- Temperature-responsive Poly(acrylamide-co-N-isopropyl acrylamide) Hydrogel: Synthesis, Characterization, and Sorption Application
Department of Chemical Engineering, Firat University, 23279, Elazig, Turkey
- 온도민감형 Poly(acrylamide-co-N-isopropyl acrylamide) 수화젤의 합성, 분석 그리고 흡습성
Acrylamide-based hydrogels
were used to remove cationic Basic Blue 3 (BB3) dye from aqueous solutions, in
this study. First, acrylamide-based hydrogel was synthesized and characterized
using SEM, FTIR and swelling analysis. The effects of various parameters were
investigated to determine the removal of the BB3 dye from aqueous solutions.
The sorption yield of BB3 dye of the hydrogel was determined to be 99%. The
desorption efficiency was found to be above 90%. The sorption experimental data
were evaluated using applying the Langmuir, Freundlich, Temkin, and
Dubinin-Radushkevich (D-R) isotherm models. It was determined that the Langmuir
was more suitable for isotherm the process. The maximum sorption capacity was
calculated as 28.3 mg/g. Pseudo first order, pseudo second order,
intraparticle diffusion, and Endovich kinetic models were used to calculate the
kinetic parameters. It was concluded that the pseudo second-order kinetic model
was described by the adsorption data very well.
The acrylamide-based hydrogels were synthesized by
chemical polymerization The temperature-dependent equilibrium water swelling
ratios of hydrophilic gels were determined by gravimetric methods in water. The
acrylamide-based hydrogels were used as a sorbent for the removal of Basic Blue
3 from aqueous solutions.
Keywords: hydrogel, swelling ratios, sorption, kinetic and thermodynamic evaluation
This study was funded by Firat University Scientific Research Projects Unit (FUBAP) within the scope of the individual project (Project No: MF.12.29).
The detailed information about experimental procedure for the sorption application of Basic blue 3 dye molecules was contained in the supporting information document. Sorbent composition, sorption conditions, kinetic and thermodynamic model equations, FTIR spectrum of sorbent (before and after sorption) and the summary of the literature related to the sorption of basic dye molecules were presented at http://journal.polymer-korea.or.kr.
PK_2020_044_01_49_Supporting_Information_template.pdf (523 kb)
Supplementary Information
Various dyestuffs and pigments are used for coloring various industrial
products, such as textile products, plastics, carpets, papers, printings, cosmetics,
and foods.1-4 The dye and textile industries are among the most
polluting sectors, and they produce the most toxic waste among all of the
industrial sectors. Approximately 10-15% of the dyestuffs used in textile
industries is contained in the effluent, resulting in the production of
significant amounts of wastewater. The spreading of this wastewater into the
environment results in the coloration of water, limited oxygenation capacity,
and decreased photosynthetic activities in water environments as a result of
decreased penetration by sunlight, which lead to chronic and acute poisoning.1,2
Also the wastewater can cause serious human health issues, including
hypersensitive reactions, genotoxic effects, and carcinogenic effects.2,5
The dyestuffs, which are commercially available in many varieties, are
classified in various ways, according to the structure, color, and methods of
application. Generally, dyes are classified as anionic (reactive, acid and
direct dyes), cationic (all basic dyes) and non-ionic (disperse dyes) dyestuffs
according to the particle load they produce during their dissolution in the
aqueous medium food.1-3 Cationic dyes, which are used extensively in
the paint industry, also are used in the textile industry, especially for the
dyeing of acrylic fibers.3 Cationic dyes, which interact with the
negatively-charged surfaces of cell membranes, enter cells, and accumulate in
the cytoplasm, are known to be more toxic than anionic dyes. Due to their
intended use, the dyestuffs have a low biodegradation rate and are resistant to
environmental conditions.1,6 Therefore, the wastewater that contains
these dyes must be treated before being released into the environment.4
However, cationic dyes cannot be removed completely by conventional treatment
methods, such as physical methods (coagulation, irradiation, ion exchange,
membrane separation), chemical methods (chemical oxidation, degradation,
electrochemical destruction), and biological treatment methods.1-5,7,8
In addition, the toxic effects of the secondary components that result from the
degradation of paint pose risks to the environment. In treating wastewater that
contains dyestuffs, the sorption process is generally more preferred over other
removal processes. Because sorption process has important advantages. The
design of sorption process is simple, has a low installation cost, is easy to
use and has low sensitivity to toxic substances.1,5,7
Several studies have been conducted on the sorption of various cationic
dyes on various adsorbent materials.9-28 But, as is known from
published research papers, the molecular structure of the adsorbate in a
sorption process and its related properties (e.g., molecular size and shape,
polar character, hydrophobic character, ionic or nonionic, ionic load centers
number and type) directly affect the sorption yield and the capacity of the
adsorbent.2,3,5 Therefore, the sorption behaviors of dye molecules
may be different depending on their different molecular structures, sorption
amount, sorption kinetics, and thermodynamics.3,5 Thus, work must be
done to determine the sorption yield and capacity for each dye molecule and
adsorbent pair.
Hydrogels are cross-linked polymeric structures with hydrophilic
character.29-32 They do not dissolve in water, and they can take a
large amount of water into their structures and swell.30-34
Hydrogels can be sensitive to various environmental factors, such as temperature,
pH, electric and magnetic fields, and ionic strength, and they react to changes
in these factors by changes in their volumes that are reversible. The water
sorption behavior of hydrogels is one of the most important factors that
determine their properties and areas of usage. The hydrogel structure in
contact with water passes from the glassy form to the rubber form with the
swelling. Because of this ability to change and their high water-holding
capacity, hydrogels are used in agriculture, the production of hygienic
products, tissue engineering, controlled drug release, separation processes, and
wastewater
treatment.31,33,34 Gels, which are hydrophilic and also known as
polyelectrolytes, are prepared from monomers that contain hydrophilic groups
and are water-ionizing. The hydrophilicity of gels generally is known
to increase with the presence of water-soluble groups, such as -OH, -COOH,
-CONH2, -SO3H.30,31 In addition, sorption in
hydrogels usually is due to the presence of the ionizable functional groups of
the monomers that form hydrogels. These functional groups have a complex
forming role in the removal of dye molecules from wastewater.32
Recently, hydrogels have been used extensively for the preparation of membranes
and sorbents in water purification and separation.29-39
In this study, acrylamide(AAm)-based hydrogels were synthesized using chemical
polymerization and characterized by instrumental analysis methods. And the
structural and morphological properties before and after the sorption process
of hydrogel employed as a sorbent in the experiments were defined using SEM and
FTIR analysis. The temperature-dependent equilibrium swelling ratios of
hydrophilic gels were determined with gravimetric methods in water. Also, they
were used as a sorbent for the removal of BB3 dye from aqueous solutions.
Various parameters that are effective in the removal of dyes, i.e., hydrogel
composition, initial pH, contact time, temperature, and initial dye
concentration were examined to determine their effects on sorption efficiency
and sorbent capacity. In addition, the sorption mechanism was analyzed using various
kinetic models and thermodynamic models. The desorption efficiency of hydrogels
was investigated.
. We purchased the
following chemical compounds from Sigma-Aldrich (USA). Acrylamide [CH2= CHCONH2,
AAm (99%)], N-isopropyl acrylamide [(H2C= CHCONHCH(CH3)2,
NIPAm (≥99%)], N,N,N,N tetramethylethylenediamine [(CH3)2NCH2CH2N(CH3)2,
TEMED (99%)], ethylene glycol dimethacrylate [CH2=C(CH3)COOCH2CH2-OCOC(CH3)=CH2,
EGDM (98%)], ammonium persulfate [(NH4)2S2O8,
APS (98%)].
The chemicals used in the synthesis process were used without further
purification. All of the supplied chemicals are either analytical grade or
highest purity.
Synthesis
of Hydrogels. The AAm-based hydrogels were synthesized in solution
medium according to the free radical polymerization mechanism. The
multi-functional cross-linking agent ethylene glycol dimethacrylate (EGDM) was
used in the preparation of the polymeric gels. To synthesize the AAm co-NIPAm
polymeric gels, the calculated amount (x g) of AAm monomer was dissolved in
1 mL of distilled water using a magnetic stirrer. The calculated amount
(1-x) of NIPAm comonomer were added to the reaction mixture and dissolved
completely. The total amount of the monomers that was used in the synthesis was
kept constant at one gram, and calculations were made using this amount. Then,
we added 0.25 mL/0.013 mmol of EGDM, followed by the addition of 0.2
mL/0.044 mmol of APS as the initiator and the addition of
0.25 mL/0.017 mmol TEMED solution (% 1) as the accelerator. The
prepared reaction mixture was placed in 2 mL glass pipettes and placed in an
oven at 60±1 oC. At the end of the polymerization, the glass
pipettes were broken carefully, and the polymeric gels were removed from the
pipettes. The gels were sliced thicknesses of 0.5 cm and placed in distilled
water. Any unreacted monomers and reactants were removed by washing for 48 h.
Before the gels were used for the water sorption and dye sorption experiments,
the gel samples were dried at 60±1 oC in an oven.
The compositions of the hydrogels that were synthesized are provided in
Table S1.
Analyses
and Characterization Studies. The structural
properties before and after the sorption process of hydrogel employed as a
sorbent in the experiments were defined using a Shimadzu IRSpirit Fourier
transform infrared spectrophotometer (FTIR). To reveal the surface morphology
of networked polymeric gel before and after the adsorption process, SEM
analysis was performed using the ZEISS scanning electron microscope. Before SEM
analysis, all of the hydrogel samples were coated with gold.
During the experiments, pH measurements were performed with a Thermo
Scientific Orion 1112000 3-star pH meter. Spectrophotometric measurements were
conducted using a Bausch & Lomb Spectronic 20 UV/vis spectrophotometer.
Weighing operations were done with an Ohaus brand Analytical Plus model balance
with ± 0.0001 grams sensitivity.
Determination
of Water Sorption Capacities. The swelling of
the synthesized polymeric gels was characterized using applying dynamic
swelling tests to the cross-linked polymeric samples at 25, 35, 45, and
55 oC. The AAm-based polymeric gel in the dry state was weighed
with a precision of 0.0001 g, placed in a beaker with distilled water at a
constant temperature, and allowed to swell. The moment the polymeric gel
entered the water was taken as t = 0, and the polymeric gels
were removed from the water at certain time intervals and it weighed. The
weighing process was continued regularly until constant weights were obtained
over time. At the end of the dynamic swelling tests, graphs depicting the
percentage of swelling as a function of time were prepared, and inflatable
isotherms were formed. It was observed that the percentage swelling values calculated
using the help of eq. (1) of the polymeric samples eventually reached a stable
equilibrium. This constant value is expressed as the equilibrium water
absorption capacity (Se)(gwater/ggel)
of the hydrogels, and it is calculated as follows: 
where; Se is the equilibrium water absorption capacity,
and Wwet and Wdry are the weight of swollen
and dried hydrogel samples, respectively. Dynamic swelling experiments were
performed in three replicates, and the averages of the data were presented.
Preparation
of Colored Wastewater. Basic dye Basic
Blue 3 (BB3, dye content: 25%, λmax: 654 nm, molecular weight:
359.89) was supplied from Sigma-Aldrich (USA). The chemical structure of BB3
was given in Figure S1. The stock solution of BB3 dye was prepared with a
concentration of 1000 mg/L. All of the working solutions and dilutions were
prepared using distilled water.
Determination
of Dye Sorption Capacities. The effect of gel
composition (crosslinker ratio and comonomer ratio), initial pH, temperature
and dye concentration were investigated in sorption experiments that were
conducted at a constant agitation rate in a temperature-controlled, shaking
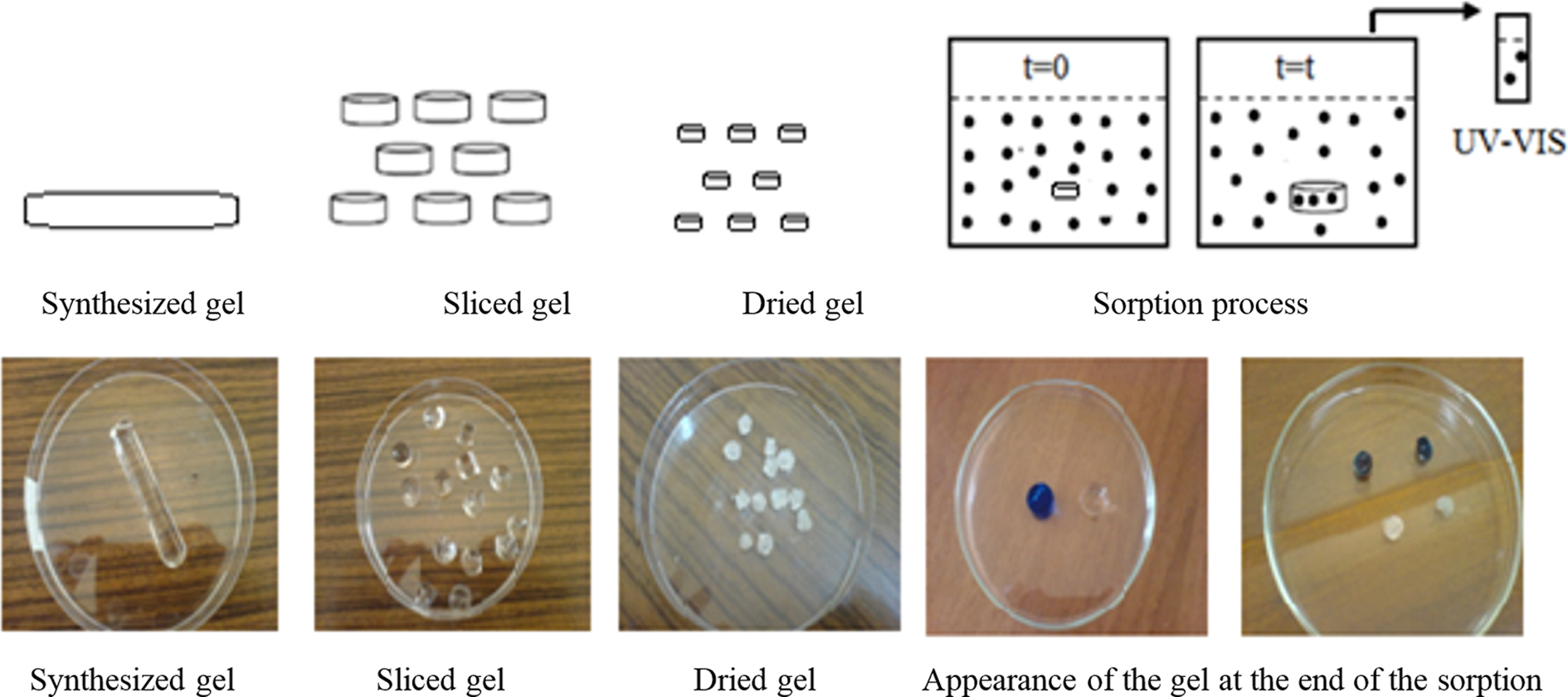
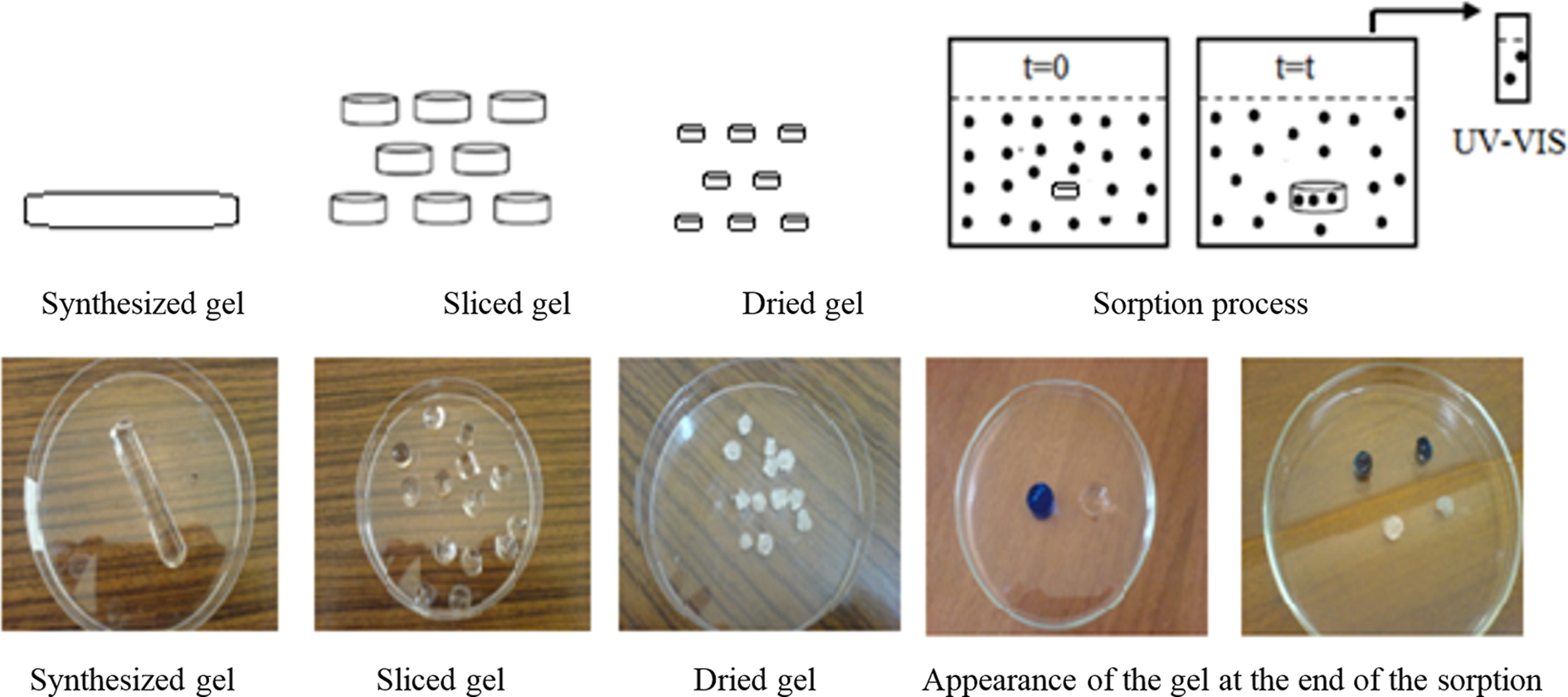
water bath. Figure 1 shows a schematic representation of the sorption process
of the BB3 dye molecules. Table 1 summarizes the effects of the parameters and
the study intervals.
Samples were analyzed at specific time intervals during the sorption
period, and the concentration of dye remaining in solution was measured
spectrophotometrically at the wavelength, λmax = 654 nm, using a
Bausch & Lomb Spectronic 20 UV/vis spectrophotometer. The sorption process
continued until equilibrium was reached. The sorption capacity (qe)
and the sorption efficiency (Ya) of the hydrogel based on the
experimental conditions were calculated using eq. (2) and eq. (3),
respectively:
where m (g) is the mass of the hydrogel; V (L) is the volume
of the aqueous medium; C0, Ce, and Ct
are the initial, equilibrium, and final concentrations of the BB3 dye molecules
in the aqueous medium, respectively; and qe is the
equilibrium sorption capacity, which represents the amount of BB3 dye molecules
adsorbed by the activated peanut shells (in mg/gm) at equilibrium conditions.
Kinetic
and Thermodynamic Analysis of the Sorption Process. To better
understand the mechanisms of sorption and to design a sorption system, the
capacity of sorption and the mechanism of sorption must be evaluated. The
structure of the sorption process was elucidated and calculated kinetic and
thermodynamic parameters by analyzing the obtained experimental data in five
different kinetic models and in four different isotherm models. Kinetic and
thermodynamic model equations used in the study were presented in Table 2 and
Table S3. The kinetic and isotherm studies were investigated using the gels
that were prepared for the NIPAm/monomer ratio of 0/1, the EGDM/monomer ratio
of 1/4.
Determination of Desorption Efficiency. The desorption
studies were investigated using the gels that were obtained as a result of
isotherm studies. After the sorption experiments, the BB3 dye molecules-loaded
hydrogel particles were collected from the solutions. The hydrogels were then
washed with 0.1 M HCl solution using magnetic stirring for 24 h. The washing
solution was renewed every 4 h. And the dye solutions obtained after
washing were collected in a glass vial to determine the concentration. The
collected dye solution concentration (Cs) was determined and
desorption efficiency was calculated using eq. (4) and eq. (5).
where Ca is the BB3 concentrations which were sorbed
from hydrogel at the equilibrium, and Cs is the BB3
concentration of washing solution which was obtained desorption process.
|
Figure 1 Schematic representation of the dye sorption process. |
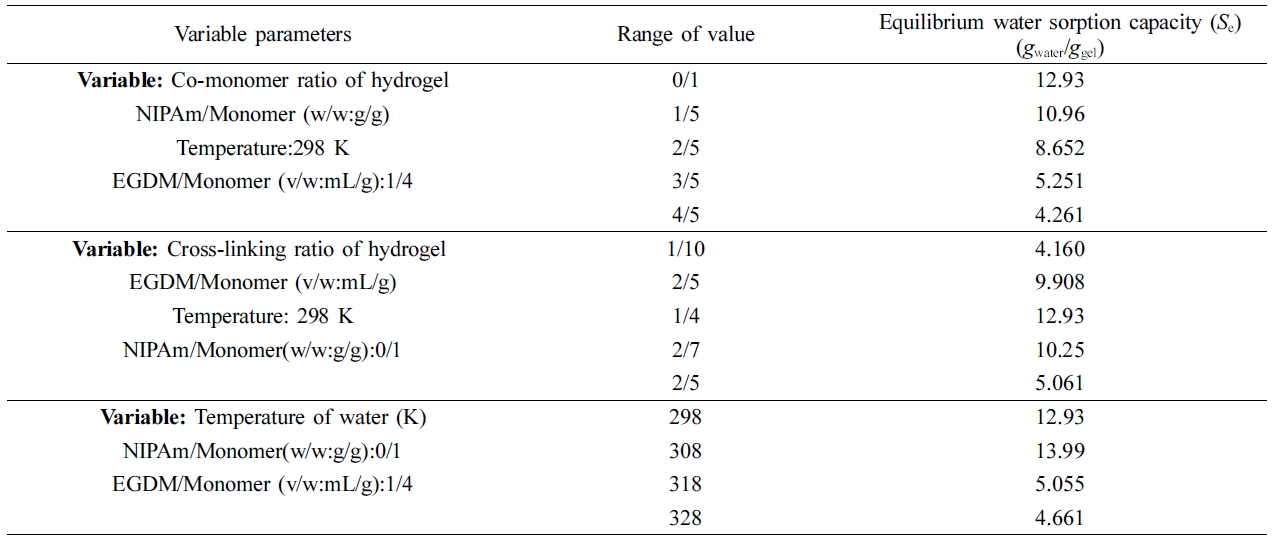
Water Sorption Capacity of Hydrogel. It is well known that the ability of the polymers to swell depends on their chemical structures, the solvent, the molar mass of the polymer, the elasticity of the polymer chain, the cross-linking density of the network, the hydrophilic character of polymeric network, the heterogeneity of the chemical structure of the polymeric chain, and the temperature of the solvent. Thus, in this study, we examined the effect of the temperature of the solvent, the crosslinking density of the hydrogel, and the hydrophilicity of the gel on the equilibrium swelling ratio. Table 2 shows the effect of these parameters on the equilibrium swelling ratio.
The experimental results indicated that the water sorption capacity of the gel decreased as the ratio of the co-monomer of the hydrogel (NIPAm/monomer (w/w: g/g)) increased because the hydrophilicity of the gel was decreased. The highest swelling ratio (12.9 gwater/ggel) was achieved in the sorption experiment in which the gel was synthesized with the NIPAm/monomer ratio of 0/1. Therefore, the effect of cross-linking density on the equilibrium swelling ratio was investigated using gels that were prepared using preserving that ratio.
Until the cross-linking density reaches the EGDM/monomer (v/w: mL/g) ratio of 0.25/1, the potential swelling ratio of the gel increased from 4.16 to 12.9 gwater/ggel as the cross-linking density of the gel was increased. However, after this critical value, the increase in the cross-link density led to a decrease in the water sorption capacity. This occurred because cross-linking above the critical value caused the gel to lose its elasticity and to form a fragile structure. Therefore, the effect of the temperature of the solvent on the equilibrium swelling ratio was investigated using gels that were prepared using the critical cross-linker ratio.
Generally, it is well known that the swelling ratio of polymer networks may increase or decrease as the temperature increases. The temperature at which the AAm-based gel films absorbed the maximum amount of water was determined to be 308 K. It was observed that the water sorption capacity of the gel at this temperature was about 14 gwater/ggel. As the temperature of the solution increased and exceeded 308 K, the water sorption capacity of the gel decreased significantly because the gel tended to shrink. It was determined that the swelling rate of the gel decreased to 4.7 gwater/ggel at 328 K.
The equilibrium swelling ratios of the hydrogels that were synthesized were compared with values available in the literature. It was observed that the degree of swelling of the AAm-based gel that we synthesized was somewhat lower than was reported in the literature. This result occurred because the gels that we synthesized had higher cross-linking densities than the gels reported in the literature.
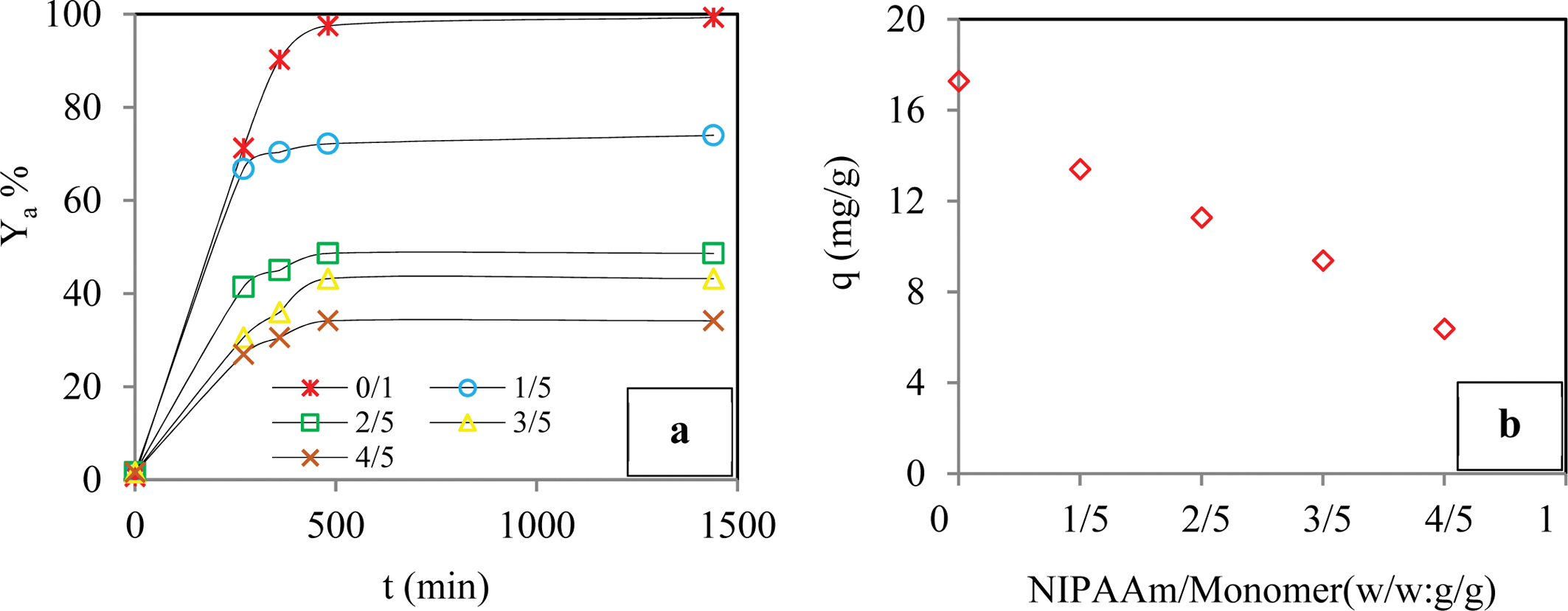
Removal Yield of BB3 Dye Molecules. Effect of the co-Monomer Ratio: To investigate the effect of the co-monomer ratio, i.e., hydrogel NIPAm/monomer (w/w: g/g), on the sorption process, sorption experiments were conducted in which the ratio was varied from 0/1 to 4/5 g/g, while other experimental conditions were kept constant. Figure 2 shows the sorption curves that were obtained, and, when these curves were examined, it was apparent that the sorption yield of the dye molecules and the sorbent capacity of hydrogel decreased as the co-monomer ratio that was used in the synthesis of the hydrogel increased. When the ratio was increased from 0/1 to 4/5, the removal yield decreased from about 99% to 34%, and the sorption capacity decreased from about 17.3 to 6.4 mg/g after 1440 min. Therefore, the effect of cross-linking density on the sorption yield was investigated using the gels that were prepared using preserving the NIPAm/monomer ratio of 0/1.
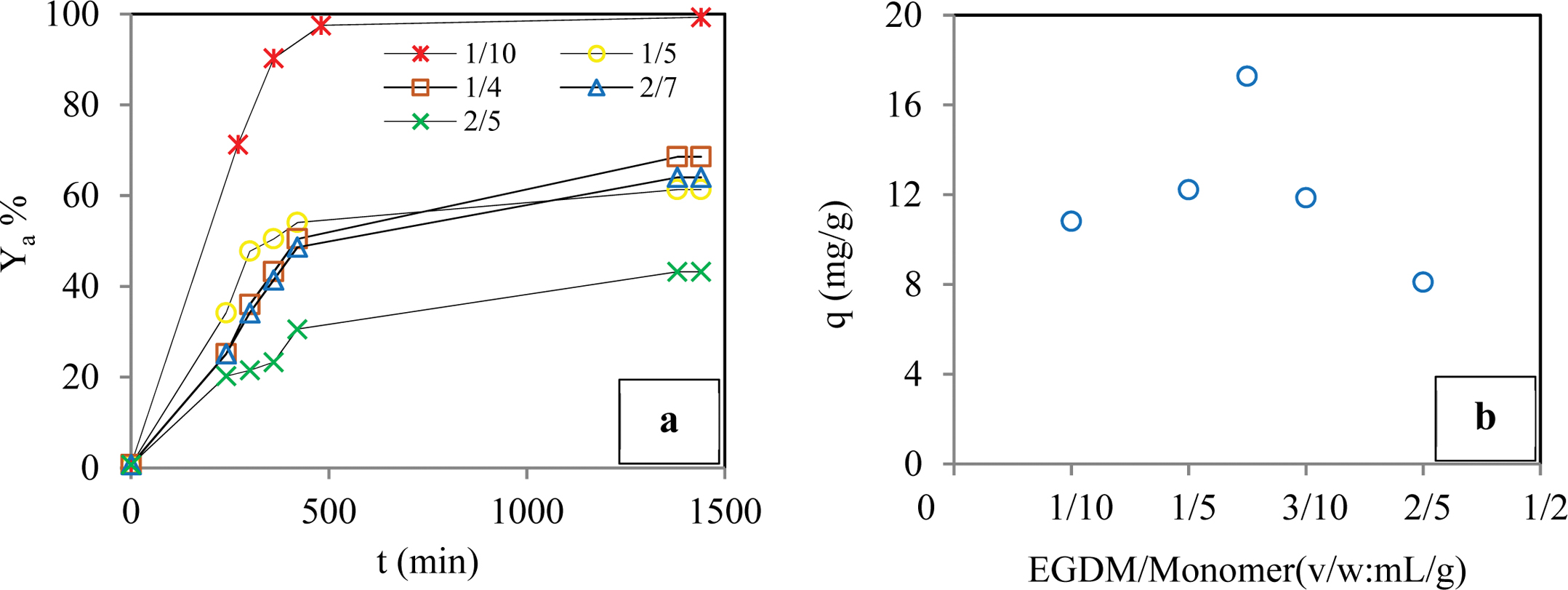
Effect of the Cross-linker Ratio: In order to evaluate the effect of the cross-linker ratio on the sorption process, we conducted sorption experiments using varying the cross-linker ratio of hydrogel EGDM/monomer (v/w: mL/g) from 1/10 to 2/5 mL/g while keeping the other experimental conditions constant. Figure 3 shows that the removal efficiency of the BB3 molecules was increased from about 61% to 93% by increasing the cross-linking dose to 1/4 mL/g at 1/10 mL/g. However, at higher cross-linker ratios above 1/4 (mL/g), significant reductions in the sorption yields were determined due to a decrease in the swelling capacity of the gel. A similar trend was observed for the sorption capacity. These results showed that the cross-linker ratio of 1/4 (mL/g) was sufficient to achieve maximum sorption yield for the experimental conditions we used. Therefore, the effects of other sorption parameters on the sorption yield were investigated using the gels that were prepared using preserving the EGDM/monomer ratio of 1/4.
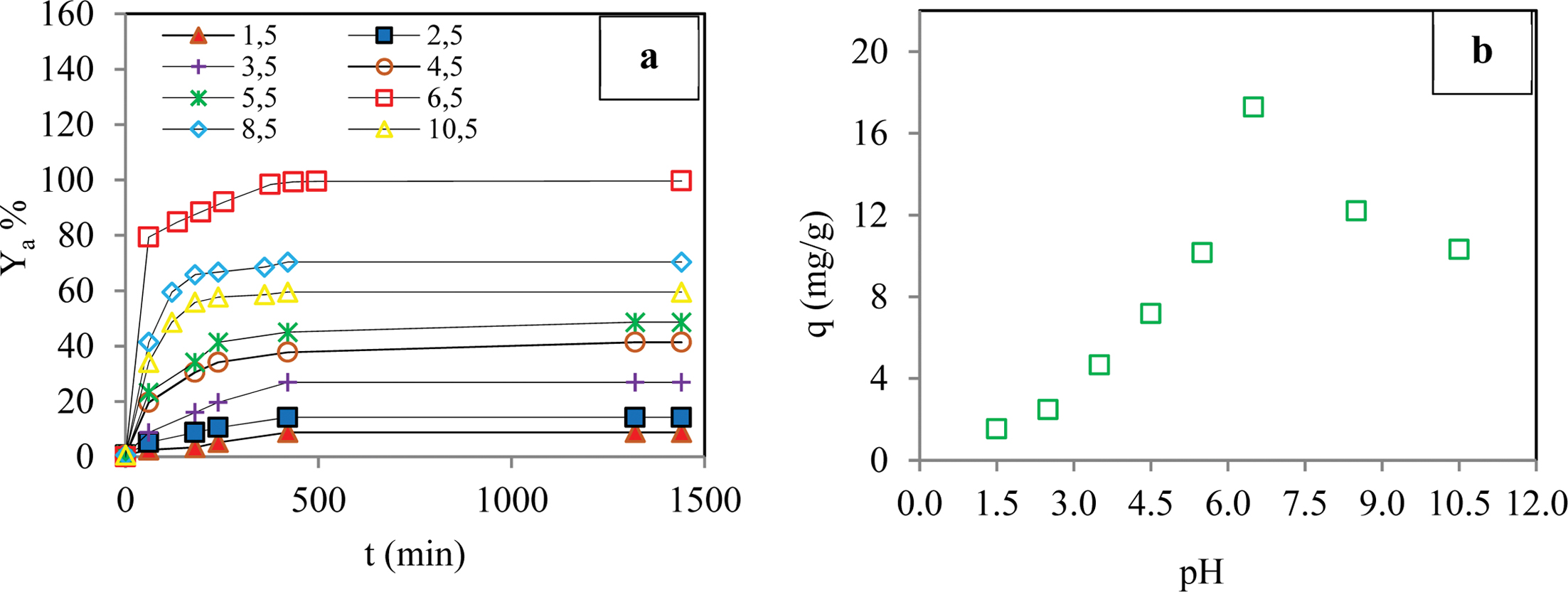
Effect of the Initial pH of Aqueous Medium: The acidity of the initial solution is among the most important factors that affect the sorption of dye molecules. The effect of pH on the sorption of BB3 molecules by AAm-based hydrogel was investigated in the pH range of 1.5 to 10.5 while keeping the other experimental conditions constant. Figure 4 shows that the sorption capacity of the gel increased in the pH range of 1.5 to 6.5, and it reached its maximum value (17.3 mg/g) at a pH value of 6.5. When the pH exceeded 6.5, a rapid decrease in sorption capacity occurred as a result of the decrease in the degree of ionization of the functional groups of the gel.
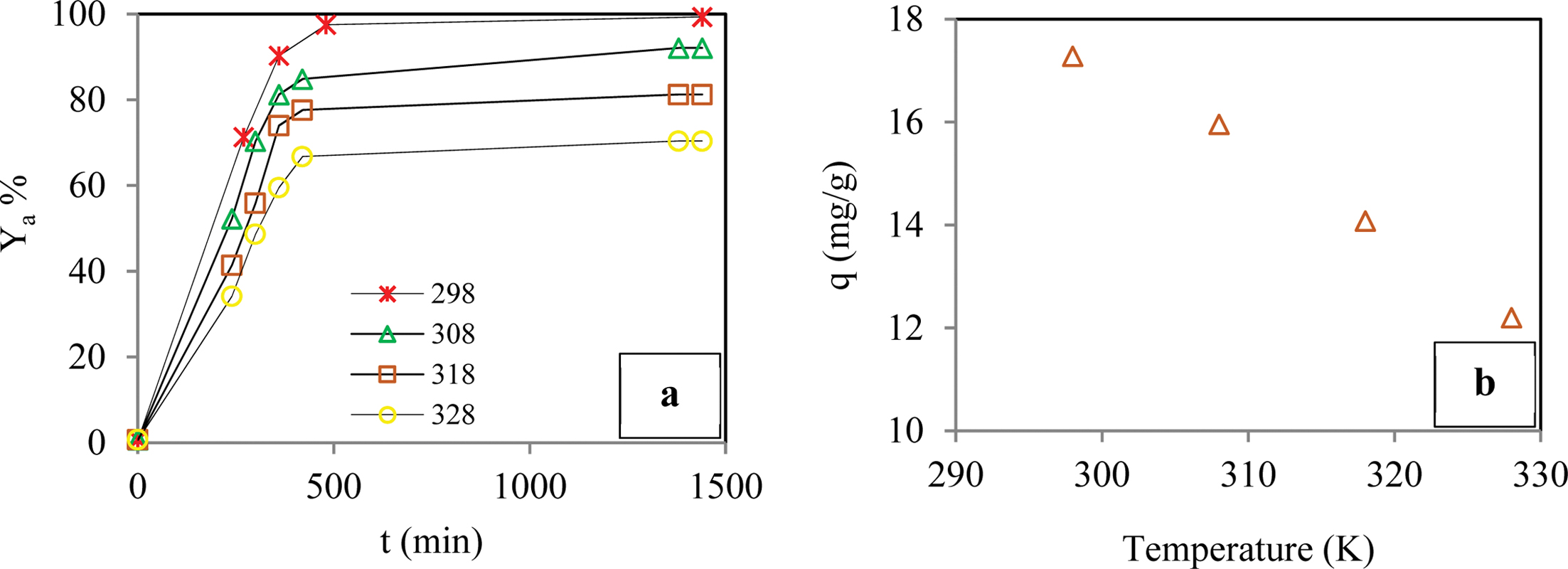
Effect of Contact Time and the Temperature of the Solution: Kinetic evaluation experiments were performed at four different temperatures, i.e., 298, 308, 318, and 328 K, to investigate the effect of temperature on BB3 sorption when other experimental conditions were kept constant. Examination of the sorption curves in Figure 5 shows that the dye removal efficiency and adsorbent capacity decreased as the temperature increased. When the temperature was increased from 298 K to 328 K, the removal yield decreased from about 99.3% to 66.7%, and the sorption capacity decreased from about 17.3 to
12.2 mg/g after 420 min. It was observed that the increase in the contact time resulted in increased removal of the dye molecules. As a result, in the studies that were conducted at 298 K, the highest removal efficiency, i.e., 99.3%, was attained at the end of the 440 min sorption period. Extension of the contact time beyond 440 min did not have an important effect on the yield of the removal. Therefore, it was concluded that the optimum contact time and temperature were 440 min and 298 K, respectively, given the exothermic nature of the sorption of BB3 dye molecules.
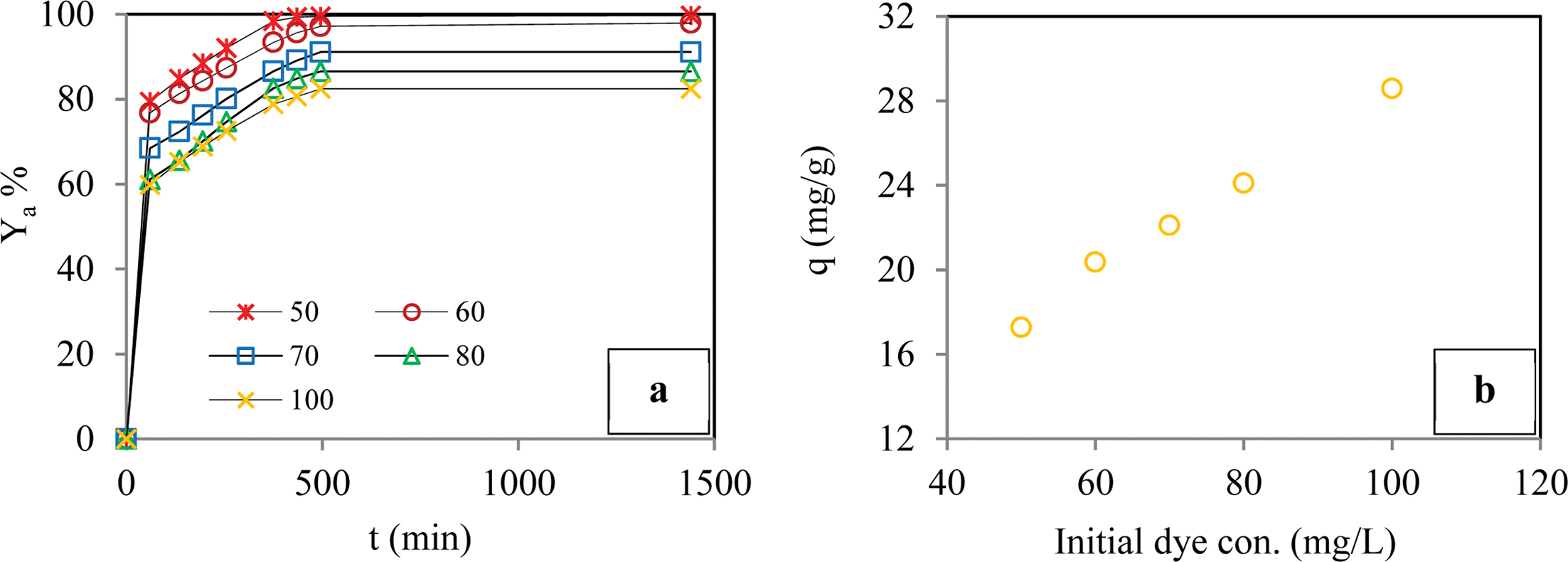
Effect of the Initial Concentration of Dye Molecules: Figure 6 shows that the removal yield of BB3 dye molecules was reduced as the initial concentration of the dye was increased. In contrast, the sorption capacity of the hydrogel increased as the initial dye concentration increased. This result was due to the saturation of the active centers of the hydrogel. While the highest absorption capacity (28.6 mg/g) was reached with an initial dye concentration of 100 mg/L, the highest removal yield (99.6%) was reached with an initial dye concentration of 50 mg/L.
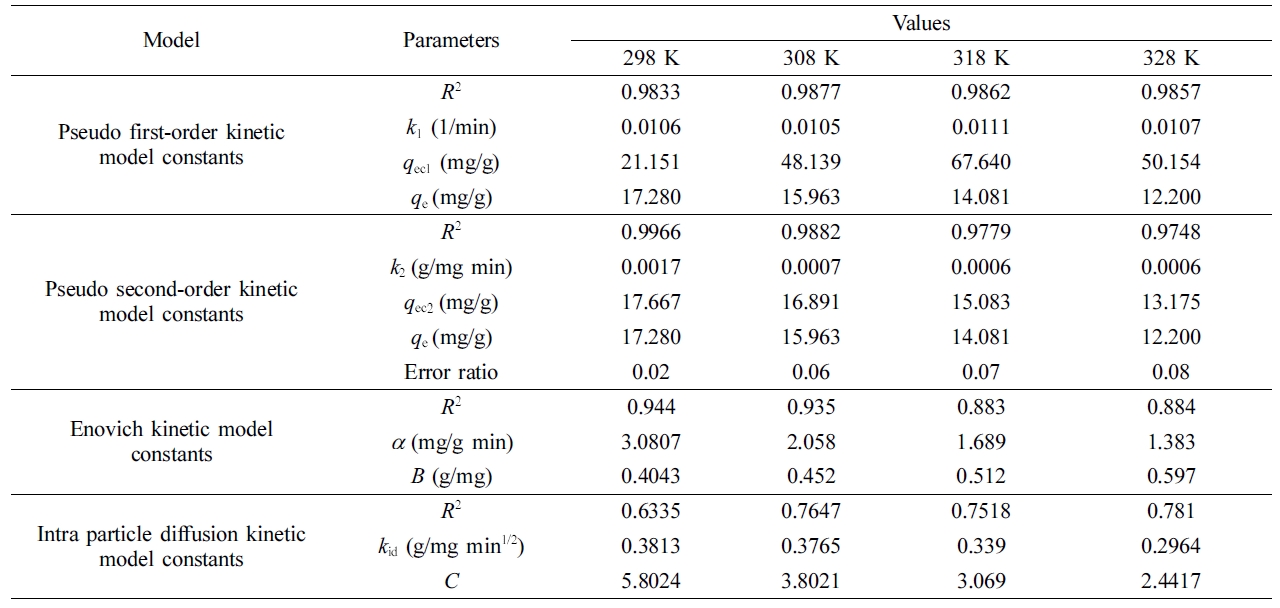
Determination of Kinetic Parameters. In order to determine the most suitable kinetic model, the sorption data were evaluated using four different kinetic models, i.e., pseudo first order, pseudo second order, intraparticle diffusion, and Endovich kinetic models. The parameters of the kinetic models, which were obtained based on the slopes and intercepts of the plotted graphs (Figure S2), are summarized in Table 3.
When we examined the data presented in Table 3, it was apparent that the calculated maximum sorption capacity values (qc) using the pseudo second order kinetic model equation were closer to the experimentally-determined maximum sorption capacity (qe), and the correlation coefficient was closer to one. In contrast, the sorption capacities calculated using the so-called first order kinetic model were far different from the experimental data. In addition, we observed that the correlation coefficient was lower than that of the pseudo second order kinetic model. As a result, it was concluded that the pseudo second-order model mechanism was more suitable for modelling the sorption process. Using the equation for the pseudo second-order kinetic model, the maximum sorption capacities (qc) for 298, 308, 318 and 328 K were calculated as 17.7, 16.9, 15.1, and 13.2 mg/g, respectively
The error ratios for 298, 308, 318, and 328 K were obtained as 0.02, 0.06, 0.07, and 0.08, respectively. The decreases in the values of qc and k2 (g/mg.min) with temperature indicated that the dye molecules were adsorbed favorably by the gel at lower temperatures.
Similar results for the sorption of basic dye molecules onto different adsorbents have been reported in the literature, e.g., raw pine,3 acid-treated pine cone powder,3 pine cones,9 chitosan-ethyl acrylate (Ch-g-Ea),10 N, F co-doped, flower-like TiO2 microspheres,11 metal hydroxide sludge (MHS),12 NaOH-pretreated rice husks,13 milled sugarcane bagasse,14 activated carbon,15 graphene oxide,15 multi-walled carbon nanotubes,15 surfactant modified bentonite clay (organoclay),21 copper nanowires loaded on activated carbon,22 brick waste,24 mixed silica–alumina oxide,25 pineapple (Ananas comosus) plant stems,26 sawdust,27 3‐allyloxy‐2‐hydroxy‐1‐propanesulfonic acid sodium salt.28
Figure S2(d) shows that the sorption process was divided into two parts and that the rate of sorption was different for the two parts. The first step, which might be attributable to external surface sorption, had a higher sorption rate than the second step at all of the temperatures. The second step could be viewed as a gradual sorption stage in which intraparticle diffusion is initiated, thereby decreasing the sorption rate in this step. The intraparticle diffusion stage controls and limits the rate of sorption. Table 3 provides the total sorption rate (kid) as the average of the rates for the two steps for all of the temperatures. The kinetic calculations indicated that the values of kid increased as the temperature increased.
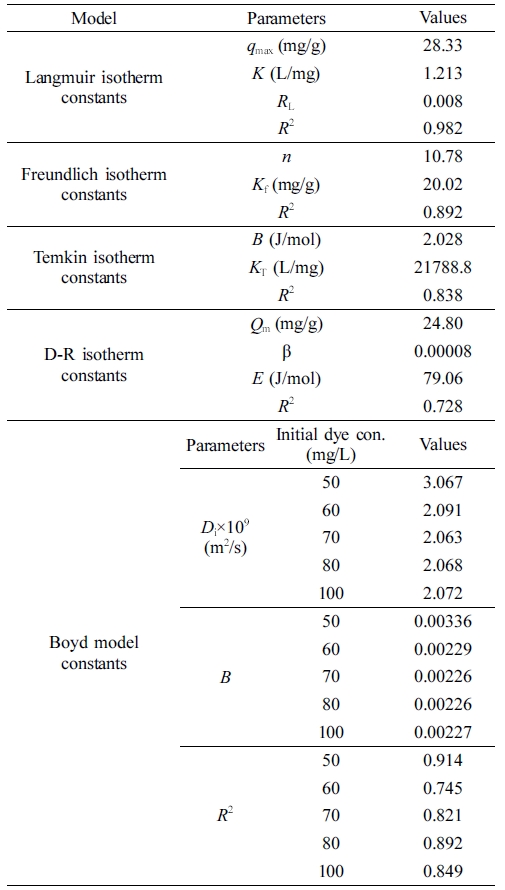
Determination of Equilibrium Parameters. In order to determine the most suitable thermodynamic model, the sorption data at 298 K were evaluated using five different thermodynamic models, i.e., the Langmuir, Freundlich, Temkin, Boyd, and Dubinin-Radushkevich isotherm models. Parameters of the thermodynamic models, which were obtained using the slopes and intercepts of the plotted graphs (Figure S3), are summarized in Table 4.
Since the correlation coefficients for the Langmuir isotherm were closer to one (0.98), it was concluded that the Langmuir isotherm model was more suitable than the other models for modeling the process of removing the BB3 dye molecules. These results were in agreement with other studies, which also showed that the Langmuir model provided a good fit for the sorption of basic dye molecules. Similar sorption results have been reported in the literature.9-11,13,15,18-20,22,24,25,29,34,39 Using the equation for the Langmuir isotherm model, the maximum monolayer sorption capacity (qmax) for 298 K was calculated as 28.3 mg/g. The numerical value of the RL is important in the process of deciding either the shape of the isotherms is favorable, unfavorable, linear or irreversible. If RL=0 it is irreversible, 0
The experimental results indicated that the water sorption capacity of the gel decreased as the ratio of the co-monomer of the hydrogel (NIPAm/monomer (w/w: g/g)) increased because the hydrophilicity of the gel was decreased. The highest swelling ratio (12.9 gwater/ggel) was achieved in the sorption experiment in which the gel was synthesized with the NIPAm/monomer ratio of 0/1. Therefore, the effect of cross-linking density on the equilibrium swelling ratio was investigated using gels that were prepared using preserving that ratio.
Until the cross-linking density reaches the EGDM/monomer (v/w: mL/g) ratio of 0.25/1, the potential swelling ratio of the gel increased from 4.16 to 12.9 gwater/ggel as the cross-linking density of the gel was increased. However, after this critical value, the increase in the cross-link density led to a decrease in the water sorption capacity. This occurred because cross-linking above the critical value caused the gel to lose its elasticity and to form a fragile structure. Therefore, the effect of the temperature of the solvent on the equilibrium swelling ratio was investigated using gels that were prepared using the critical cross-linker ratio.
Generally, it is well known that the swelling ratio of polymer networks may increase or decrease as the temperature increases. The temperature at which the AAm-based gel films absorbed the maximum amount of water was determined to be 308 K. It was observed that the water sorption capacity of the gel at this temperature was about 14 gwater/ggel. As the temperature of the solution increased and exceeded 308 K, the water sorption capacity of the gel decreased significantly because the gel tended to shrink. It was determined that the swelling rate of the gel decreased to 4.7 gwater/ggel at 328 K.
The equilibrium swelling ratios of the hydrogels that were synthesized were compared with values available in the literature. It was observed that the degree of swelling of the AAm-based gel that we synthesized was somewhat lower than was reported in the literature. This result occurred because the gels that we synthesized had higher cross-linking densities than the gels reported in the literature.
Removal Yield of BB3 Dye Molecules. Effect of the co-Monomer Ratio: To investigate the effect of the co-monomer ratio, i.e., hydrogel NIPAm/monomer (w/w: g/g), on the sorption process, sorption experiments were conducted in which the ratio was varied from 0/1 to 4/5 g/g, while other experimental conditions were kept constant. Figure 2 shows the sorption curves that were obtained, and, when these curves were examined, it was apparent that the sorption yield of the dye molecules and the sorbent capacity of hydrogel decreased as the co-monomer ratio that was used in the synthesis of the hydrogel increased. When the ratio was increased from 0/1 to 4/5, the removal yield decreased from about 99% to 34%, and the sorption capacity decreased from about 17.3 to 6.4 mg/g after 1440 min. Therefore, the effect of cross-linking density on the sorption yield was investigated using the gels that were prepared using preserving the NIPAm/monomer ratio of 0/1.
Effect of the Cross-linker Ratio: In order to evaluate the effect of the cross-linker ratio on the sorption process, we conducted sorption experiments using varying the cross-linker ratio of hydrogel EGDM/monomer (v/w: mL/g) from 1/10 to 2/5 mL/g while keeping the other experimental conditions constant. Figure 3 shows that the removal efficiency of the BB3 molecules was increased from about 61% to 93% by increasing the cross-linking dose to 1/4 mL/g at 1/10 mL/g. However, at higher cross-linker ratios above 1/4 (mL/g), significant reductions in the sorption yields were determined due to a decrease in the swelling capacity of the gel. A similar trend was observed for the sorption capacity. These results showed that the cross-linker ratio of 1/4 (mL/g) was sufficient to achieve maximum sorption yield for the experimental conditions we used. Therefore, the effects of other sorption parameters on the sorption yield were investigated using the gels that were prepared using preserving the EGDM/monomer ratio of 1/4. Figure 8
|
Figure 2 Effect of the co-monomer ratio on adsorption yield of dye (a); adsorption capacity of dye (b) (Conditions: pH of 6.5; temperature of 298 K; dye conc. of 50 ppm; contact time of 1440 min; agitation rate 180 rpm; cross-linker ratio of 1/4 mL/g; co-monomer ratio of 0/1 - 4/5 g/g). |
|
Figure 3 Effect of the cross-linker ratio on adsorption yield of dye (a); adsorption capacity of dye (b) (Conditions: pH of 6.5; temperature of 298 K; dye conc. of 50 ppm; contact time of 1440 min; agitation rate 180 rpm; cross-linker ratio of 1/10-2/5 mL/g; co-monomer ratio of 0/1 g/g). |
|
Figure 4 Effect of the initial pH of the aqueous medium on adsorption yield of dye (a); adsorption capacity of dye (b) (Conditions: pH of 1.5-10.5; temperature of 298 K; dye conc. of 50 ppm; contact time of 1440 min; agitation rate 180 rpm; cross-linker ratio of 1/4 mL/g; comonomer |
|
Figure 5 Effect of the temperature on adsorption yield of dye (a); adsorption capacity of dye (b) (Conditions: pH of 6.5; temperature of 298-328 K; dye conc. of 50 ppm; contact time of 1440 min; agitation rate 180 rpm; cross-linker ratio of 1/4 mL/g; co-monomer ratio of 0/1g/g). |
|
Figure 6 Effect of initial concentration of dye molecules on adsorption yield of dye (a); adsorption capacity of dye (b) (Conditions: pH of 6.5; temperature of 298 K; dye conc. of 50-100 ppm; contact time of 1440 min; agitation rate 180 rpm; cross-linker ratio of 1/4 mL/g; comonomer |
|
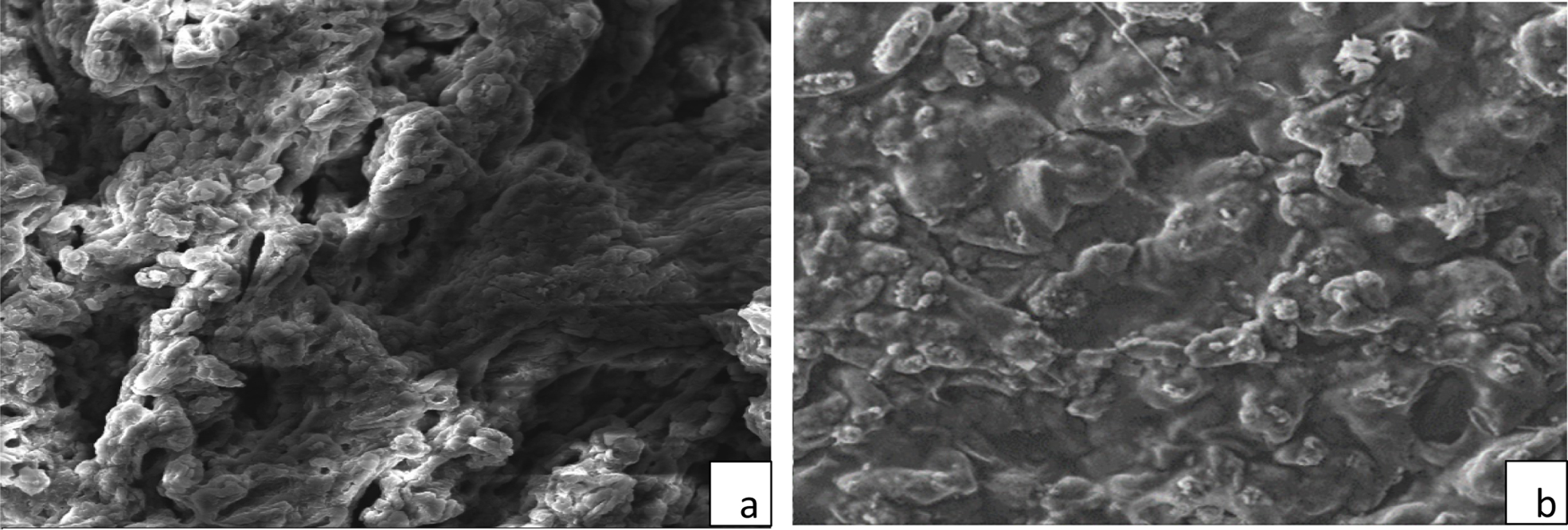
Figure 7 SEM images of the hydrogel before removal process (a); after removal process (b). ×1000. |
|
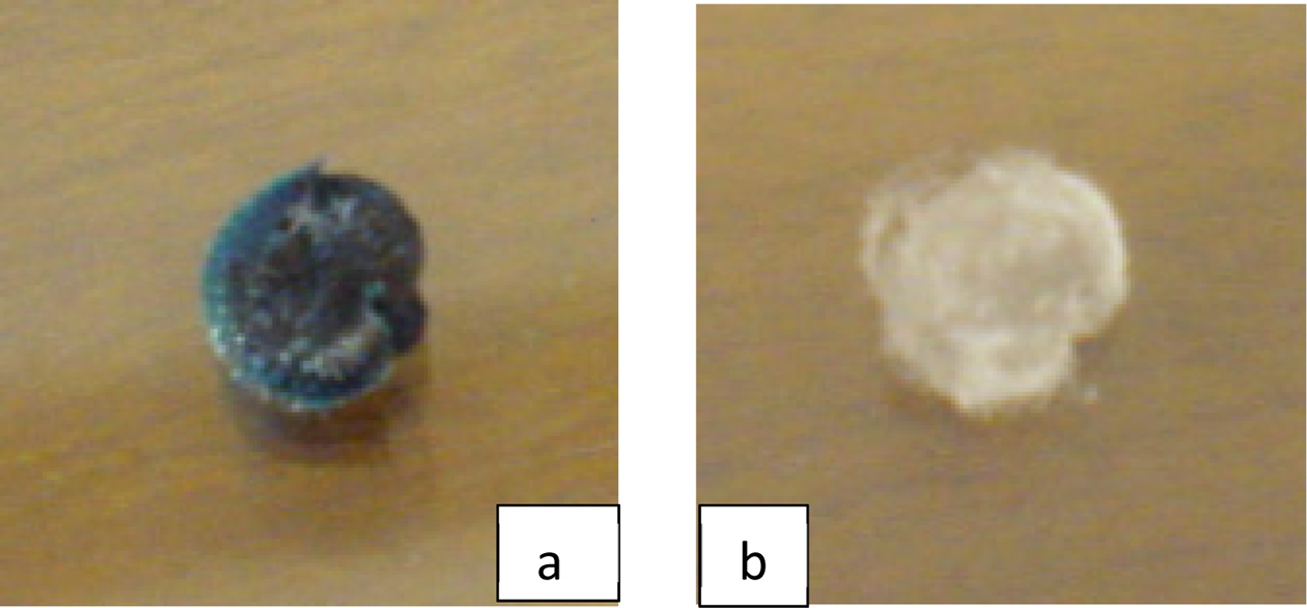
Figure 8 Photograph of the hydrogel before desorption process (a); after desorption process (b). |
|
Table 4 Parameters of the Thermodynamic Model for the Sorption
of Dye Molecules |
|
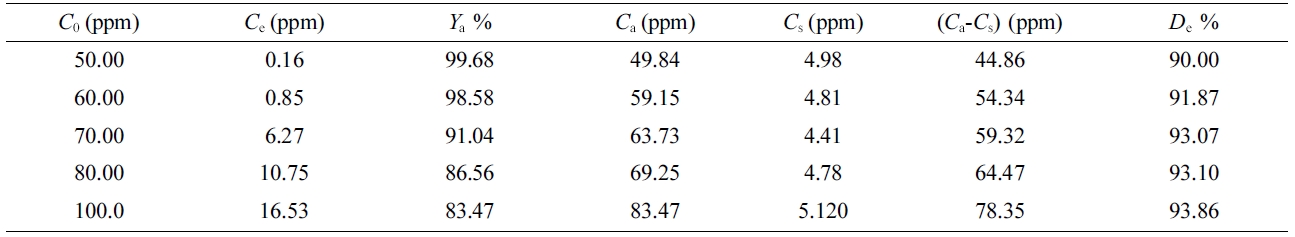
Table 6 Desorption Characteristics of BB3 dye Sorbed on
Acrylamide Based Hydrogel |
In this research paper, the sorption properties of the BB3 dye
molecules to the AAm-based hydrophilic gel structure were evaluated in
different gel compositions (co-monomer ratio, cross-linker ratio) and sorption
conditions (initial dye concentration, temperature of the solution and contact
time, initial pH of the solution). First, AAm-based hydrogels were synthesized
using chemical polymerization and characterized. Then, the
temperature-dependent equilibrium swelling ratios of the hydrophilic gels in
water were determined with gravimetric methods. Subsequently, the sorption
experiments were conducted at various conditions.
· The experimental results indicated that the water sorption capacity of
the gel decreased as the ratio of the co-monomer of the hydrogel increased.
· Until the cross-linking density reaches the critical value
(EGDM/monomer (v/w: mL/g) ratio of 0.25/1), the potential swelling ratio of the
gel increased, after this critical value, the increase in the cross-linking
density led to a decrease in the water sorption capacity.
· The temperature at which the AAm-based gel films absorbed the maximum
amount of water was determined to be 308 K.
· The water sorption capacity of gel at the optimum conditions (308 K;
EGDM/monomer (v/w: mL/g) ratio of 0.25/1; NIPAm/monomer (w/w: g/g):0/1) were
determined to be 14 gwater/ggel, respectively.
· The experimental results showed that the removal yield of BB3
increased due to the increased contact time. The trends observed for the
temperature and initial dye concentration of the solution were the opposite of
this.
· The optimum values of the co-monomer ratio, cross-linker ratio,
temperature, pH, contact time, and the initial concentration of the BB3 dye
molecules were NIPAm/monomer (w/w:g/g): 0/1; EGDM/monomer (v/w:mL/g):
0.25/1; 298 K; 6.5 ± 0.02; 440 min; and 50 ppm, respectively. The sorption
yield of dye molecules and the sorption capacity (qe) of the
hydrogel at the optimum conditions were determined to be 99% and 17.3 mg/g,
respectively.
· When the parameters obtained using four different sorption kinetic
models were examined, it was concluded that the pseudo second-order kinetic
model was described by the adsorption data very well. Using the equation for
the pseudo-second kinetic model, the maximum sorption capacities (qc)
for 298, 308, 318, and 328 K were calculated 17.7, 16.9, 15.1 and
13.2 mg/g, respectively. The decreases in the values of qc and
k2 (g/mg.min) with temperature indicated that the dye
molecules were adsorbed favorably by the gel at lower temperatures.
· When the parameters obtained using five different sorption isotherm
models were examined, it was determined that the Langmuir was more suitable for
isotherm the process. The maximum sorption capacity was calculated as 28.3 mg/g
using the Langmuir isotherm. Since the RL was between 0 and
1, it was determined that the sorption process was favorable.
Acknowledgements: This study was
funded by Firat University Scientific Research Projects Unit (FUBAP) within the
scope of the individual project (Project No: MF.12.29).
Supporting
Information: The detailed information about experimental procedure for
the sorption application of Basic blue 3 dye molecules was contained in the
supporting information document. Sorbent composition, sorption conditions,
kinetic and thermodynamic model equations, FTIR spectrum of sorbent (before and
after sorption) and the summary of the literature related to the sorption of
basic dye molecules were presented at http://journal.polymer-korea.or.kr.
- 1. M. T. Yagub, T. K. Sen, S. Afroze, and H. M. Ang, Colloid Interface Sci., 209, 172 (2014).
-

- 2. K. S. Bharathi and S. T. Ramesh, Appl. Water Sci., 3, 773 (2018).
-

- 3. S. Dawood and T. K. Sen, Water Res., 46, 1933 (2012).
-

- 4. R. K. Vital, K. V. N. Saibaba, K. B. Shaik, and R. Gopinath, J. Bioremediat. Biodegrad., 7, 1 (2016).
-

- 5. M. Kaykhaii, M. Sasani, and S. Marghzari, Chem. Mater. Eng., 6, 31 (2018).
-

- 6. S. Sivamani and B. L. Grace, I.J.BST, 2, 47 (2009).
-

- 7. M. Chincholi, P. Sagwekar, C. Nagaria, S. Kulkarni, and S. Dhokpande, IJSETR, 3, 835 (2014).
- 8. A. R. Warade, R. W. Gaikwad, R. S. Sapkal, and V. S. Sapkal, IJARIIE, 2, 3851 (2016).
- 9. A. S. Mohammadi, M. Sardara, A. Mohammadib, F. Azimia, and N. Nurieh, Archives of Hygiene Sciences, 2, 158 (2013).
- 10. M. Sadeghi-Kiakhani, M. Arami, and K. Gharanjig, J. Environ. Chem. Eng., 1, 406 (2013).
-

- 11. Y. Jiang, Y. Luo, F. Zhang, L. Guo, and L. Ni, Appl. Surf. Sci., 273, 448 (2013).
-

- 12. M. Attallah, I. Ahmed, and M. M. Hamed, Environ. Sci. Pollut. Res., 20, 1106 (2013).
-

- 13. L. Lin, S. R. Zhai, Z. Y. Xiao, Y. Song, Q. D. An, and X. W. Song, Bioresour. Technol., 136, 437 (2013).
-

- 14. Z. Zhang, I. M. O'Hara, G. A. Kent, and W. O. S. Doherty, Ind. Crop. Prod., 42, 41 (2013).
-

- 15. Y. Li, Q. Du, T. Liu, X. Peng, J. Wang, J. Sun, Y. Wang, S. Wu, Z. Wang, Y. Xia, and L. Xia, Chem. Eng. Res. Design, 91, 361 (2013).
-

- 16. G. L. Dotto, J. M. Moura, T. R. S. Cadaval, and L. A. A. Pinto, Chem. Eng. J., 214, 8 (2013).
-

- 17. B. A. Sadler and L. Metals, Removal of Methylene Blue from Aqueous Solution Using a Novel Red Mud Mixed with Cement, John Wiley & Sons Inc., Hoboken, New Jersey, p 1-133 (2013).
-

- 18. M. Roosta, M. Ghaedi, N. Shokri, A. Daneshfar, R. Sahraei, and A. Asghari, Spectrochim. Acta. A Mol. Biomol. Spectrosc., 118, 55 (2014).
-

- 19. Z. Zhou, S. Lin, T. Yue, and T. C. Lee, J. Food Eng., 126, 133 (2014).
-

- 20. P. F. Wang, M. H. Cao, C. Wang, Y. H. Ao, J. Hou, and J. Qıan, J. Appl. Surface Sci., 290, 116 (2014).
-

- 21. T. S. Anirudhan and M. Ramachandran, Process Saf. Environ. Prot., 95, 215 (2015).
-

- 22. M. Ghaedi, E. Shojaeipour, A. M. Ghaedi, and R. Sahraei, Spectrochim. Acta A Mol. Biomol. Spectrosc., 142, 135 (2015).
-

- 23. Y. Premkumar and K. Vijayaraghavan, Sep. Sci. Technol., 50, 1439 (2015).
-

- 24. F. Kooli, L. Yan, R. Al-Faze, and A. Al-Sehimi, Arab. J. Chem., 8, 333 (2015).
-

- 25. M. Wawrzkiewicz, M. Wiśniewska, V. M. Gun'ko, I. Vladimir, and V. I. Zarko, Powder Technol., 278, 306 (2015).
-

- 26. S. L. Chan, Y. P. Tana, A. H. Abdullah, and S. T. Ong, J. Taiwan Institute Chem. Eng., 61, 306 (2016).
-

- 27. S. Banerjee and M. Chattopadhyaya, Arab. J. Chem., 10, 1629 (2017).
-

- 28. A. G. Ibrahim, F. A. Hai, H. Abd El-Wahab, and H. Aboelanin, Adv. Polym. Technol., 37, 3561 (2018).
-

- 29. S. Kang, Y. Zhao, W. Wang, T. Zhang, T. Chen, H. Yi, F. Rao, and S. Song, Appl. Surf. Sci., 448, 203 (2018).
-

- 30. S. Sudarsan, D. S. Franklin, M. Sakthivel, G. Chitra, T. B. Sridharan, and S. Guhanathan, J. Polym. Environ., 26, 3773 (2018).
-

- 31. D. Saraydın, Y. Işıkver, and E. Karadağ, Polym. Eng. Sci., 58, 310 (2018).
-

- 32. T. E. Dudu, D. Alpaslan, and Y. Uzun, Int. J. Environ. Res., 11, 557 (2017).
-

- 33. K. Bello, B. K. Sarojini, B. N. Anjali, and R. K. Byrappa, Carbohydr. Polym., 181, 605 (2018).
-

- 34. N. Mohammed, N. Grishkewich, R. M. Berry, and K. C. Tam, Cellulose, 22, 3725 (2015).
-

- 35. E. Makhado, S. Pandey, P. N. Nomngongo, and J. Ramontja, J. Colloid Interface Sci., 513, 700 (2018).
-

- 36. W. Wang, Y. Zhao, H. Bai, T. Zhang, V. Ibarra-Galvan, and S. Song, Carbohydr. Polym., 198, 518 (2018).
-

- 37. F. A. Ngwabebhoh, M. Gazi, and A. A. Oladipo, Chem. Eng. Res. Design, 112, 274 (2016).
-

- 38. V. V. Panic and S. J. Velickovic, Sep. Purif. Technol., 122, 384 (2014).
-

- 39. S. Ghorai, A. Sarkar, M. Raoufi, A. B. Panda, H. Schönherr, and S. Pal, ACS Appl. Mater. Interfaces, 6, 4766 (2014).
-

- 40. R. Akkaya, and U. Ulusoy, Hacettepe J. Biol. Chem., 39, 359 (2011).
-

- 41. A. M. Dumitrescu, G. Lisa, A. R. Iordan, F. Tudorache, I. Petrila, A. I. Borhan, M. N. Palamaru, C. Mihailescu, L. Leontie, and C. Munteanu, Mater. Chem. Phys., 156, 170 (2015).
-

- 43. K. Vijayaraghavan and Y. S. Yun, Biotechnol. Adv., 26, 266 (2008).
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2020; 44(1): 49-60
Published online Jan 25, 2020
- 10.7317/pk.2020.44.1.49
- Received on Sep 16, 2019
- Revised on Nov 2, 2019
- Accepted on Nov 21, 2019
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
- Supporting Information
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Şeyda Taşar
-
Department of Chemical Engineering, Firat University, 23279, Elazig, Turkey
- E-mail: sydtasar@hotmail.com; sydtasar@firat.edu.tr
- ORCID:
0000-0003-3184-1542











 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.