- Polyethylene Glycol-based Ointment Formulations of Alnus Sibirica Extract and Their Accelerated Stability Assessments
Yoon Tae Goo#, Tae Hoon Kang#, Kye Wan Lee*, Do Hoon Kim*, Yu Hwa Park*, Byoung Deok Kim, Min Won Lee, Hyoweon Bang**, and Young Wook Choi†

College of Pharmacy, Chung-Ang University, 84 Heuksuk-ro, Dongjak-gu, Seoul 06974, Korea
*Central Research Institute, Dongkook Pharm Co. Ltd, 145 Gwanggyo-ro, Yeongtong-gu, Suwon 16229, Korea
**College of Medicine, Chung-Ang University, 84 Heuksuk-ro, Dongjak-gu, Seoul 06974, Korea- 물오리나무 엑스의 폴리에틸렌글리콜 연고제 설계 및 가속 안정성 평가
중앙대학교 약학대학, *주식회사 동국제약, **중앙대학교 의과대학
Ointment formulation development of Alnus sibirica extract (ASEx), a natural immunomodulator, has been limited because of instability problem. In this study, stabilized ASEx-containing ointments were developed using polyethylene glycol (PEG) base. By the Herschel–Bulkley model, the flow behavior indices were calculated as 0.41~0.97, indicating a shear-thinning flow. The PEG ointments were superior to other comparative formulation, white petrolatum-based ointment, in terms of moistness and removal capacity in a simplified sensory assessment by human volunteers. ASEx degradation followed first-order kinetics with temperature dependence. From accelerated stability assessments, a selective formulation (P3) was found to have longest shelf life of 527 days at 40 oC. Furthermore, by applying a Q10 value of 2.17, the shelf life of P3 at 25 oC was estimated to exceed four years. Thus, we conclude that the P3 formulation may be an appropriate candidate for development of a commercial product with good long-term stability
천연물 유래 면역 조절제인 물오리나무 엑스는 불안정성으로
인해 연고제 개발에 제한적이었다. 본 연구에서는 폴리에틸렌글리콜을 기반으로 한 안정화된 물오리나무 엑스
연고를 개발하였다. 허쉘-버클리 모델에 대입하였을 때 유동
거동 지수는 0.41~0.97로 계산되었으며, 전단박화 유동을
확인하였다. 사람을 대상으로 한 관능 평가에서 폴리에틸렌글리콜 연고가 습윤성 및 수세성 측면에서 백색바셀린
연고보다 높은 점수를 나타내었다. 물오리나무 엑스의 온도의존성 분해반응은 1차 속도식을 따랐으며, 40 oC 가속
안정성 시험에서 폴리에틸렌글리콜 연고(P3)의 유효기간이 527일로
가장 길었다. 또한 계산된 2.17의 Q10 값을
이용하였을 때, P3의 유효기간은 25 oC에서 4년을 초과하는 것으로 추정되었다. 따라서 우수한 안정성을 갖는 제품
개발에 P3 제제가 적절한 후보군이라 할 수 있다
Alnus sibirica extract (ASEx) is a natural immunomodulator. Stabilized ASEx-containing ointments were developed using polyethylene glycol base. The P3 formulation may be an appropriate candidate for development of a commercial product with good long-term stability.
Keywords: Alnus sibirica extract, polyethylene glycol, ointments, stability, zinc stearate
This study was supported by the Korea Institute of Planning and
Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET)
through the Agri-Bio Industry Technology Development Program, funded by the
Ministry of Agriculture, Food and Rural Affairs (MAFRA) (117046-3). This
research was also supported by the Chung-Ang University Research Scholarship
Grants in 2018.
Alnus sibirica (AS) is a tree of the family Betulaceae that is found in
damp areas of mountain valleys and has been used in Oriental traditional
medicines as a remedy for fever, hemorrhages, burn injuries, diarrhea, and
alcoholism.1 The extract of AS (ASEx) contains various phenolic compounds
such as flavonoids, tannins, and triterpenoids, including a series of
di-arylheptanoids.2-4 These compounds have antioxidant and
anti-inflammatory properties. In particular, oregonin (ORG) and hirsutenone
(HST) have been isolated as biologically active diarylheptanoids.5
ORG is a glucopyranoside of HST (aglycon form; 1,7-bis-(3,4-dihydroxyphenyl)-5-hydroxyheptane-3-on);
thus, ORG is water-soluble, while HST is water-insoluble. Previous studies have
reported that ORG suppressed inflammation in rabbit macrophages by regulating
NF-kB signaling,6 reduced cellular lipid accumulation and
pro-inflammatory cytokine secretion in primary human macrophages,7
and inhibited lipopolysaccharide‐induced nitric oxide synthase gene
transcription in macrophages and microglia.8 Taking advantage of
these effects, ORG and HST have been investigated as natural immunomodulators
to treat autoimmune diseases, including atopic dermatitis.9,10
Atopic dermatitis is a chronic, incurable immune disease that occurs due
to a combination of genetic and environmental factors and involves an increased
concentration of cytokines secreted by Th2 cells that activate the host’s
allergic response, ultimately upregulating B cell synthesis of Immunoglobulin
E.11 In addition, inflammation-related enzymes such as inducible
nitric oxide synthase, cyclooxygenase-2, and 5-lipoxygenase are important
factors that promote inflammatory responses in dermatitis.12 Topical
application of corticosteroids along with frequent emollient use has been the
mainstay of treatment for atopic dermatitis. Although corticosteroids are
highly effective, their associated local and systemic side effects such as skin
thinning, atrophic changes including telangiectasia, purpura, striae, and
adrenal gland suppression limit their long-term use.13
Alternatively, less toxic compounds including natural immunomodulators have
received increased focus as possible treatment approaches.14,15
Various efforts to develop topical formulations for polyphenols such as
ORG or HST have been attempted. Previously, we formulated HST-containing
oleaginous ointments and creams16 in which the stability of HST
modestly increased by adding various antioxidants; however, these compounds
remained unsatisfactory for practical development. Additionally, nanostructured
lipid carrier formulations were investigated to stabilize HST in the presence
of antioxidants.17 A Tat peptide-admixed elastic liposomal system
was formulated to improve the dermal delivery of HST.12 ORG was
formulated with various types of ointment vehicles, and the drug release
characteristics were evaluated.18 Topical cream and gel formulations
containing polyphenols extracted from Echinacea purpurea were developed and
evaluated for stability.19 However, none of these formulations were
able to attain a two-year shelf life for storage in ambient conditions, a
prerequisite for commercial development. Because of this instability problem,
the practical development of topical preparations containing these
diarylheptanoids remains limited.
Therefore, in the present study, ASEx was formulated with two ointment
base types: a polyethylene glycol (PEG) base, or white petrolatum-based
ointments in the presence or absence of zinc stearate as a stabilizing agent.
The physical characteristics of the formulations were evaluated in terms of
viscosity and sensory tests. Accelerated stability assessments of the different
ointments were conducted based on first-order kinetic analyses of ORG in ASEx.
Applying the Q10 method, the shelf lives of ASEx-containing
ointments were estimated.
Materials. ASEx (14.9% ORG
content) was supplied by Dongkook Pharm. Co., Ltd. (Seoul, Korea). ORG (purity
> 92% by HPLC) was supplied by the Pharmacognosy laboratory at the College
of Pharmacy in Chung-Ang University, Seoul, Republic of Korea. Beeswax, PEG
400, propylene glycol, sodium stearate, and zinc stearate were purchased from
Duksan Pure Chemical Co., Ltd. (Ansan, Korea). PEG 4000 was purchased from
Sanyo Chemical Industries, Ltd. (Kyoto, Japan). White petrolatum was purchased
from Samchun Pure Chemical Co., Ltd. (Pyung-taek, Korea). All other chemicals
and solvents purchased from commercial sources were of analytical reagent
grade.
Preparation
of ASEx-Containing Ointments. As listed in Table
1, PEG-based (P1–P3) and white petrolatum-based (V1 and V2) ointments
containing ASEx were prepared using the reported melt fusion method.20,21
Briefly, all constituents except for ASEx and zinc stearate were melted at
60 oC in a water bath (Sb-1300; Sunil Eyela Co., Seongnam,
Korea) and thoroughly mixed; then, ASEx was added. For comparison, zinc
stearate was added only for P3 and V2 ointments. The mixture was stirred at
60 oC with a polytetrafluoroethylene-coated magnetic bar until
homogeneous. Then, the mixture was cooled to 25 oC under gentle
stirring. Separately, the P3 formulation was further varied with different
contents of zinc stearate or sodium stearate, with the final volume
complemented with distilled water. All ointments were subjected for ageing at
25 oC for 24 h before use.
HPLC
Determination of ORG in ASEx. ASEx was
dissolved in distilled water (1 mg/mL) and assayed for ORG content using HPLC
analysis. For the calibration curve, an ORG-dissolved stock solution (0.5
mg/mL) was prepared and serially diluted. Solution samples were filtered
through a 0.45 μm polyvinylidene difluoride (PVDF) membrane filter
(Whatman International Ltd., Kent, UK); then, 20 μL of filtrate was
injected into an Agilent Infinity 1260 HPLC system (Agilent Technologies, Santa
Clara, CA, USA) consisting of a 1260 Quat pump VL, 1260 TCC (Column heater),
and 1260 DAD WR (detector), and the data were collected using Agilent Empower
Pro software. Using a C18 column (250×4.6 mm, 5 µm; Shiseido, Tokyo,
Japan), the mobile phase system consisted of A (0.1% phosphoric acid) and B
(acetonitrile), with a varied gradient according to the following program: 10
min (95% A), 35 min (75% A), 40 min (55% A), 45 min (40% A), and 55 min (95%
A). Analyses were performed at a flow rate of 1.0 mL/min at 25 oC,
and the column eluent was monitored at 280 nm. The intra- and inter-day
precision and accuracy of the ORG assay in ASEx solution were determined in
triplicate.
Assay Validation of ASEx in Ointments. Defined amounts of ASEx-containing
ointments (P3 and V2) were accurately weighed and dissolved in a 10-fold volume
of an extractant composed of methanol and distilled water (50:50, [v/v]). For
complete extraction, the dissolved solution was heated at 60 oC
in a water bath and sonicated for 30 min at 40 oC using a water
bath-type sonicator (47 kHz; Branson 2210R-DTH; Branson Ultrasonics
Corporation, Danbury, CT, USA). The solution was filtered through a 0.45 μm
PVDF membrane filter and injected for the HPLC assay. The intra- and inter-day
precision and accuracy of the ASEx assay for the different ointments were determined
in triplicate.
Rheological
Observation. Rheological assessments were carried out using the
Advanced Rheometric Expansion System (ARES; Rheometric Scientific, U.K)
equipped with parallel plates (25 mm in diameter) with a gap of 1.0 mm.
Experiments were conducted under a steady shear flow at 25 oC.
Shear rates ranged from 0.1-100 s-1. Separately, viscosity
measurements for the P3 formulations with different contents of zinc stearate
or sodium stearate were performed at 25 oC using a rheometer
(MCR 102; Anton Paar, Ostfildern, Germany) with a 25 mm parallel plate (PP25;
Anton Paar, Ostfildern, Germany) at a shear rate of 1.0 s-1.
All experiments were performed in triplicate and averaged.
Sensory Evaluation in Human Volunteers. Informed consent was received from
all subjects before participation in the study. The study was conducted in accordance
with the Declaration of Helsinki, and the protocol was approved by the Ethics
Committee of Chung-Ang University (Protocol number:
ID-1041078-201912-HRBM-366-01). Twelve healthy volunteers aged 25-55 living in
and around Seoul were chosen for the panel and were familiarized with the
terms, evaluation procedures, and rating scales. The panel members were
educated on the general concept of the study through a detailed explanation of
the test, such as descriptions of the scales for the evaluation parameters and
reference points, during tutorial sessions. Subsequently, panelists evaluated
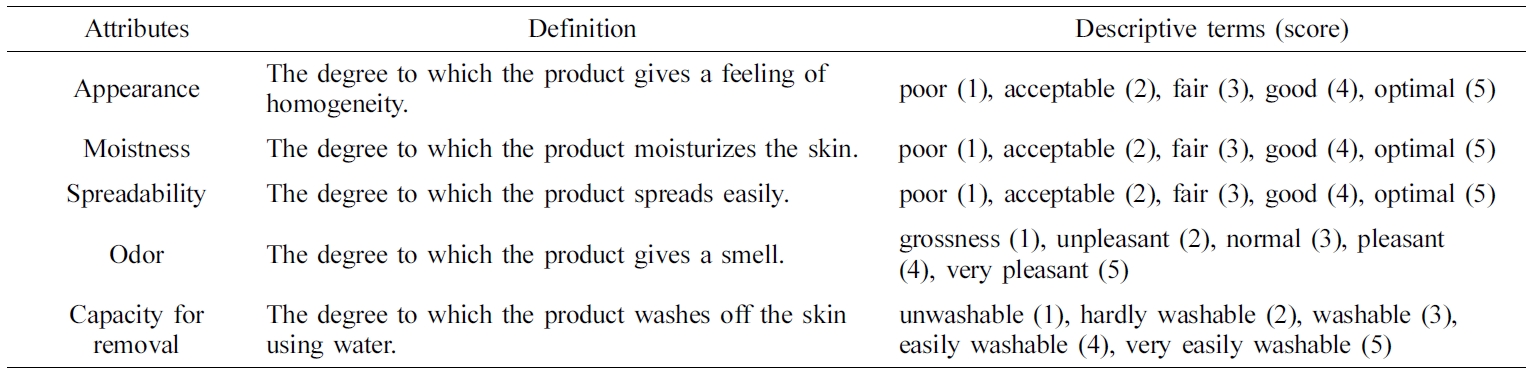
the various ointment samples according to the samples’ sensory attributes:
appearance, moistness, spreadability, odor, and capacity for removal, as shown
in Table 2. For each assessment, the panelists evaluated five ointment samples
presented in the same containers labeled with three-digit code numbers. This
assessment was carried out in triplicate, and the values were averaged.
Aqueous
Stability of ASEx at Different Temperatures. Aliquots of ASEx
dissolved in aqueous solution (1 mg/mL) were transferred to 10 mL
Teflon-capped vials and sealed with parafilm to shut off the evaporation of
water; then, the samples were stored at different temperature of 25, 40, and
50 oC for 10 days. At predetermined time points, 300 µL of
sample solution were removed from the vial and filtered through a 0.45 μm
PVDF membrane filter, and the filtrates were subjected to HPLC analysis as
described above. The first-order degradation rate constants were calculated
from the semi-logarithmic plot of the amount of remaining ORG in ASEx against
time, and degradation’s temperature dependence was analyzed by the Arrhenius
plot.
Accelerated
Stability Tests of ASEx-Containing Oint-ments. For the accelerated
stability test, ASEx-containing ointments were kept in glass vials and stored
at 40 oC and 75% relative humidity using the Climacell 707
display cabinet (MMM Medcenter Einrichtungen, Munich, Germany). At
predetermined time points (0, 30, 90, and 120 day), ORG was extracted from the
ointments as described above. The extract was filtered through a 0.45 μm
PVDF membrane filter, and an aliquot (20 μL) of filtrate was introduced
into HPLC. Separately, changes in apparent consistency were examined for , all
ointments that were kept in 50 mL Falcon tubes and stored in the same
conditions for 120 days.
|
Table 1 Compositions of ASEx-Containing Ointments |
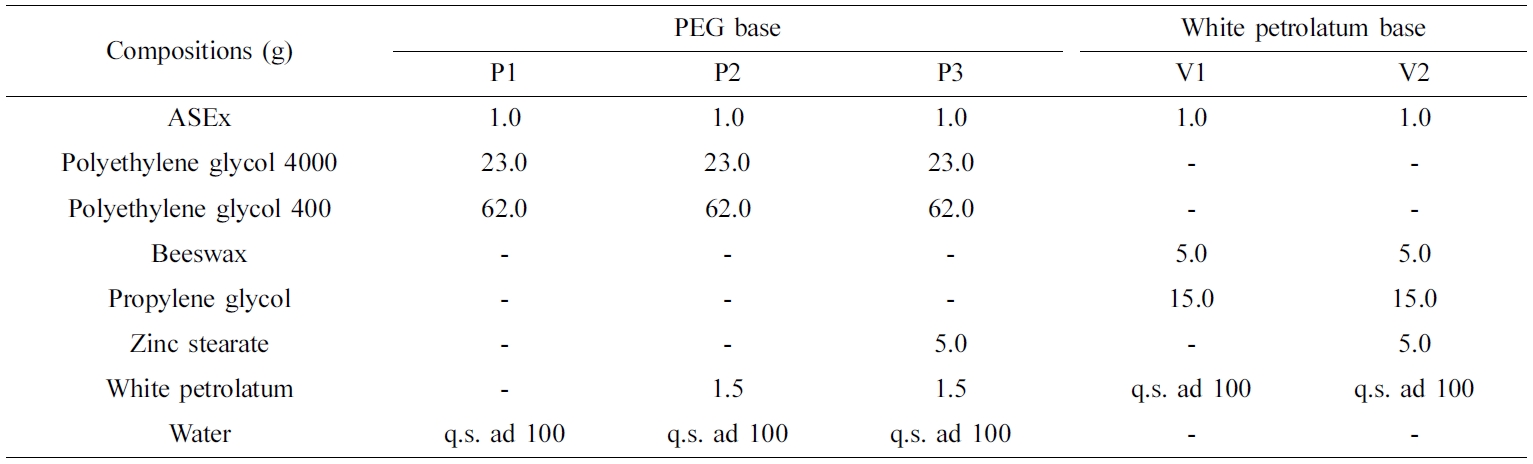
ASEx, Alnus sibirica extract. |
|
Table 2 Sensory Attributes and Descriptive Terms for Simplified Sensory Evaluations |
Characterization of ASEx-Containing Ointments. ASEx-containing ointments were successfully prepared.
PEG-based ointments (P1, P2, and P3) and white petrolatum-based ointments (V1
and V2) were prepared as hydrophilic and oleaginous types, respectively. For
the PEG-based ointments, PEG 400 and PEG 4000 were used as the main
constituents (P1), and a small amount of white petrolatum and/or zinc stearate
was added (P2 and P3) as a viscosity modifier and stabilizer. For the white
petrolatum-based ointments, V1 was composed of white petrolatum, propylene
glycol, and beeswax, while V2 further contained a small amount of zinc
stearate. Throughout all ointment formulations, content uniformity was
maintained in the range of 100 ± 5% ASEx content.
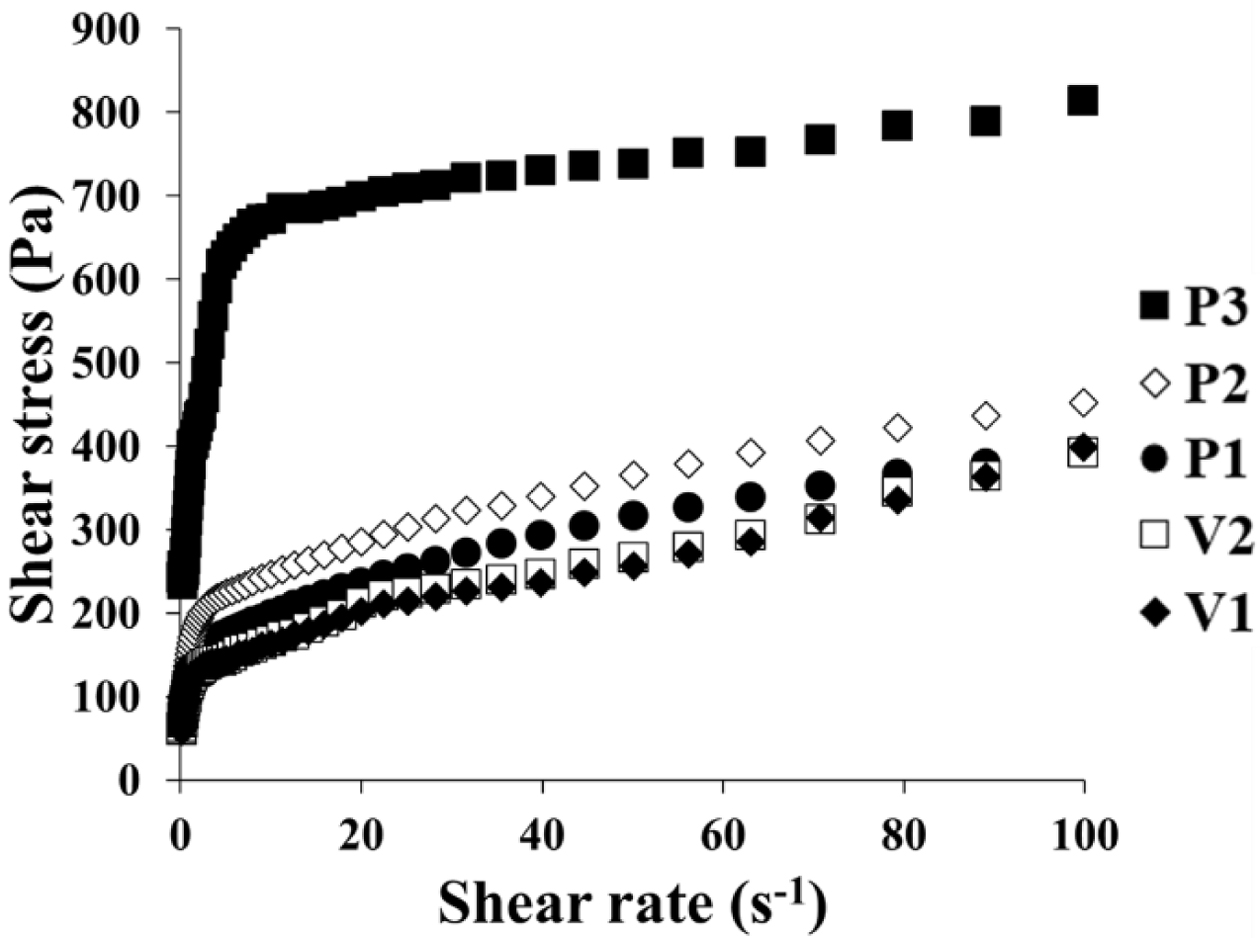
Flow curves of the different ASEx-containing ointments are shown in
Figure 1. All ointments demonstrated non-Newtonian flow behavior. To further
analyze the rheological behavior of the ointments, the Herschel–Bulkley model
was employed with the following eq. (1):22 
where τ is shear stress, τ0 is yield stress, g is shear rate, κ is the consistency index, and n
is the flow behavior index. The flow curves fit well with good correlation
coefficients (R2 > 0.95), and the yield stress was
determined from the intercept of the linear correlation between the first three
or four plot points.23 The values for these rheological parameters
are listed in Table 3. P3 showed a much greater τ0 value
compared to the other ointments; the τ0 values for the
ointments are ranked P3 > P2 ≥ V2 > P1 > V1. The consistency index (κ)
represents the viscous nature of the semi-solid formulations24 and
was greatest in the P3 formulation. The flow behavior index (n)
indicates the degree of shear sensitivity, and lower n values indicate greater
viscosity reliance on the shear rate.25 Flow behavior with n = 1
corresponds to Newtonian flow, n < 1 indicates a
shear-thinning fluid, while n > 1 indicates a dilatant
fluid.26 All ointments demonstrated values in the range of
0.26~0.97, indicating shear-thinning flow behavior.
Sensory
Evaluation of ASEx-Containing Ointments. In recent years,
several techniques have been developed to objectively evaluate skin properties
in dermato-cosmetic research.27 Quantitative descriptive analyses of
cosmetic products have been widely used to evaluate the sensory characteristics
of cosmetics and cosmetic ingredients.28 However, given the
diversity of sensory attributes, it would be too comprehensive, time-consuming,
lengthy, and expensive to conduct such analyses.29 Thus, in this
study, a simplified sensory assessment was performed by employing the sensory
attributes of appearance, moistness, spreadability, odor, and capacity for
removal.
Twelve panelists were recruited and instructed to grade their feelings on
a score of 1 (poor) to 5 (optimal). These results are listed in Table 4.
Despite the limited number of panel members, several significant results were
found. For the appearance attribute, none of the panelists gave any of the
compounds a score of 1, and the average scores ranged from 3.17–3.42,
indicating a fair to good impression. For the moistness attribute, the
PEG-based ointments were superior to the white petrolatum-based ointments. Two
panelists gave a score of 5 (optimal) for P3, while two panelists gave a score
of 1 (poor) to V1 and V2. Although both PEG and white petrolatum have been
reported as good moisturizing agents,30,31 the white
petrolatum-based ointments showed lower moistness scores in this experiment.
This might be attributed to individual panel members’ variations in sensation.
For the spreadability attribute, P3 showed the lowest score of 2.83 (acceptable
to fair), while V2 was given a score of 5 (optimal) by one panelist. These
results were considered to be closely related to rheological properties: P3 has
a high viscosity and therefore yielded a lower score compared to other
ointments with low viscosity. For the odor attribute, none of the panelists
gave scores of 1 (grossness) or 5 (very pleasant), yielding a score of
approximately 3 (normal) for all ointments. Small standard deviations were also
observed, indicating little difference between the panelists’ evaluations.
Finally, regarding the capacity for removal by washing with water, PEG-based
ointments showed a value of 3.17– 3.50 (washable to easily washable), which
clearly surpassed the white petrolatum-based ointments that were graded with
values of 1.75–1.83 (unwashable to hardly washable). Because PEG polymers are
water-soluble and do not hydrolyze or support mold growth, PEG makes a good
base for washable ointments and can be formulated to have a soft-to-hard
consistency.32 In contrast, white petrolatum-based ointments are
mainly composed of oleaginous ingredients, consequently making them hard to
remove by washing with water alone.
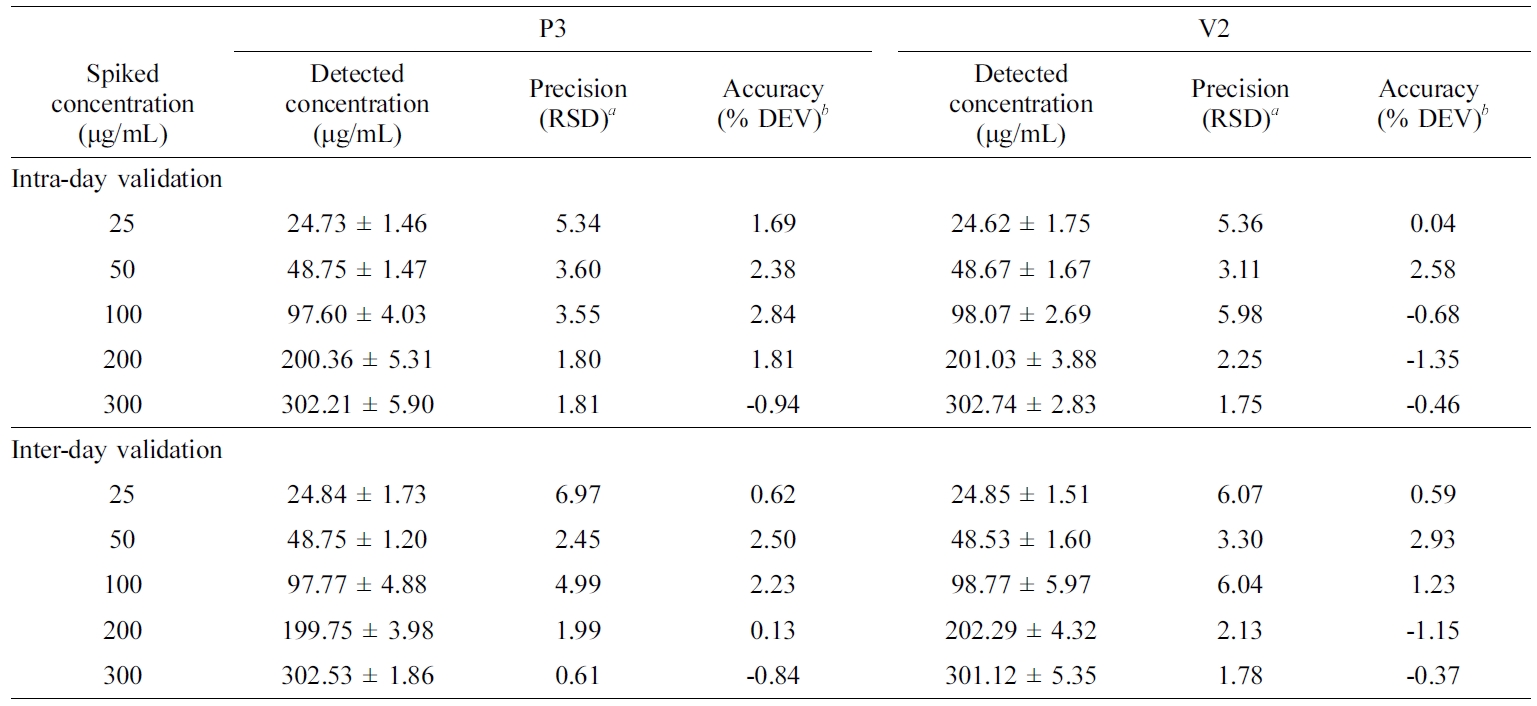
HPLC
Assay Validation of ORG in ASEx. A typical
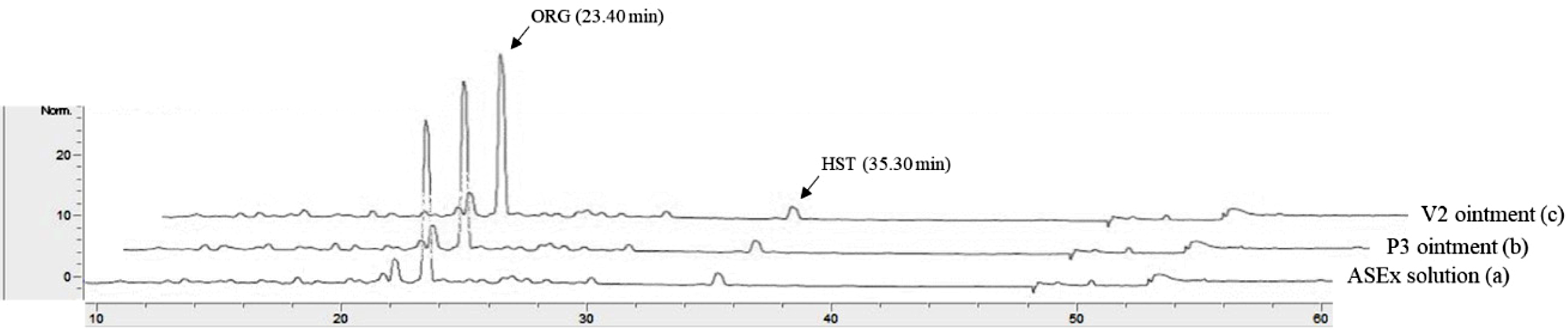
chromatogram of an ASEx-dissolved stock solution is shown in Figure 2
(chromatogram a). ORG was separated with an acceptable peak resolution at a
retention time of 23.40 min, while the trace amount of HST was identified with
a noise peak at a retention time of 35.30 min. Upon integration, the peak
area of ORG was 11-fold greater than that of HST (data not shown); thus, ORG
was selected as a marker compound for the stability-indicating assay in the
quality control analyses. This peak pattern was identical in both the P3 ointment
(chromatogram b) and the V2 ointment (chromatogram c) as representative
PEG-based and white petrolatum-based ointments, respectively, indicating no
interference of the ointment base on the ORG assay. The calibration curve of
the peak area ratio of ORG versus ORG concentration resulted in a
regression equation of y = 10252x – 16234 (R2
= 0.9998) in the range of 1–400 μg/mL. Intra- and inter-day validations for
accuracy and precision analysis for the ASEx solutions and ointments were
established. For ASEx solutions in the range of 25–300 μg/mL, the intra-
and inter-day accuracies ranged from -1.7 to 4% and from -0.6 to 3.6%,
respectively. Intra- and inter-day precisions ranged from 2.1 to 6.8% and from
1.3 to 5.4%, respectively. ORG assays for both the P3 and V2 ointments were
also validated (Table 5). For both ointments, the intra- and inter-day
validation data were mostly within a 6% deviation. All validation data were
acceptable for both ointments, as the criteria for accuracy were within the 15%
deviation, and precision was within a 15% relative standard deviation from the
normal values.33
Degradation
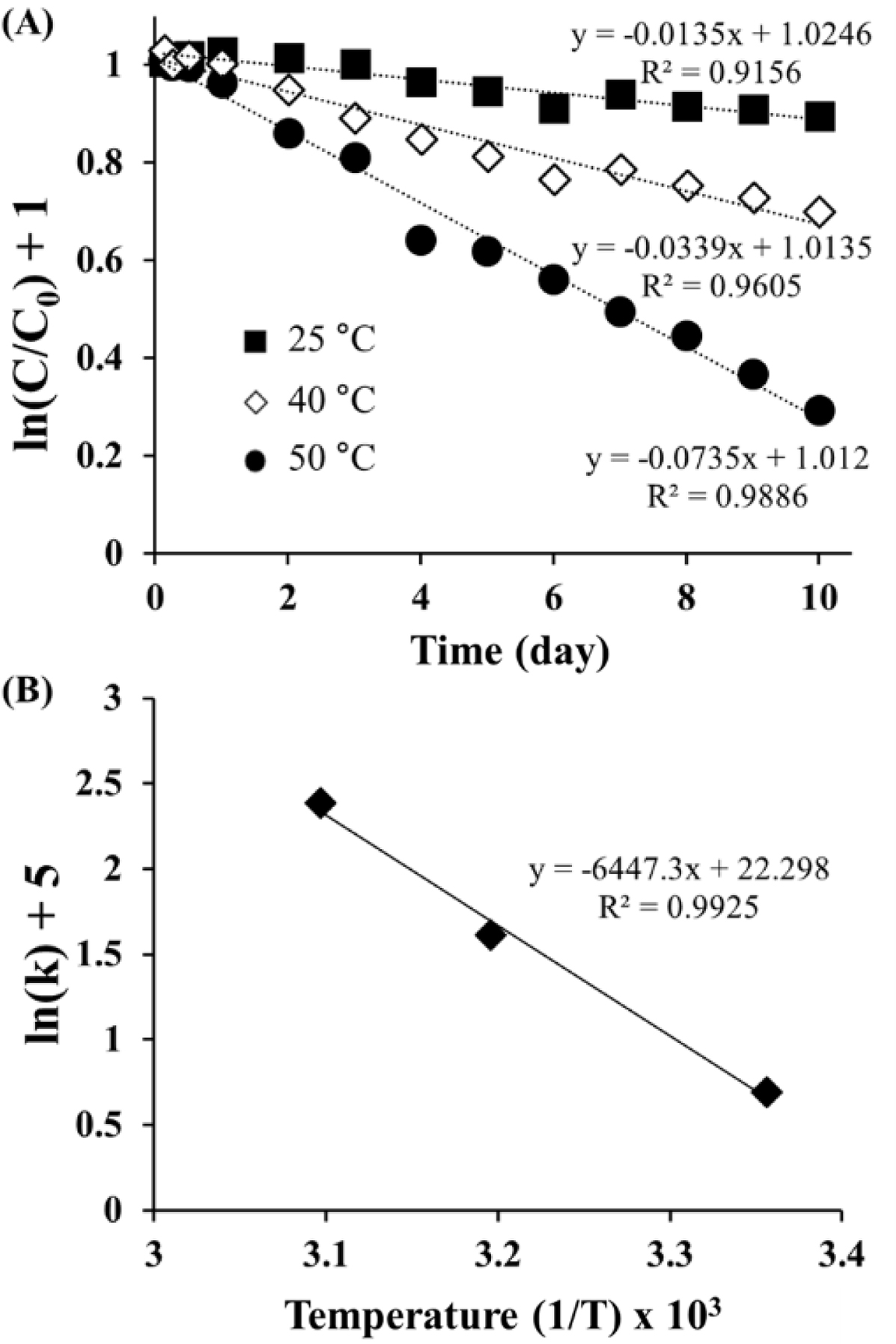
Kinetics of ORG in the ASEx Solution. As shown in Figure
3(A), the semi-log plot of the remaining ORG versus time gave a straight
line with good linearity (R2 > 0.9), indicating the
first-order degradation of ORG following eq. (2):

where C/C0 is the fraction of remaining ORG in
the aqueous solution at the predetermined time point t, and k is
the rate constant (day-1). Using the regression function of
Microsoft Excel 2019, k values (×10-2) were obtained as 1.35
(25 °C), 3.39 (40 °C), and 7.35 (50 °C). ORG was unstable in
aqueous solution, with a short half-life of 51.3 days even at 25 °C. The
degradation of ORG was accelerated as temperature increased. The temperature
dependence of ORG degradation was further analyzed using the following
Arrhenius eq. (3):

where Ea is the activation energy, A is the
frequency factor, T is the absolute temperature, and R is the gas
constant. As shown in Figure 3(B), the semi-log plot of the rate constants
versus reciprocal absolute temperature showed a linear relationship with a good
correlation (R2 = 0.9925). The activation energy of
ORG, calculated from the slope of the regression line, was 53.60 kJ/mole, which
is greater than that of HST (16.53 kJ/mole) as reported in the literature.34
The higher the activation energy, the less likely that the degradation reaction
is influenced by temperature.35 The difference in the Ea
values suggests that ORG is more stable than HST, possibly due to its
glycosylated structure.
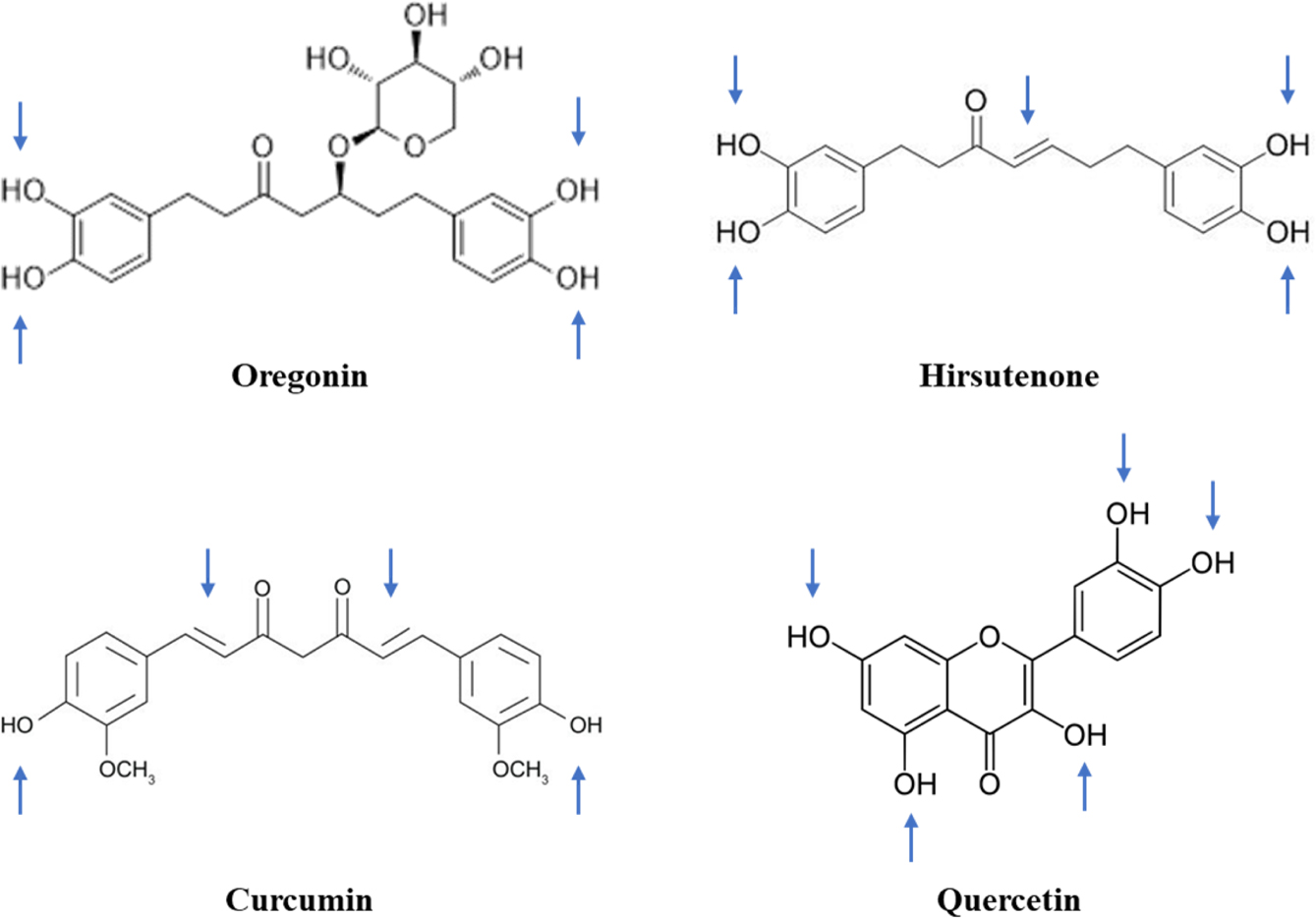
The instability of these polyphenolic compounds can be further explained
by structural comparisons as depicted in Figure 4. As marked by arrows, the
3,4-dihydroxyphenyl groups are commonly prone to oxidation. Moreover, the
existence of any double bonds in an aliphatic hydrocarbon chain could
accelerate oxidative degradation. As a representative, HST is extremely
unstable due to both the 3,4-dihydroxyphenyl group and double bonds within its
structure.36 Various antioxidants including ascorbic acid, sodium edetate,
and ascorbyl palmitate have been screened to overcome the instability of HST,
but no stability enhancement has been practically attained.34
Numerous studies have shown that, due to its structural similarity to HST,
curcumin is degraded by double-bond oxidation.37 However, in the
case of ORG, which possesses a glucopyranoside, aliphatic chains are saturated
and subsequently stabilized. There is a general consensus that glycosylation
increases the stability of polyphenols during ambient storage, even against
exposure to oxygen, pH imbalances, temperature and/or light, while also
enhancing gastrointestinal absorption.38-40 Nevertheless, ORG is
still unstable in aqueous solution, and its degradation is accelerated with
increasing temperature. ORG degradation might be attributed to oxidation and/or
enzymatic hydrolysis. As hydrolytic degradation requires presence of
glycosidase, ORG degradation in enzyme-free ASEx solutions should instead occur
via oxidative pathways. In comparison, quercetin is a labile compound that
belongs to the polyphenol class but does not include sugar moieties or
aliphatic chains. Quercetin has been reported to undergo thermally accelerated
oxidative degradation in aqueous solutions with a half-life of 0.08 days at
37 °C.41 Therefore, we suggest that ORG degradation is
primarily mediated by oxidation of the dihydroxyphenyl groups.
Accelerated
Stability Assessment of Ointments. The stability of
ASEx-containing ointments was evaluated under an accelerated condition of
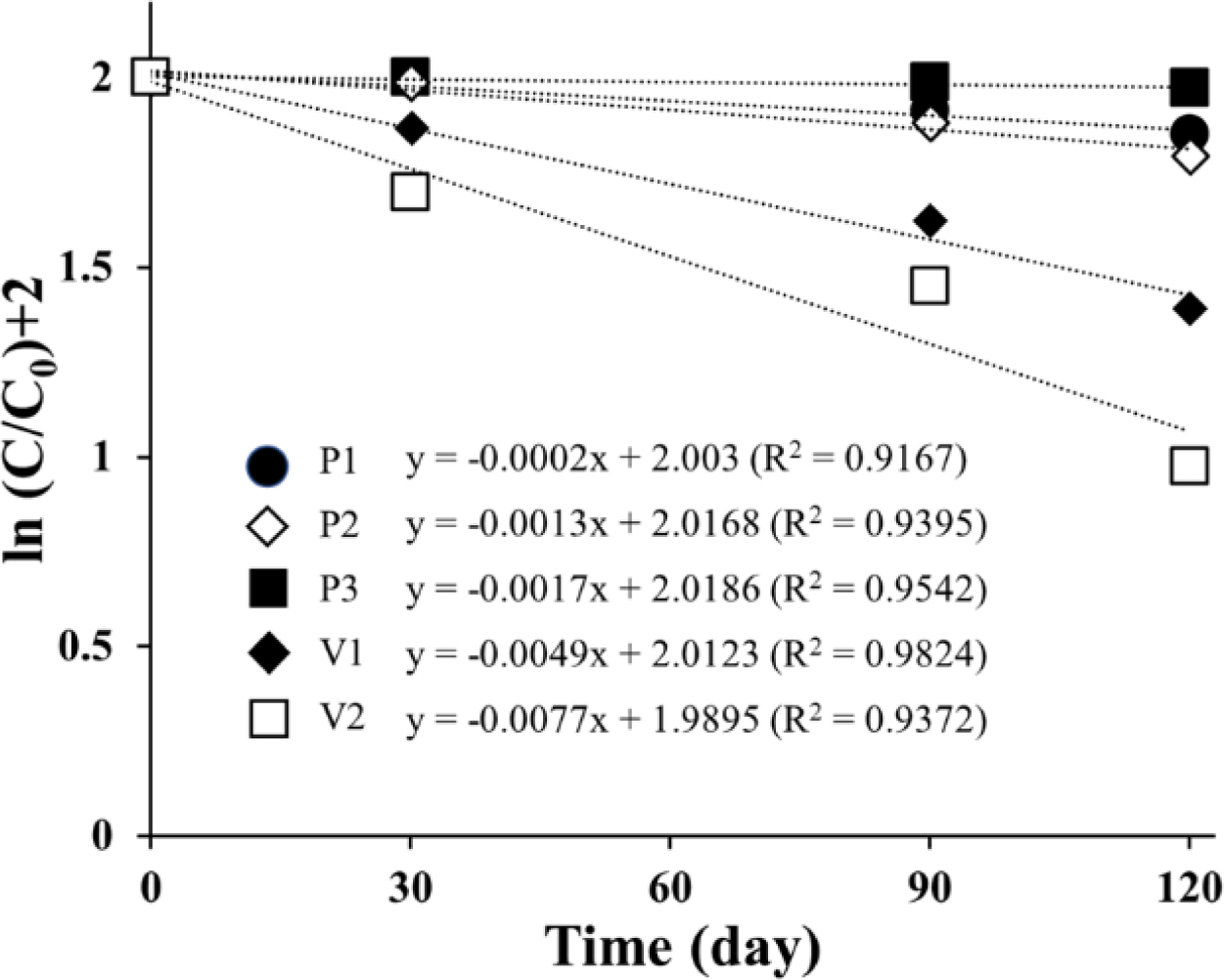
40 °C for 120 days. As shown in Figure 5, linearity was observed in the
semi-log plot of the remaining level of ASEx as a function of time, indicating
that ASEx degradation in the ointments followed first-order kinetics. In
comparison to the PEG-based ointments, white petrolatum-based ointments showed
a rapid decrease in ASEx content. The calculated rate constants and half-lives
are listed in Table 6. The half-lives of PEG-based ointments were in the range
of 400 to 3460 days, much longer than those of white petrolatum-based ointments
ranging from 90 to 140 days. In the literature, the PEG molecule can act as
both a hydrogen donor and acceptor.42 Hydrogen bonds between PEG and
hydroxyl group-rich molecules such as felodipine, polyvinyl alcohol, and
chitosan have been widely reported.43-45 In addition, polyphenolic
compounds such as ORG have also been found to contribute to hydrogen bonding.46
Thus, we assumed the presence of a hydrogen bond between ORG and PEG could
consequently improve the stability of ORG in ASEx-containing PEG-based
ointments. This stabilization effect might be related to the changes in the
consistency of the ointments during the acceleration tests. As shown in Figure
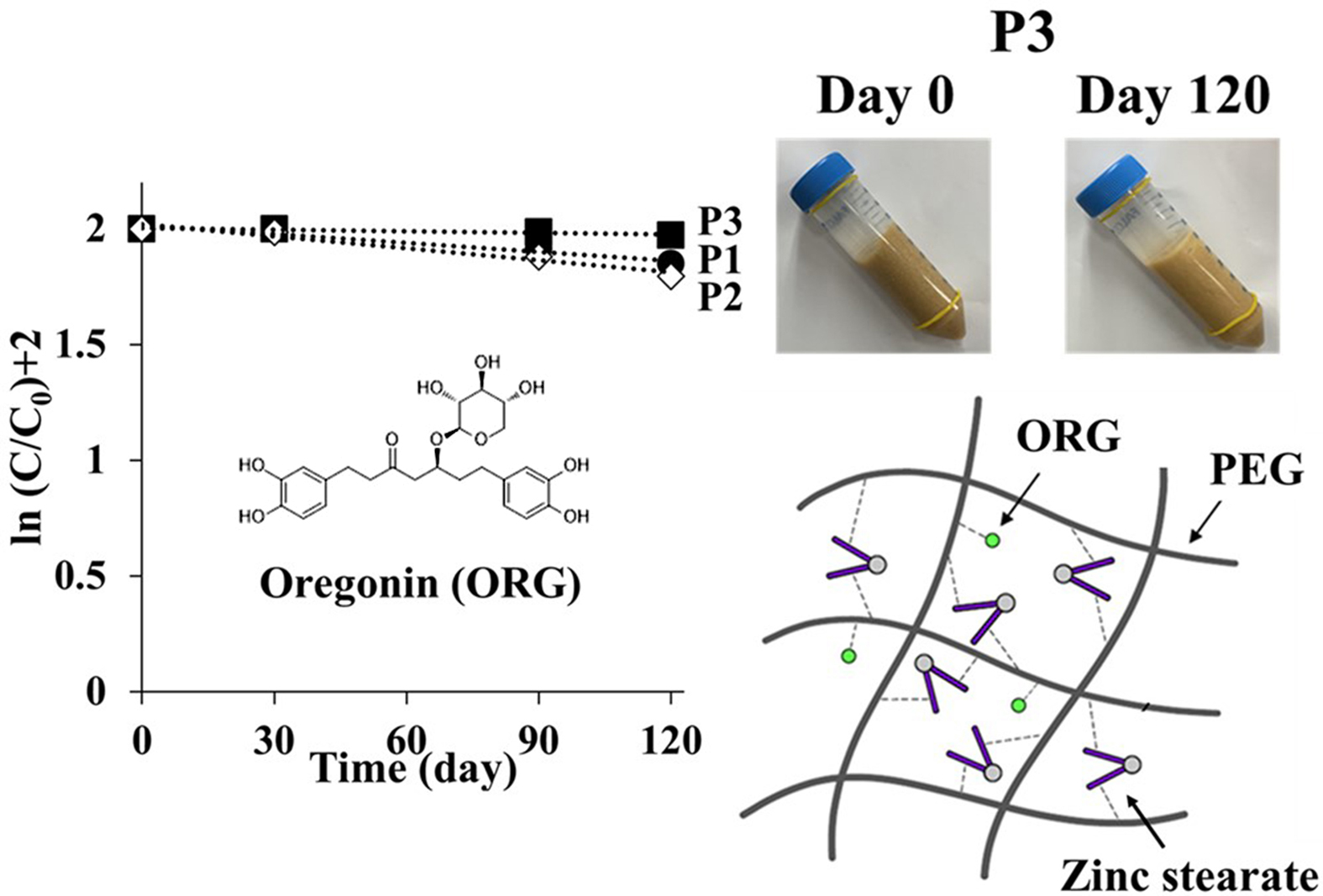
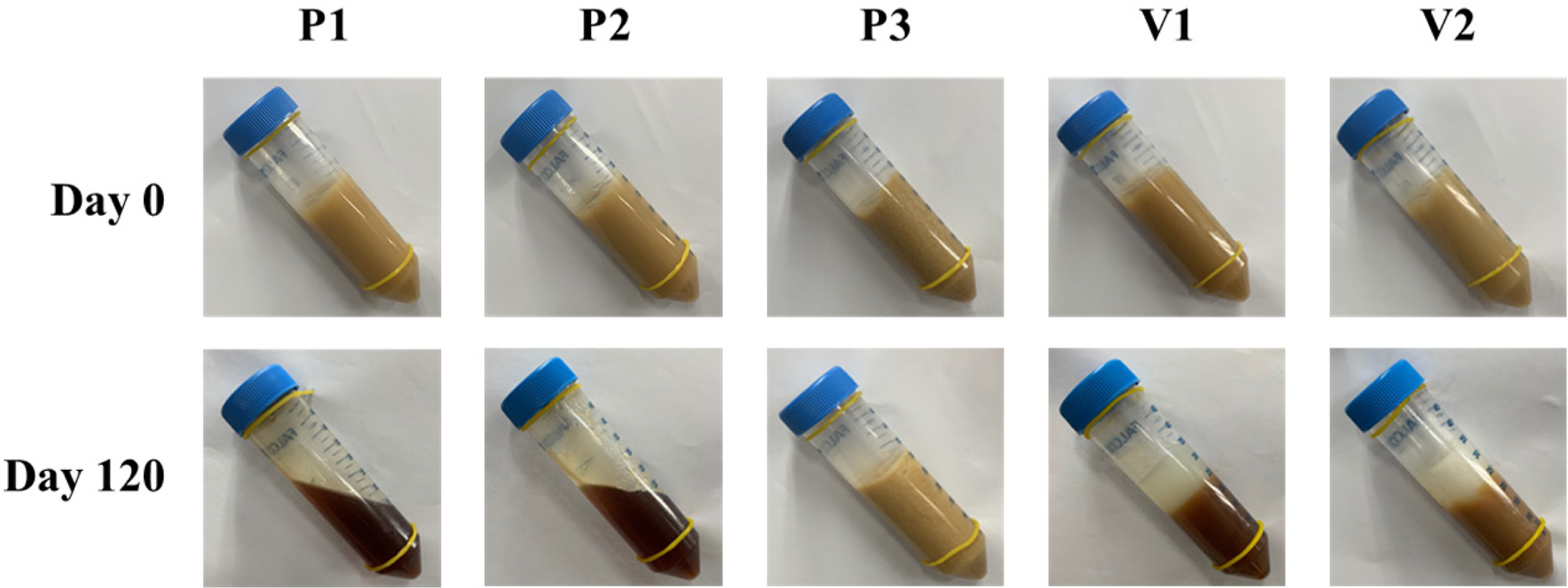
6, after 120 days of storage under the accelerated condition, changes in color
and apparent consistency were observed. In the beginning (day 0), all samples
exhibited a semisolid property with a yellowish-brown color that reflected the
inherent color of ASEx, regardless of ointment type. However, at day 120, all
ointments except P3 demonstrated physical instability through liquefaction
and/or melt-down in P1 and P2, phase separation in V1 and V2, and dark brown
color changes in P1, P2, V1, and V2. The color changes may be an indirect
indicator of ASEx degradation. The ointment matrices with low viscosity allow
for greater molecular movement, consequently providing more chances for
molecular aggregation and/or collisions to facilitate reactions.47
P3 showed much greater stability compared to the other ointments and exhibited
higher viscosity than either P1 or P2. This peculiar stability may be
attributed to not only the hydrogen bond formation between ORG and PEG
molecules, but also complex formation induced by the zinc stearate.
We assumed that PEG molecules could form a complex with ORG and zinc stearate
via hydrogen bonding to increase ORG stability, along with the viscosity of
PEG-based ointments. Thus, to further investigate the effects of zinc stearate,
P3 compositions were formulated with different contents of zinc stearate or
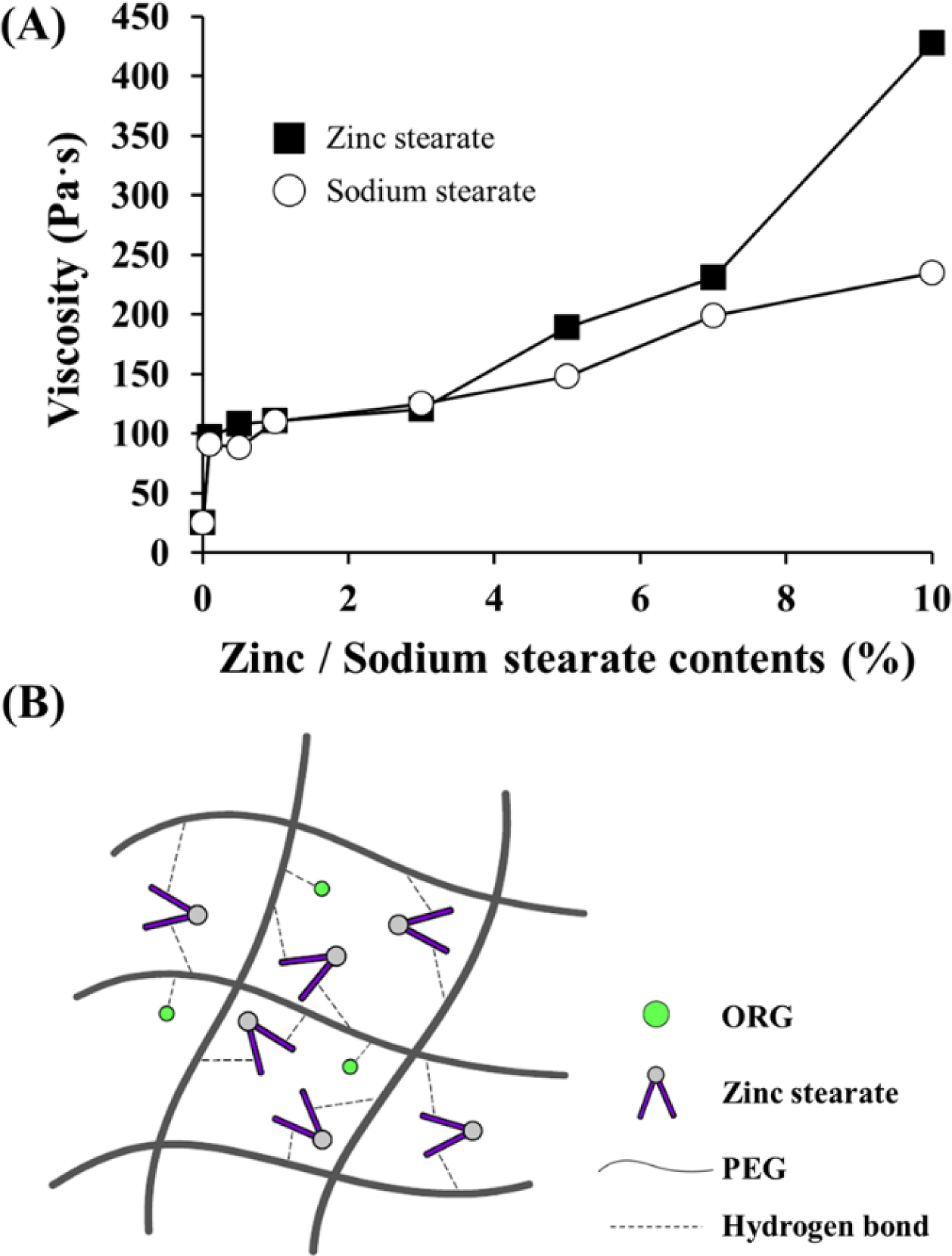
sodium stearate. As shown in Figure 7(A), viscosity was increased as the amount
of zinc stearate or sodium stearate increased. High concentrations of solid
particles provide stiffness to semi-solid formulations.48 With
additions up to 3%, no noticeable differences were observed between sodium
stearate and zinc stearate. Beyond 3%, sodium stearate moderately increased
viscosity, whereas steep increases in viscosity values occurred with zinc
stearate to approximately 190 Pa·s (5%), 230 Pa·s (7%), and 430 Pa·s (10%). Due
to its divalent cation nature, zinc stearate has a cone-shaped structure and
can offer a cross-bridged network structure as depicted in Figure 7(B),
subsequently increasing viscosity. In comparison, sodium stearate provides only
a linear-structured linkage, which does not increase viscosity. Reversible
macromolecular interactions such as hydrogen bonding can generate a networked
matrix structure,49 and hydrogen bonds between stearic acid and PEG
molecules were found to increase viscosity.50 When viscosity exceeds
200 Pa·s, a very stiff and less spreadable ointment is formed,51
which might be disadvantageous for topical application. In this regard, P3
formulations that contain 5% zinc stearate may be a suitable for developing an
ointment with improved ASEx stability.
Shelf
Life Estimation Using the Q10 Method. Based on the
accelerated stability analysis of ASEx in ointments, the expected shelf lives
at room (25 °C) and refrigerated (4 °C) temperatures were calculated
by applying the Q10 rule. The Q10 rule is a
simple approach to predict the valid shelf life at different temperatures by
using eq. (4):

where t90(T2) is the predicted shelf
life at temperature T2, t90(T1)
is the observed shelf life at temperature T1, and Q10
is a rate constant quotient related to activation energy.52 For
pharmaceutical predictions, the value of Q10 is commonly set
at 2, 3, or 4 because these correspond to a reasonable activation energy.
Because the reaction rate increases as the temperature is raised by ten
degrees, the Q10 value is calculated by eq. (5):

where k is a rate constant at a specified temperature. In this
experiment, by introducing the rate constants of ORG degradation in ASEx
solutions at 40 °C and 50 °C, the Q10 value was
calculated as 2.17. With this value, the shelf lives of the different ointment
formulations were calculated (Table 7). The results suggested that the shelf
lives of PEG-based ointments were longer than those of white petrolatum-based
ointments. Specifically, P3 showed the longest shelf life of more than four
years at 25 °C while other ointments resulted in a shelf life of less than
a year. However, under a refrigerated storage condition, P1 and P2 demonstrated
shelf lives of more than two years, indicating the potential for development of
a commodity. In contrast, V1 and V2 revealed shelf lives of less than one year
even when stored at 4 °C, indicating inadequate storage stability. Thus,
P3 would be the preferable option for the development of a commercial product
with sufficient long-term stability.
The Q10 rule is commonly used to estimate the shelf
life of drugs in solutions or drug powders for which the degradation process is
temperature-dependent.53 Although accelerated stability assessments
can be applied to ointment formulations, there is no assurance that the
obtained results can be extra-polated to different storage conditions or
quantitative expiry dating. Changes in temperature may alter other factors that
affect drug stability. For example, the crystallization and viscosity characteristics of
emulsions change as temperature increases.54 Thus, further studies
are necessary not only to observe any potential changes in physicochemical
stability but also to determine in vivo efficacy for atopic dermatitis
treatment.
|
Figure 1 Flow curves of ASEx-containing ointments. |
|
Figure 2 Typical HPLC chromatograms of ASEx in a stock solution (a); P3 ointment (b); V2 ointment (c). |
|
Figure 3 inetic a nalyses of ORG d eg radation: (A) F irst-order plots of remaining ORG in ASEx solution at different temperatures; (B) Arrhenius plot of the degradation rate constant versus the reciprocal absolute temperature. |
|
Figure 4 Comparison of the structural similarity of polyphenolic compounds. Blue-colored arrows indicate the possible points of degradation in the molecules. |
|
Figure 5 Degradation of ORG in ointment formulations during storage under accelerated conditions at 40 °C. |
|
Figure 6 Changes in apparent consistency assessment of the different ointments during storage under accelerated conditions at 40 °C for 120 days. |
|
Figure 7 Effects of zinc stearate on the viscosity and network structure of the P3 ointment: (A) Viscosity changes as a function of zinc stearate and sodium stearate concentrations (0.1~10%); (B) Schematic illustration of a cross-bridge network structure via hydrogen bonds between PEG molecules and ORG or zinc stearate. |
|
Table 3 Characteristics of ASEx-Containing Ointments |
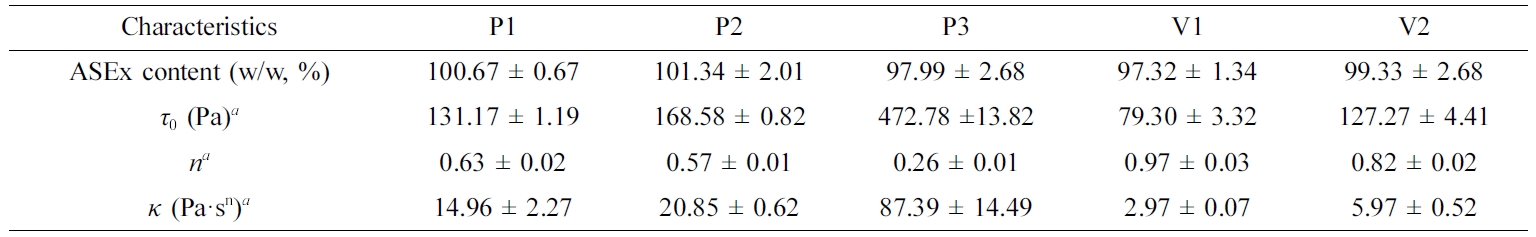
aCalculated by the Hershel–Bulkley equation: t0, yield stress; n, flow behavior index; κ, consistency index. Data are presented as the mean ± SD (n=3).ASEx, Alnus sibirica extract. |
|
Table 4 Sensory Assessment Results for Various Ointments |
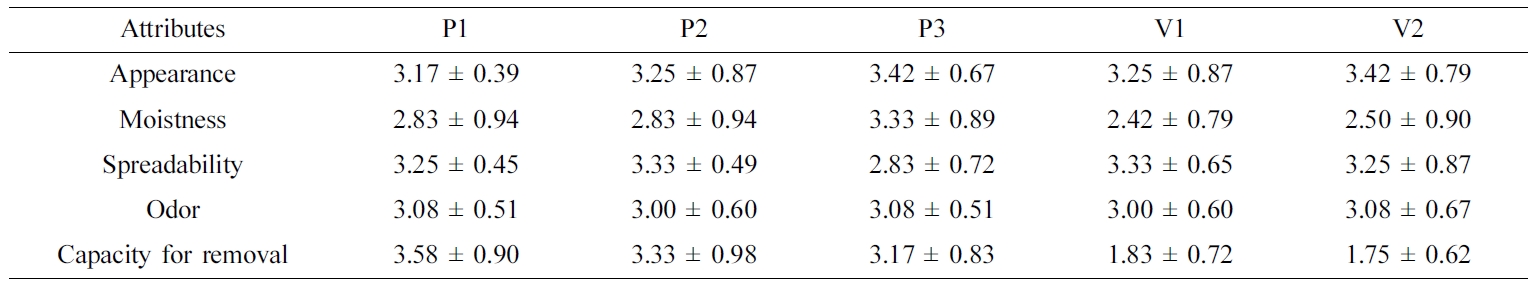
Data are presented as the mean ± SD (n=3). |
|
Table 5 Intra- and Inter-day Validation of the ORG Assay in a Stock Solution and Representative Ointments of P3 and V2 |
aCalculated by SD×100/mean. bCalculated by (spiked concentration – measured concentration)×100/spiked concentration. Data are presented as the mean ± SD (n=3). RSD, Relative standard deviation; % DEV, percentage deviation. |
|
Table 7 Estimated Shelf Lives of ASEx-containing Ointments at Different Temperatures |

Values represent the shelf life (days) calculated by employing the Q10 value o f 2.17. |
ASEx-containing ointments were successfully formulated in the presence or
absence of zinc stearate as a stabilizer. The rheological behavior of both the
PEG-based and white petrolatum-based ointments displayed a shear-thinning
system, indicating good flow properties for topical application. PEG molecules
are able to form complexes with ORG and zinc stearate via hydrogen bonding,
improving ASEx stability and providing high viscosity to the ointment matrix.
Based on the Q10 method, the shelf life of P3 was estimated
to be more than four years at 25 °C, suggesting that the P3 formulation
containing 5% zinc stearate may be a suitable candidate for topical
formulations with improved stability of ASEx. However, further studies are
needed to evaluate the formulation’s in vivo efficacy for the treatment
of atopic dermatitis.
- 1. W. S. Lee, J. R. Kim, K. R. Im, K. H. Cho, D. E. Sok, and T. S. Jeong, Planta Med., 71, 295 (2005).
-

- 2. T. Aoki and S. Ohta, Phytochem., 29, 3611 (1990).
-

- 3. M. Nomura, T. Tokoroyama, and T. Kubota, Phytochem., 20, 1097 (1981).
-

- 4. X. Ren, T. He, Y. Chang, Y. Zhao, X. Chen, S. Bai, L. Wang, M. Shen, and G. She, Molecules, 22, 1383 (2017).
-

- 5. J. Choi, S. Moon, H. Bae, Y. W. Kim, D. Lee, S. Kim, Y. Seo, H. S. Wang, Y. W. Choi, and M. W. Lee, Molecules, 24, 2883 (2019).
-

- 6. J. M. Han, W. S. Lee, J. R. Kim, J. Son, O. H. Kwon, H. J. Lee, J. J. Lee, and T. S. Jeong, J. Agric. Food Chem., 56, 92 (2007).
-

- 7. A. Lundqvist, L. U. Magnusson, C. Ullstrom, J. Krasilnikova, G. Telysheva, T. Dizhbite, and L. M. Hulten, Biochem. Biophys. Res. Commun., 458, 693 (2015).
-

- 8. C. J. Lee, S. S. Lee, S. C. Chen, F. M. Ho, and W. W. Lin, Br. J. Pharmacol., 146, 378 (2005).
-

- 9. S. E. Choi, M. S. Jeong, M. J. Kang, D. I. Lee, S. S. Joo, C. S. Lee, H. Bang, M. K. Lee, S. C. Myung, Y. W. Choi, K. S. Lee, S. J. Seo, and M. W. Lee, Exp. Dermatol., 19, e37 (2010).
-

- 10. M. S. Jeong, S. E. Choi, J. Y. Kim, J. S. Kim, E. J. Kim, K. H. Park, D. I. Lee, S. S. Joo, C. S. Lee, H. Bang, M.-K. Lee, Y. W. Choi, K.-s. Li, N. J. Moon, M. W. Lee, and S. J. Seo, Clin. Dev. Immunol., 2010, 618517 (2010).
-

- 11. L. B. Bacharier and H. Jabara, Int. Arch. Allergy Immunol., 115, 257 (1998).
-

- 12. M. J. Kang, J. Y. Eum, M. S. Jeong, S. E. Choi, S. H. Park, H. I. Cho, C. S. Cho, S. J. Seo, M. W. Lee, and Y. W. Choi, Biol. Pharm. Bull., 33, 100 (2010).
-

- 13. D. M. Ashcroft, P. Dimmock, R. Garside, K. Stein, and H. C. Williams, BMJ, 330, 516 (2005).
-

- 14. A. Marini, K. Reinelt, J. Krutmann, and A. Bilstein, Skin Pharmacol. Physiol., 27, 57 (2014).
-

- 15. J. C. Heo, D. Y. Nam, M. S. Seo, and S. H. Lee, Int. J. Mol. Med., 28, 733 (2011).
-

- 16. S. R. Kim, K. H. Jeong, S. G. Lee, J. B. Kang, C. J. Kim, M. Y. Kim, K. H. Yoon, D. I. Lee, S. S. Joo, M. W. Lee, S. Lee, and Y. W. Choi, Bull. Korean Chem. Soc., 36, 1688 (2015).
-

- 17. S. G. Lee, D. J. Shin, E. S. Lee, Y. T. Goo, C. H. Kim, H. Y. Yoon, M. W. Lee, H. Bang, S. J. Seo, and Y. W. Choi, Bull. Korean Chem. Soc., 39, 1287 (2018).
-

- 18. T. J. Im, I. Y. Oh, Y. M. Park, J. H. Park, M. W. Lee, J. Y. Cho, M. W. Lee, and Y. W. Choi, J. Pharm. Investig., 37, 211 (2007).
- 19. S. Yotsawimonwat, J. Rattanadechsakul, P. Rattanadechsakul, and S. Okonogi, Int. J. Cosmet. Sci., 32, 340 (2010).
-

- 20. M. Al-Ghabeish, X. Xu, Y. S. Krishnaiah, Z. Rahman, Y. Yang, and M. A. Khan, Int. J. Pharm., 495, 783 (2015).
-

- 21. X. Xu, M. Al-Ghabeish, Y. S. Krishnaiah, Z. Rahman, and M. A. Khan, Int. J. Pharm., 494, 31 (2015).
-

- 22. K. Welin-Berger, J. A. Neelissen, and B. Bergenståhl, Eur. J. Pharm. Sci., 13, 309 (2001).
-

- 23. H. S. Lim and G. Narsimhan, LWT-Food. Sci. Tech., 39, 344 (2006).
-

- 24. B. Sheng, L. Li, X. Zhang, W. Jiao, D. Zhao, X. Wang, L. Wan, B. Li, and H. Rong, Molecules, 23, 495 (2018).
-

- 25. M. Khakbiz, A. Simchi, and R. Bagheri, Mater. Sci. Eng. A, 407, 105 (2005).
-

- 26. M. H. Esfe, H. Rostamian, M. Rejvani, and M. R. S. Emami, Physica E Low Dimens. Syst. Nanostruct., 102, 160 (2018).
-

- 27. M. R. Pena Ferreira, P. Costa, and F. M. Bahia, Skin Res. Technol., 16, 444 (2010).
-

- 28. M. E. Parente, G. Ares, and A. V. Manzoni, J. Sens. Stud., 25, 685 (2010).
-

- 29. M. Lukic, I. Jaksic, V. Krstonosic, N. Cekic, and S. Savic, Int. J. Cosmet. Sci., 34, 140 (2012).
-

- 30. A. Müller, M. Zink, N. Hessler, F. Wesarg, F. A. Müller, D. Kralisch, and D. Fischer, RSC Adv., 4, 57173 (2014).
-

- 31. R. Ghadially, L. Halkier-Sorensen, and P. M. Elias, J. Am. Acad. Dermatol., 26, 387 (1992).
-

- 32. A. O. Barel, M. Paye, and H. I. Maibach, Handbook of cosmetic science and technology, CRC Press, Boca Raton, Florida, USA, 2014.
-

- 33. V. P. Shah, K. K. Midha, S. Dighe, I. J. McGilveray, J. P. Skelly, A. Yacobi, T. Layloff, C. Viswanathan, C. E. Cook, R. D. McDowall, K. A. Pittman, and S. Spector, J. Pharm. Sci., 81, 309 (1992).
-

- 34. K. Y. Moon, B. K. Ahn, S. G. Lee, S. H. Lee, D. W. Yeom, and Y. W. Choi, J. Pharm. Investig., 41, 331 (2011).
- 35. X. B. Suo, Y. J. Deng, H. Zhang, and Y. Q. Wang, Arch. Pharm. Res., 30, 876 (2007).
-

- 36. B. K. Ahn, S. G. Lee, S. R. Kim, D. H. Lee, M. H. Oh, M. W. Lee, and Y. W. Choi, J. Pharm. Investig., 43, 453 (2013).
-

- 37. Y. J. Wang, M. H. Pan, A. L. Cheng, L. I. Lin, Y. S. Ho, C. Y. Hsieh, and J. K. Lin, J. Pharm. Biomed. Anal., 15, 1867 (1997).
-

- 38. J. L. Gonzalez-Alfonso, P. Peñalver, A. O. Ballesteros, J. C. Morales, and F. J. Plou, Front. Nutr., 6, Article 30 (2019).
-

- 39. M. Nadim, D. Auriol, N. Lamerant‐FayeL, F. Lefevre, L. Dubanet, G. Redziniak, C. Kieda, and C. Grillon, Int. J. Cosmet. Sci., 36, 579 (2014).
-

- 40. W. Szeja, G. Grynkiewicz, and A. Rusin, Curr. Org. Chem., 21, 218 (2017).
- 41. J. Wang and X. H. Zhao, J. Serb. Chem. Soc., 81, 243 (2016).
-

- 42. I. W. Kim, M. D. Jang, Y. K. Ryu, E. H. Cho, Y. K. Lee, and J. H. Park, Anal. Sci., 18, 1357 (2002).
-

- 43. E. Karavas, E. Georgarakis, M. P. Sigalas, K. Avgoustakis, and D. Bikiaris, Eur. J. Pharm. Biopharm., 66, 334 (2007).
-

- 44. J. Dutta, Am. J. Chem., 2, 6 (2012).
- 45. J. Wu, W. Wei, L. Y. Wang, Z. G. Su, and G. H. Ma, Biomater., 28, 2220 (2007).
-

- 46. Y. Sun, J. No, G. Lee, W. Li, S. Yang, G. Yang, T. Schmidt, J. Kang, and Y. Kim, Molecules, 21, 480 (2016).
-

- 47. S. K. Bae, J. C. Kim, U. K. Jee, and J. D. Kim, Korean J. Chem. Eng., 16, 56 (1999).
-

- 48. J. Bortz, R. Levinson, M. Kirschner, and R. Cuca, US Patent 2006/0140990 A1 (2006).
- 49. H. Moynihan and A. Crean, Physicochemical basis of pharmaceuticals, Oxford Univ. Press, Oxford, UK, 2009.
- 50. M. Vijayaraghavan, S. Stolnik, S. M. Howdle, and L. Illum, Int. J. Pharm., 438, 225 (2012).
-

- 51. F. A. Oladimeji, E. O. Akinkunmi, A. I. Raheem, G. O. Abiodun, and V. O. Bankole, Afr. J. Tradit. Complement. Altern. Med., 12, 135 (2015).
-

- 52. T. P. Labuza and B. Fu, J. Ind. Microbiol., 12, 309 (1993).
-

- 53. S. Kristensen, Y. Lao, J. Brustugun, and J. Braenden, Pharmazie- Int. J. Pharm. Sci., 63, 872 (2008).
-

- 54. G. Zografi, J. Soc. Cosmet. Chem., 33, 345 (1982).
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2020; 44(2): 208-218
Published online Mar 25, 2020
- 10.7317/pk.2020.44.2.208
- Received on Jan 7, 2020
- Revised on Feb 5, 2020
- Accepted on Feb 5, 2020
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Young Wook Choi
-
College of Pharmacy, Chung-Ang University, 84 Heuksuk-ro, Dongjak-gu, Seoul 06974, Korea
- E-mail: ywchoi@cau.ac.kr
- ORCID:
0000-0003-2431-3995









 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.