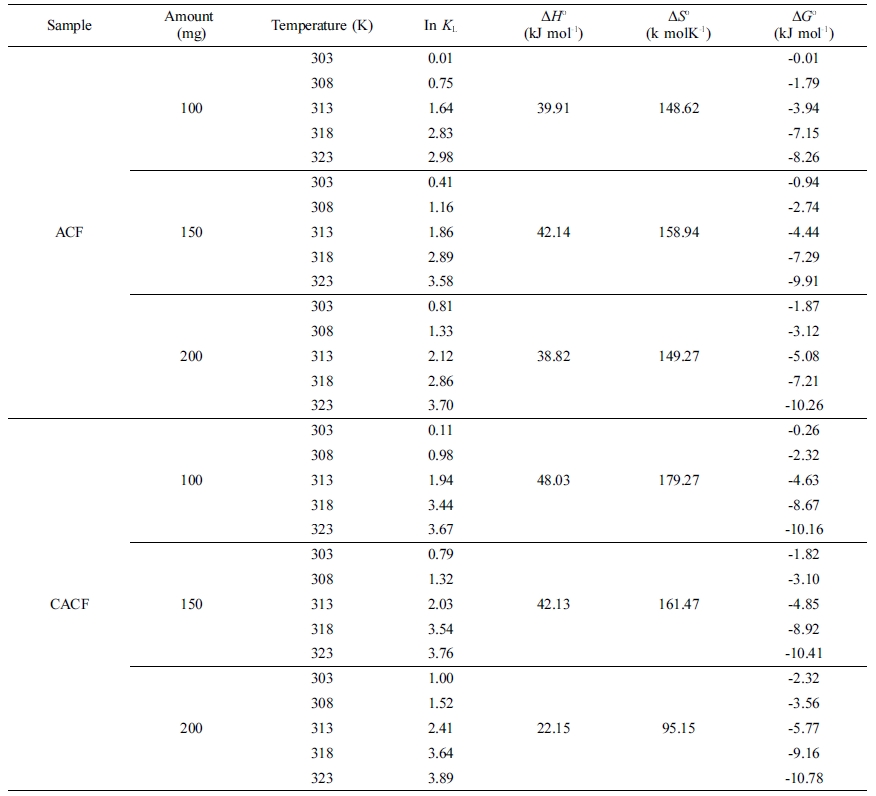
Standard energy computations point out that physiochemical approaches
hold the promise of allowing significant contributions to understand the
adsorption behavior of Cr(VI) onto ACF and CACF which could be summarized as
follows;
(1) Physiochemical analyses observed that a green modification onto ACF
leads to a decrement in the specific surface area by 9.58%, but also increases
33.40% of surface functional moieties.
(2) CACF revealed the maximum Cr(VI) adsorption capacity of 6.36% more
than ACF with 200 mg of initial adsorbent amount at 323 K.
(3) The Langmuir isotherm was fitted with all samples. Observed natures
of the adsorption behavior of Cr(VI) onto ACF and CACF were found to be
spontaneous and feasible over certain temperature. Herein, the physio-sorption
was more favorable than the chemisorption.
We also noted that there are discovered technical limitations of unevenly
dispersed surface functional moieties, or more opportunities which could pave a
way for widespread applications with the aid of further developments. In
conclusion, Cr(VI) onto carbonaceous surface has a possibility to be
effectively managed by controlling the balance of physiochemical environment via
green aid.
- 1. M. Yoshinaga, H. Ninomiya, M. M. A. Al Hossain, M. Sudo, A. A. Akhand, N. Ahsan, M. A. Alim, M. Khalequzzaman, M. Iida, I. Yajima, N. Ohgami, and M. Kato, Chemosphere, 201, 667 (2018).
-

- 2. M. A. Torkmahalleh, M. Karibayev, D. Konakbayeva, M. M. Fyrillas, and A. M. Rule, Air Qual. Atmos. Hlth, 11, 1059 (2018).
-

- 3. J. H. Lee, S. H. Lee, and D. H. Suh, Carbon Lett., 30, 99 (2020).
-

- 4. S. K. Reddy and S. Balasubramanian, Chem. Commun., 50, 503 (2014).
-

- 5. H. S. Harned and R. Davis, Jr., J. Am. Chem. Soc., 65, 2030 (1943).
-

- 6. M. Galib and G. Hanna, Phys. Chem. Chem. Phys., 16, 25573 (2014).
-

- 7. D. Pines, J. Ditkovich, T. Mukra, Y. Miller, M. P. Kiefer, S. Daschakraborty, T. J. Hynes, and E. Pines, J. Phys. Chem. B, 120, 2440 (2016).
-

- 8. H. Wang, J. Zeuschner, M. Eremets, I. Troyan, and J. Willams, Sci. Rep., 6, 19902 (2016).
-

- 9. H. P. Boehm, Carbon, 32, 759 (1994).
-

- 10. K. Kadirvelu, C. Faur-Brasquet, and P. L. Cloirec, Langmuir, 16, 8404 (2000).
-

- 11. S. Mor, K. Ravindra, and N. R. Bishnoi, Biores. Technol., 98, 954 (2007).
-

- 12. C. Namasivayam and R. T. Yamuna, Chemosphere, 30, 561 (1995).
-

- 13. A. Özer and D. Özer, J. Hazard. Mater., 100, 219 (2003).
-

- 14. D. S. Tong, C. H. C. Zhou, Y. Lu, H. Yu, G. F. Zhang, and W. H. Yu, Appl. Clay Sci., 50, 427 (2010).
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2020; 44(3): 295-300
Published online May 25, 2020
- 10.7317/pk.2020.44.3.295
- Received on Jan 13, 2020
- Revised on Mar 9, 2020
- Accepted on Mar 10, 2020
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Sang Sun Choi
-
Department of Environmental Engineering, Anyang University, Anyang 14028, Korea
- E-mail: choiss@anyang.ac.kr
- ORCID:
0000-0002-3133-7808









 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.