- Synthesis of P Doped EDTA-Co Structure and Synergistic Effect of ORR as Electrochemical Catalyst
School of Chemical Engineering & Materials Science, Chung-Ang University, Seoul 06971, Korea
*Department of Mechanical and Aerospace Engineering, The Hong Kong University of Science and Technology, Hong Kong
- 인이 함유된 EDTA-Co 구조 제조 및 전기화학적 촉매로서 산소 환원 반응 적용 시 상승효과
중앙대학교 공과대학 화학신소재공학부, *홍콩과학기술대학교 기계 & 항공우주공학과
In this study, we successfully
developed an electrochemical cathodic catalyst with a previously unreported
structure. In essence, a new material was fabricated by doping EDTA-Co with P.
EDTA has six ligand sites and forms an octahedral structure through six coordination
bonds with transition metals. In addition, by doping hetero atoms, the atomic
and spin charge density of the catalyst surface are changed to lower the
adsorption energy of oxygen, which leads to more favorable reactions and
enhances the catalytic properties. Co and P were added to EDTA to synthesize a
successful catalyst, and testing confirmed an increase in electrochemical
performance due to the addition of Co and P. Moreover, this work demonstrates
that the synergy effect exceeds that of simply adding Co and P.
이번 연구를 통해서 이전에 보고되지 않은 구조를 가진 전기화학
음극 촉매를 성공적으로 개발했다. 이는 EDTA와 Co의 결합에 P를 도핑함으로써 제작되었다. EDTA는 6개의 리간드 부위를 통하여 전이금속과 6개의 배위결합을 진행하여 팔면체의 구조를 형성한다. 또한 헤테로
원자를 도핑함으로써, 촉매 표면의 원자 및 스핀전하 밀도를 변화시켜 산소의 흡착에너지를 낮추어 산소환원반응에
보다 유리하게 변화시켜 촉매의 특성을 향상시킨다. 성공적인 촉매를 합성하기 위해 간단한 1단계 열분해 반응을 통하여 Co 및 P를 EDTA와 합성하였으며, 그
결과 Co 및 P의 첨가로 인해 전기화학적 특성의 증가가
증명되었다. 또한 이 연구는 단순히 Co와 P가 각각 더해지는 효과 그 이상의 상승효과를 보였다.
A new material (as EDTA-Co with P) was fabricated by
coordination bonding with Co and by doping N. Cobalt and EDTA undergo six
coordination bonds. In addition, the oxygen chemical adsorption energy of the
catalyst surface was changed when N doping, and in each case, an
electrochemical synergy occurred, and when the two cases were mixed, excellent
electrochemical characteristics were shown.

Keywords: oxygen reduction reaction (ORR), ethylenediaminetetraacetic acid (EDTA), phosphorus (P) doping micro-porous carbon, high specific surface area, six coordination between Co and EDTA
This research was supported by
the Chung-Ang University Research Grants in 2019 and also supported by the
Korea Agency for Infrastructure Technology Advancement under the Ministry of
Land, Infrastructure and Transport of the Korean government (Project Number:
19SCIP-B108153-05).
Due to the continuously increasing energy consumption and depletion of
environmentally harmful fossil fuels, the development of eco-friendly and
renewable energy is attracting significant attention from the scientific
research community.1 Among the various energy storage media options,
metal-air batteries have high energy storage density and theoretically have an
air electrode serving as the cathode electrode, which allows an infinite supply
of resources. In addition, metal-air batteries have the advantage of being very
similar in structure and function to the widely used Li-ion battery, which
eliminates any large changes in energy storage industrial processing.2-4
The electrochemical performance of a metal-air battery is determined by
its reactivity with oxygen in the air electrode’s cathode. The more oxygen that
is adsorbed and desorbed on the electrode’s surface, the more electric charge
that gets generated, and the higher the energy storage density. Therefore, in
order to obtain a metal-air battery with high energy storage density, the
cathode electrode’s oxygen reactivity must be elevated.5,6 However,
this poses a challenge, as the cathode has a very low reactivity with oxygen,
and thus requires a catalyst material to increase the reactivity. Various
studies have been conducted to determine the optimal electrochemical catalyst
required at the cathode. Several studies have shown Pt/C to be the best
performing catalyst.7 However, on account of Pt/C has resource
scarcity and high cost, carbon-based materials have been studied as
alternatives, due to their low cost and specialized properties. However, the
catalyst composed only of carbon has low reactivity with oxygen and is low in
efficiency as an electrochemical catalyst. In order to overcome this, doping
with hetero atom (B, N, S, P) improves performance.8,9 Moreover,
synergistic effects are produced through bonding with metals.10
Many previous studies have conducted material-based experiments that
consist only of carbon such as carbon nanotube (CNT) and graphene. In this
study, however, ethylenediaminetetraacetic acid (EDTA) was selected as the
carbon-based catalyst material. EDTA contains two amine-like structures that
contain N. This has the advantage of having pyridinic N, pyrrolic N, graphitic
N and oxidized N structures of N-doped material even without N doping. EDTA has
six ligand functional groups; when it encounters transition metals, it
undergoes coordination bonding to form octahedral structures.11,12
Throughout this process, the EDTA maintains a porous texture and high surface
area, which is highly advantageous for an electrochemical catalyst. The
electrolyte and air move between the pores and the coexistence of all
three-phases (gas-liquid-solid) increases oxygen reactivity and provides a
smooth passage for charge transfer.13,14 In this work, we report the
development of a novel electrochemical catalyst through the combination of EDTA
and cobalt (Co), a member of the transition metal family. Furthermore, the
addition of P generates supplemental synergistic effects that demonstrates the
efficacy of our EDTA-Co and P catalyst15— a solution, that to the
best of our knowledge, has yet to be proposed.
Synthesis
of EDTA-Co Bond Precursor. All reagents were
used without further purification. 1.6 g of EDTA (≥ 99%, Sigma-Aldrich) was
added to 20 mL of DI water and stirred for 2 h. Next, the solution was
ultrasonicated and dispersed for 4 h (VCX-750, Ultrasonic Processor) using a
750 W output. 0.3 g of Co(Cl)2·6H2O (97%, Deajung
Chemical Co., Korea) was added to the dispersed EDTA solution to induce bonding
between the EDTA and Co constituents. Finally, the solution was stirred for 20
min and allowed to stand for 2 h until bonding completed. The EDTA-Co bonded
precursor was obtained after washing and filtering with DI water.
Synthesis
of Porous Electrocatalytic Samples. 1.6 g of EDTA or EDTA-Co,
0.8 g of triphenylphospine (≥95%, Sigma-Aldrich) 0.8 g of potassium
hydroxide (85%, Sigma-Aldrich), and 0.8 g of melamine (99%, Deajung Chemical
Co., Korea) were mixed in a mortar until it transformed into a semi-solid state
with an adhesive-like viscosity. The resulting mixture was transferred to an alumina
boat and calcined at a heating rate of 10 oC/min to 750 oC
for 2 h under N2 flow. After the sample cooled, it was refluxed
in 200 mL of 0.1 M sulfuric acid (H2SO4, 0.1 N
Standard Solution, Alfa-Aesar) at 80 oC for 12 h to remove
impurities. Afterwards, the products were washed with DI water and ethanol,
filtered, and dried overnight in a 70 oC oven. Using this
experimental method, the product samples’ EDTA-Co bond were determined
depending on the initial EDTA and EDTA-Co material selection, and the P doping
can be controlled depending on the presence or absence of triphenylphospine.
Sample
Characterization Methods. The sample
materials’morphology and porous structure was determined by field-emission
scanning electron microscopy (FE-SEM, SIGMA, Carl Zeiss) and field-emission
transmission electron microscopy (FE-TEM, JEM-F200) with an energy dispersive
spectroscopy (EDS) detector. The crystal structures’EDTA-Co bonds were characterized
via X-ray diffraction (XRD, Bruker-AXS) with a 2θ = 20o
to 80o diffraction range. The diffractometer operated at 40 kV
and was equipped with a Cu Kα radiation source.
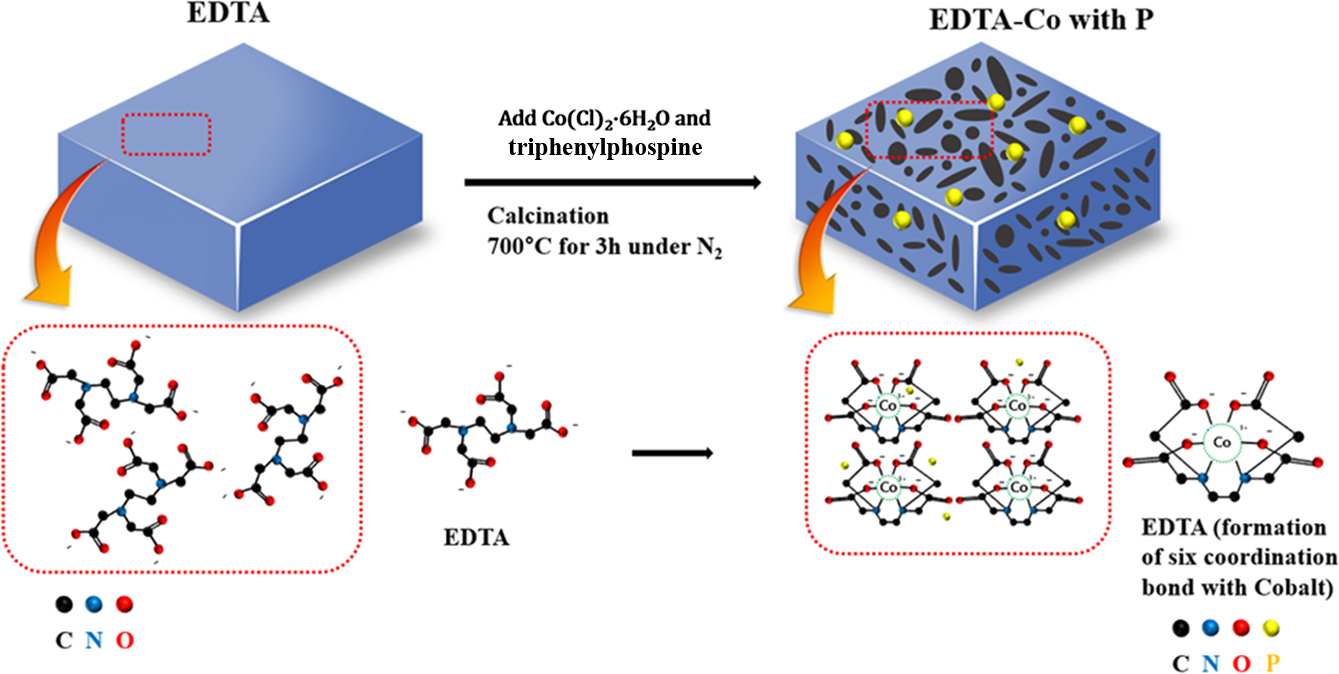
Schem 1. Fabrication process of EDTA-Co with P.
Electrochemical Property Measurements. Measurements
of the electrocatalytic (Produced y the method shown in Schem 1) bifunctional
properties (Oxygen Reduction Reaction - ORR & Oxygen Evolution Reaction -
OER) and three-electrode mechanism system were carried out with a potentiostat
(CH Instruments 600E, USA) and a rotating-disk electrode (RDE). Platinum wire
(counter electrode), Ag/AgCl electrode (reference electrode), and a glassy
carbon electrode (GCE, diameter: 5.0 mm, working electrode) were used for taking
measurements. Prior to the measurements, the GCE surface was cleaned on the
polishing pad with 1 μm of polishing diamond and 0.05 μm of polishing
alumina; then rinsed with DI water. Next, a catalytic ink (1000 μL
mixture) was fabricated by adding 4.0 mg of powdered sample to 5% Nafion
solution, isopropanol, and DI water in a solution volume ratio = 1:3:6. After
sonication for 1 h, 7 μL of ink was dropped onto the GCE surface and
allowed to dry for 40 min at 40 oC. 0.1 M KOH electrolyte
was purged with oxygen and nitrogen for 1 h and then the bifunctional test
was performed. The bifunctional properties of electrocatalytic activity were
evaluated by linear sweep voltammetry (LSV) and cyclic voltammetry (CV) at 10
mV/s of scan rate in a voltage range from 0.2 to -1.0 V (ORR with O2
sat) and 0.2 to 1.0 V (OER with N2 sat) with rotation speeds from
1600 rpm.
The electrocatalytic samples’ porous properties and the morphological
structure of EDTA with Co and P were examined via FE-SEM. In this study, EDTA
was used as the main base-carbon material, to which a suitable amount of Co and
P were added. The carbon-based material has high stability and durability, but
it improves reactivity by doping hetero atom to improve the disadvantage of low
reactivity with oxygen. N is the most widely used hetero atom, but in this
study, P was used to induce synergistic effects with cobalt. Phosphorus is
located in the same group as nitrogen and has as much charge as nitrogen, so it
is expected to increase the reactivity with oxygen by redistributing charge
when doping carbon material. In addition, when Co and P co-doping, the spin
density and charge energy density on the catalyst surface change, leading to
more positive effects than simply adding the effects of cobalt and P,
respectively.
Figure 1(a) depicts low magnification SEM images of EDTA, which shows
carbon planks similar to a plate. However, when combined with Co, EDTA exhibits
a porous appearance (see Figure 1(b)). EDTA interacts with the transition metal
to form six coordination bonds; it also exhibits an octahedral structure.
Throughout this process, EDTA maintains a porous texture and develops a wider
specific surface area. Figure 1(c) and 1(d) are high magnification images of P
doped EDTA-Co. Note the rough surface and porous structure, which indicates
that the Co and P particles were cultivated next to carbon’s porous structure.
In addition, the high magnification SEM image shows pore sizes in the micropore
range, i.e., ≤ 2.0 nm
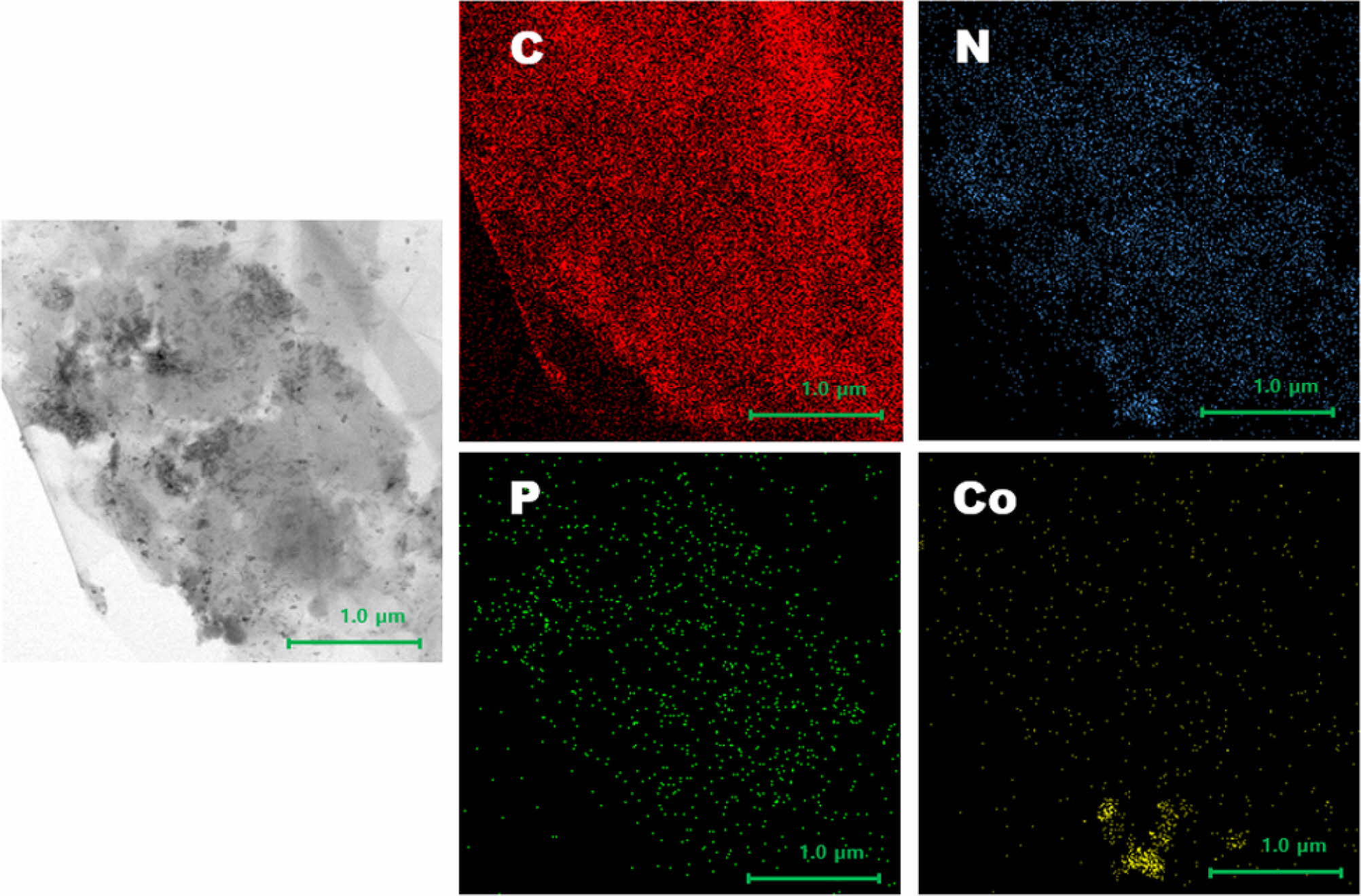
The electrocatalytic samples’ EDS mapping image (in Figure 2) shows that
the C, N, P and Co elements are grown in high concentrations. Because the
primary material is carbon-based, carbon’s EDS mapping range is the most clear
and distinct. In addition, the image confirms that P is uniformly dispersed
throughout the material, which demonstrates that P can facilitate the C and Co
bonding process. The EDS mapping image also depicted cultivation of Co
particles, indicating that the amount of Co particles was contained. These
results demonstrate that the cathode catalytic samples were successfully
synthesized.
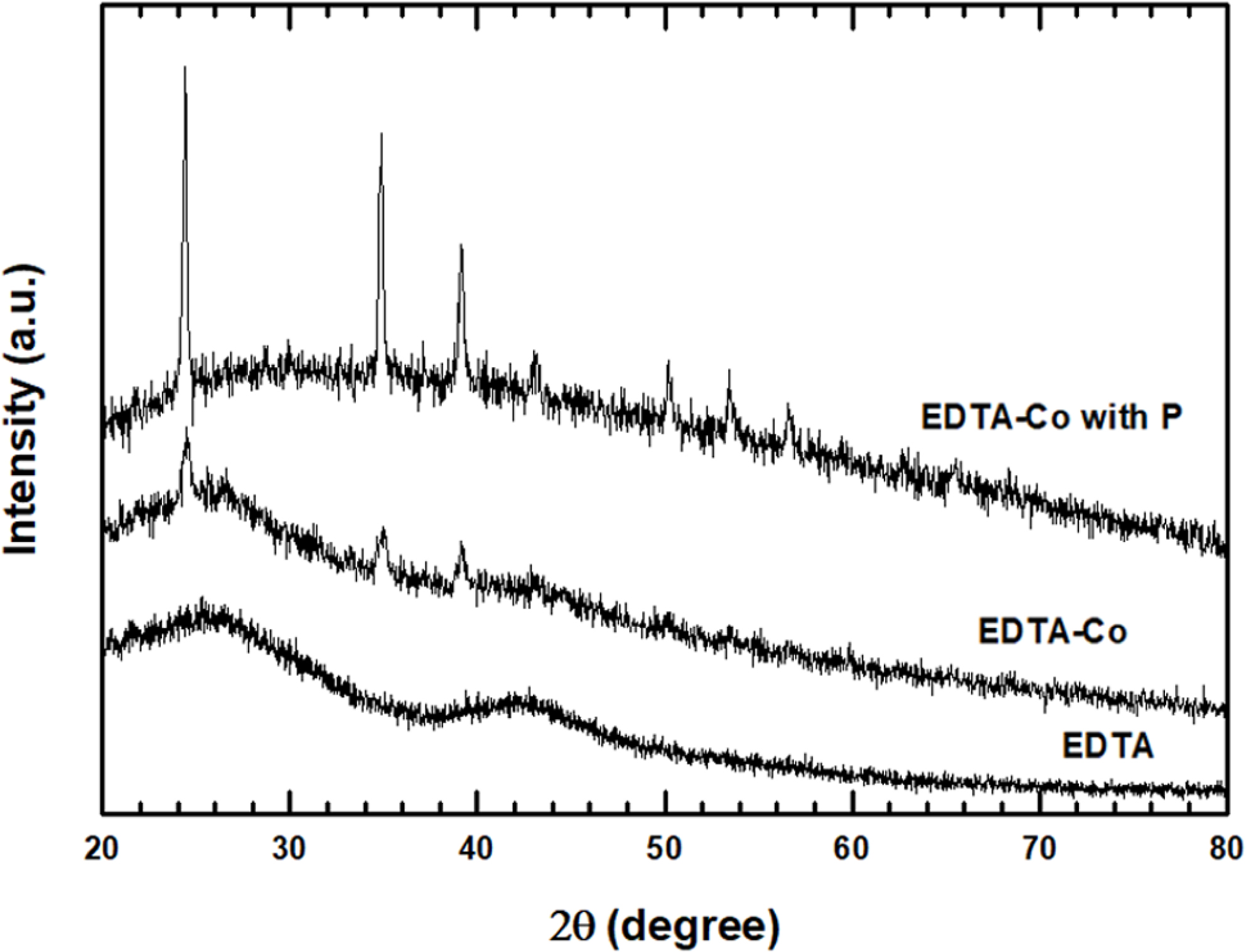
In Figure 3, the XRD pattern was acquired to confirm the morphology and
formation/extent of EDTA-Co bonding. Various diffraction peaks were identified,
including C and Co, and an amorphous characteristic. The 2θ = 24o
peak with most pronounced crystallinity is a reflection of the original carbon
phases.16 Furthermore, the intensity and width of the diffraction
peaks increase with increasing Co and P content. In addition, the peak at 35o
verifies the formation of an orthorhombic phase perovskite-like
structure.17 The peak at 39o is a Co related peak. As P is
added, the peak is incresed, indicating that P adds a synergistic effect to the
binding of EDTA and Co.18
Materials comprised of EDTA-Co supplemented with P have a high
electrocatalytic activity performance, e.g., ORR and OER, owing to their large
surface area and the combination of P and Co elements enhancing the synergy
effect of the C graphitic properties. Electrochemical measurements (CV, ORR,
and OER) of the samples were evaluated via the RDE method using a typical
three-electrode system. ORR and OER experiments on the cathodic catalyst
samples and Pt/C material were performed by immersing them in O2 and
N2 saturated 0.1 M KOH and taking RDE measurements at 1600 rpm
and a 10 mV/s scan rate to confirm the electrochemical characteristics of
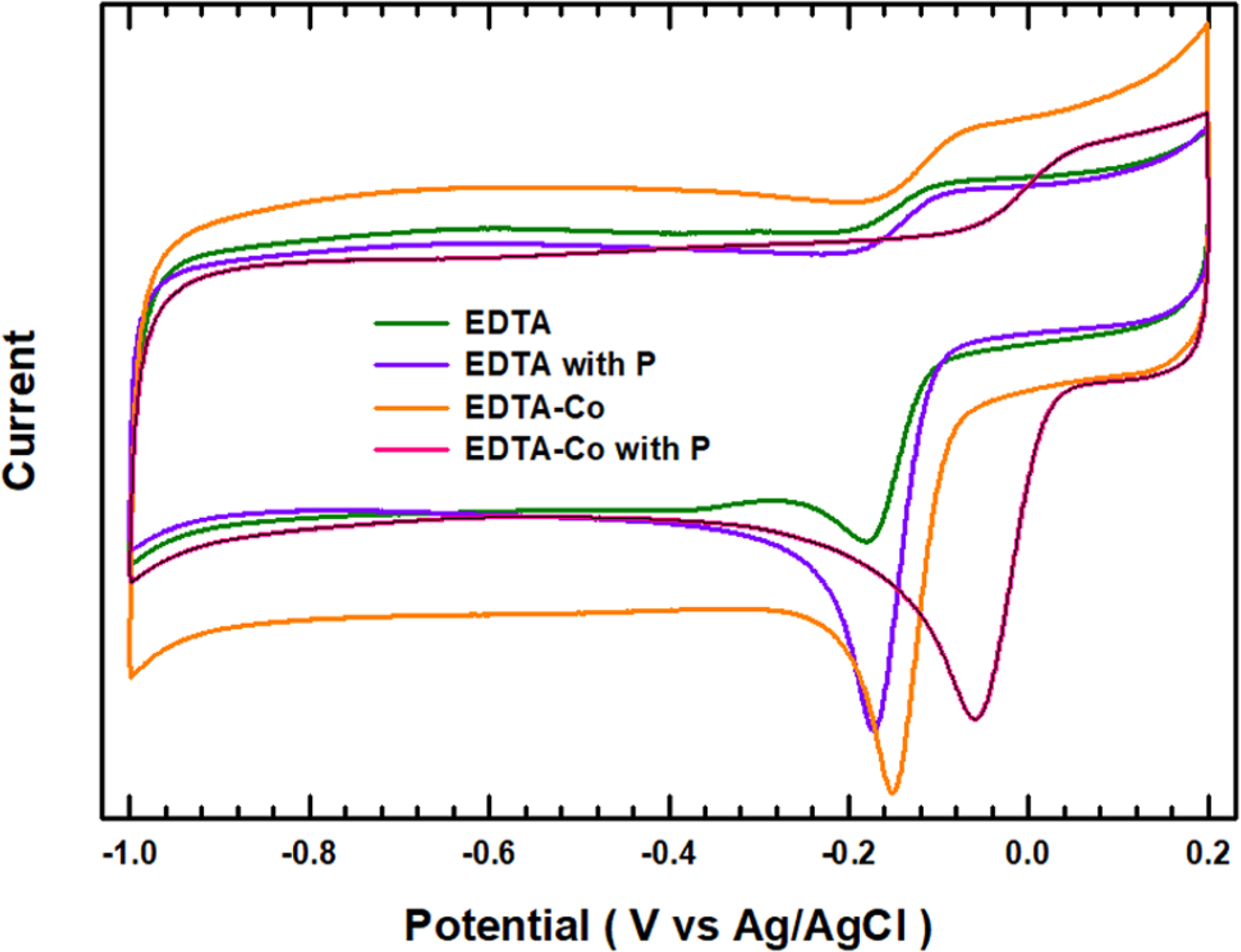
the catalysts. Cyclic voltammetry (CV) curves (see Figure 4) focused on the
oxygen reduction peak of the samples and Pt/C material in a voltage range from
-1.0 to 0.2 V (vs. Ag/AgCl). The corresponding CV curve exhibits
reversible characteristics that enables both charge and discharge. Catalysts
not containing Co and P show a very weak reaction peak and low onset potential
(-0.185 V). The peak intensity increases as the amount of additive Co, P and Co
with P increases, and the onset
potential gradually increases (faster) as well (EDTA: -0.185 V, EDTA with P:
-0.173 V, EDTA-Co: -0.152 V, EDTA-Co with P: -0.059 V).
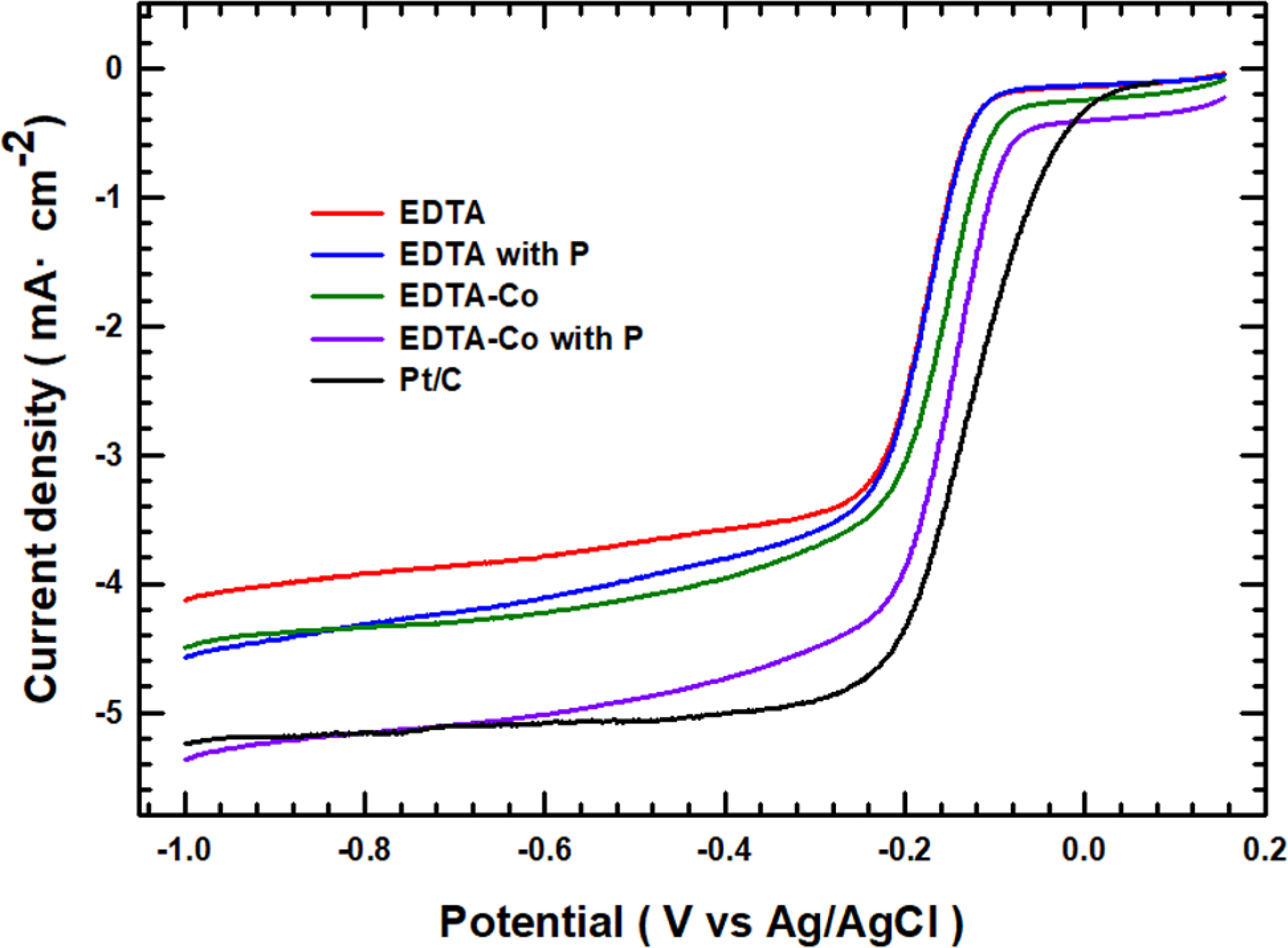
The LSV curve also demonstrates the enhancement in electrochemical
properties with the addition of Co and P. The electrocatalytic performance was
obtained by RDE measurement at 1600 rpm and 10 mV/s of scan rate using oxygen
saturated 0.1 M KOH solution. Analysis of the LSV curve in Figure 5 shows that
the onset potential and the half-wave potential of EDTA-based materials and
P-containing EDTA materials are almost identical (Eonset:
-0.123 V, Ehalf-wave: -0.178 V). P shows that charge
redistribution occurs due to changes in charge density and spin density, and
the current density is higher as compared to the EDTA material alone (-4.12 to
-4.58 mA/cm2). Co exhibited a greater synergistic effect with EDTA
material than P (Eonset: -0.105 V, Ehalf-wave:
-0.163 V). Co has an octahedral structure and six coordination bonds with EDTA,
as well as a high surface area, both of which lead to high performance in an
electrochemical catalyst. A material in which Co and P are jointly added has
more than just two effects (Eonset: -0.087 V, Ehalf-wave:
-0.149 V). These results are caused by the P charge redistribution effect
and the high specific surface area through the six EDTA-Co coordination bonds.
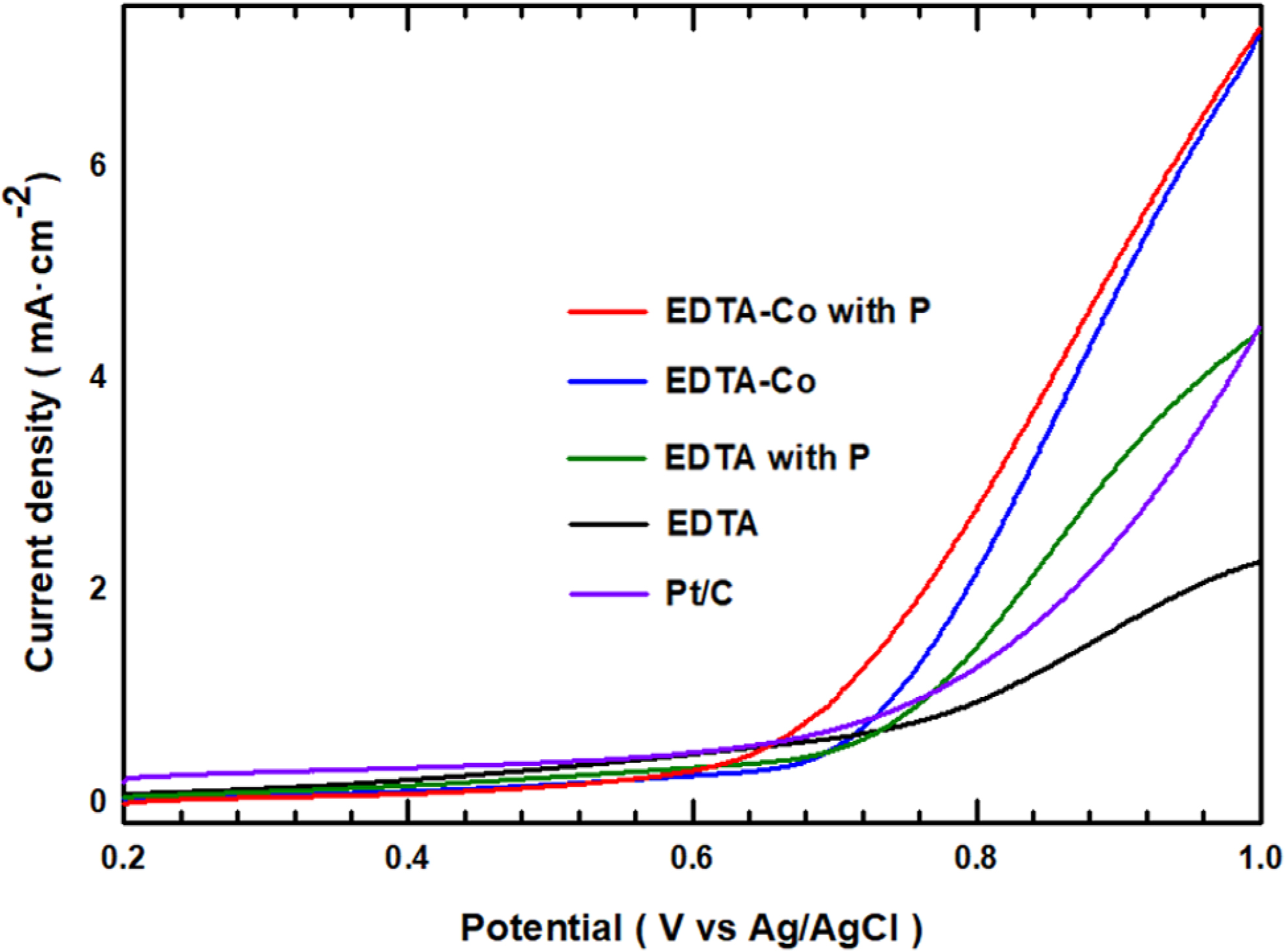
The OER curve showing the electrochemical catalyst characteristics with
ORR is shown in Figure 6. The RDE was measured using a rotating speed of 1600
rpm and a scan rate of 10 mV/s with nitrogen saturated 0.1 M KOH solution.
The results are in good agreement with the CV and LSV curves, and shows that
the performance increases with the addition of P and Co in OER activities. Only
EDTA (0.748 V), EDTA with P (0.676 V), EDTA-Co (0.662 V) and EDTA-Co with P
(0.607 V) show an increase in onset potential. Furthermore, with the exception
of only EDTA material, the samples show higher onset potential than Pt/C (0.683
V), which is itself an excellent electrochemical catalyst. In addition, the
catalytic samples reached a current density of 4 mA/cm2 at a faster
potential than Pt/C (see Table 1). Table 1 compares the samples’
electrochemical properties (OER) and those of Pt/C. Thus, through the
above-mentioned electrochemical experiments, we confirmed the positive effect P
and Co individually contribute, and demonstrated the development of a
successful electrochemical catalyst based on a superior synergistic effect when
P and Co are simultaneously added.
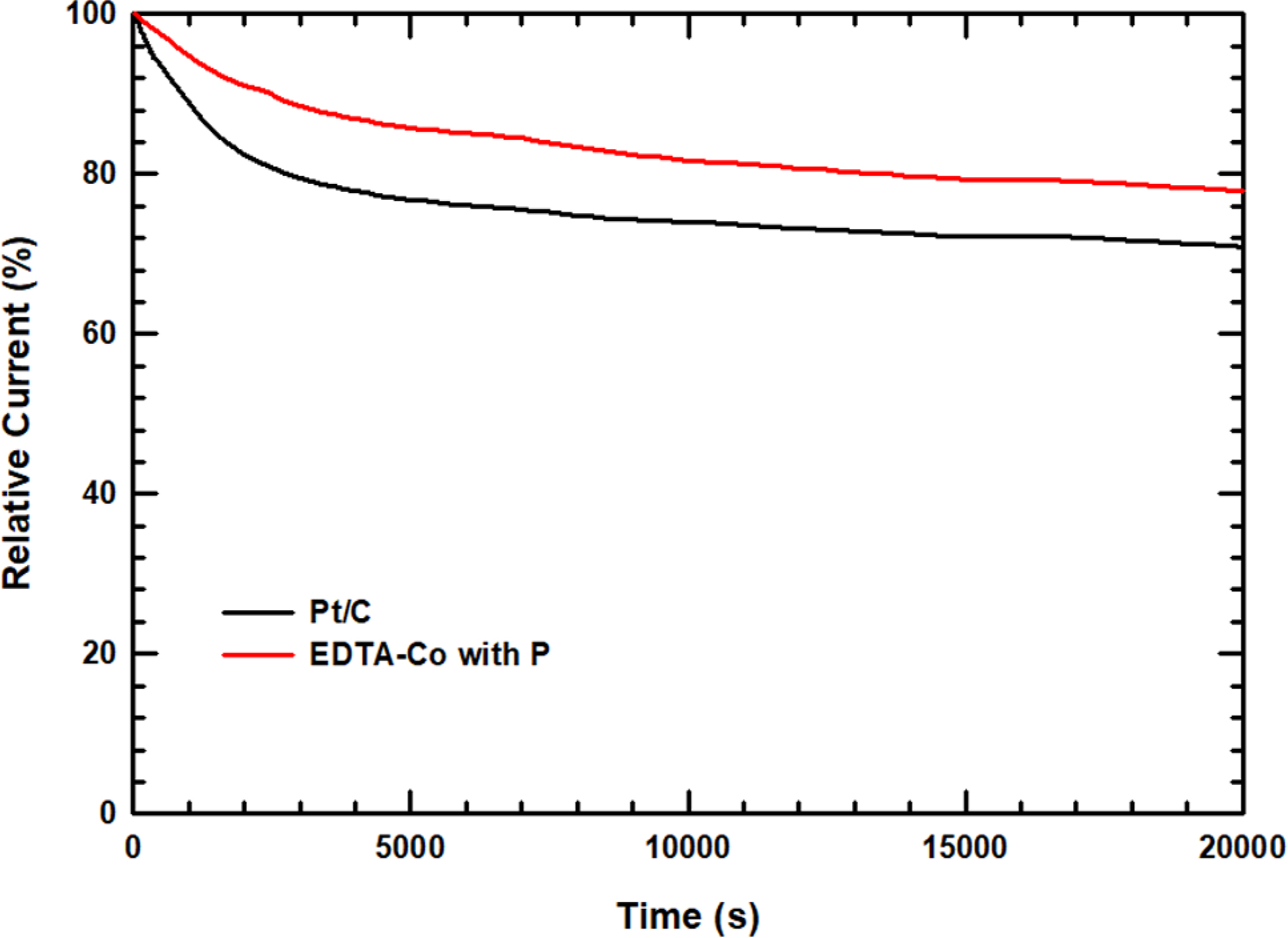
In this work, these changes were evaluated using a chronoamperometric
approach where measurements were performed at -0.6 V for 20000 s in an O2
saturated 0.1 M KOH media in Figure 7. Pt/C shows a sharp drop in the initial
stage of operation, and then decreases steadily, demonstrating that decreases
by 70.3%. However, EDTA-Co with P electrocatalyst has no rapid drop in relative
current and shows high durability of 78.8% after running for 20000 s.

|
Figure 1 FE-SEM images of (a) low-magnification EDTA; (b) EDTA-Co; (c) and (d) high-magnification EDTA-Co with P. |
|
Figure 2 EDS mapping images (element of C, N, P, Co) by FE-SEM. |
|
Figure 3 XRD patterns of EDTA, EDTA-Co and EDTA-Co with P catalyst. |
|
Figure 4
Cyclic
voltammetric profile for electrochemical catalyst in O2-saturated
0.1 M KOH electrolyte at scan rate of 10 mV s-1. |
|
Figure 5 LSV curves (ORR) of EDTA, EDTA with P, EDTA-Co,
EDTA-Co with P and Pt/C at rotating speed of 1600 rpm with O2
saturated 0.1 M KOH media. |
|
Figure 6 LSV curve (OER) of EDTA, EDTA with P, EDTA-Co and
EDTA-Co with P at rotating speed of 1600 rpm with N2 saturated 0.1 M
KOH electrolyte. |
|
Figure 7 Relative current density of EDTA-Co with P and Pt/C catalyst for 20000 s at -0.6 V. |
|
Table 1 Comparison of OER Catalytic Performance for EDTA, EDTA with P, EDTA-Co, EDTA-Co with P and Pt/C |
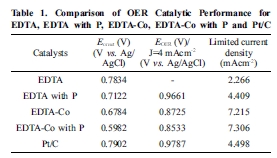
In this work, we successfully demonstrated the synergistic effect of
coupling between P, Co and EDTA. The six ligand sites of EDTA bind to the
transition metal with coordinate bonding and form an octahedral structure. In
addition, the P particle is located between Co and EDTA bond, triggering atomic
and spin charge density changes that result in charge redistribution on the
catalyst’s surface. This process changes the chemical adsorption energy of O2,
and therefore affects the material much more so than simple Co and P doping.
P-doped EDTA and Co structures were verified by FE-SEM and FE-TEM and further
confirmed by XRD analysis. The location of EDTA-Co with P of electrochemical
properties was measured by chronoamperometry and potentiostat methods. CV and
LSV curves confirmed that P and Co samples had a synergistic effect on the
electrochemical.
- 1. B. C. Steele and A. Heinzel, in MaterialsFor Sustainable Energy, A Collection of Peer-Reviewed Research and ReviewArticles from Nature Publishing Group, World Scientific, pp. 224-231(2011).
- 2. A.Rabis, P. Rodriguez, and T. J. Schmidt, ACS Catal., 2, 864(2012).
-

- 3. Y.Bing, H. Liu, L. Zhang, D. Ghosh, and J. Zhang, Chem. Soc. Rev., 39,2184 (2010).
-

- 4. X.Wang, J. S. Lee, Q. Zhu, J. Liu, Y. Wang, and S. Dai, Chem. Mater., 22,2178 (2010).
-

- 5. J. S.Lee, S. T. Kim, R. Cao, N. S. Choi, M. Liu, K. T. Lee, and J. Cho, Adv.Energy Mater., 1, 34 (2011).
-

- 6. E. M.Erickson, M. S. Thorum, R. Vasić, N. A. S. Marinković, A. I. Frenkel, A. A.Gewirth, and R. G. Nuzzo, J. Am. Chem. Soc., 134, 197 (2011).
-

- 7. J.Zhang and L. Dai, ACS Catal., 5, 7244 (2015).
-

- 8. L.-y. Feng, Y.-j. Liu, and J.-x. Zhao, J.Power Sources, 287, 431 (2015).
-

- 9. Q.Liu, S. Chen, Y. Zhou, S. Zheng, H. Hou, and F. Zhao, J. Power Sources, 261,245 (2014).
-

- 10. Z.Yang, Z. Yao, G. Li, G. Fang, H. Nie, Z. Liu, X. Zhou, X. A. Chen, and S.Huang, ACS Nano, 6, 205 (2011).
-

- 11. X.Solans, M. Font-Altaba, J. Oliva, and J. Herrera, Acta Crystallogr. Sect. C:Cryst. Struct. Commun., 39, 435 (1983).
-

- 12. Z.Liu, G. Zhang, Z. Lu, X. Jin, Z. Chang, and X. Sun, Nano Res., 6,293 (2013).
- 13. H.-W.Liang, W. Wei, Z.-S. Wu, X. Feng, and K. Müllen, J. Am. Chem.Soc., 135, 16002 (2013).
- 14. J. Wu,C. Jin, Z. Yang, J. Tian, and R. Yang, Carbon, 82, 562 (2015).
-

- 15. S.Jiang, C. Zhu, and S. Dong, J. Mater. Chem. A, 1, 3593 (2013).
-

- 16. R. Li,Z. Wei, and X. Gou, ACS Catal., 5, 4133 (2015).
-

- 17. B.Zhang, M. Pan, D. Zhao, and W. Wang, Appl. Phy. Lett., 85, 61(2004).
-

- 18. J. Wu,Z. Yang, X. Li, Q. Sun, C. Jin, P. Strasser, and R. Yang, J. Mater. Chem.A, 1, 9889 (2013).
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2019; 43(6): 940-945
Published online Nov 25, 2019
- 10.7317/pk.2019.43.6.940
- Received on Aug 20, 2019
- Revised on Oct 1, 2019
- Accepted on Oct 2, 2019
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Jinglei Yang*, Jooheon Kim
-
School of Chemical Engineering & Materials Science, Chung-Ang University, Seoul 06971, Korea
*Department of Mechanical and Aerospace Engineering, The Hong Kong University of Science and Technology, Hong Kong
- E-mail: ooxxx223@gmail.com, jooheonkim@cau.ac.kr
- ORCID:
0000-0002-9413-9016, 0000-0002-6644-7791










 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.