- Study on Mechanical Properties and Viscoelastic Properties of Bio-polyurethanes
*School of Energy, Materials and Chemical Engineering, Korea University of Technology and Education, Cheonan, Chungnam 31253, Korea
**Research Center of Eco-friendly & High Performance Chemical Materials, Cheonan, Chungnam 31253, Korea- 바이오 폴리우레탄의 물성 및 점탄성에 대한 연구
*한국기술교육대학교 에너지, 신소재, 화학공학부, **친환경고성능화학소재연구소
The bio-polyester polyol has been prepared by azelaic acid and 1,3-propanediol(1,3-PD) from biomass with esterification synthesis method, and MDI (4,4'-methylenebis (phenyl isocyanate)), H12MDI and IPDI (isophorone diisocyanate) were used as isocyanates, 1,4-butanediol(1,4-BD) was used as chain extender. It also had been set the general polyurethane with SS-106 polyol, and bio-polyurethane without chain extender as control groups. The viscoelastic behaviors of the bio-polyurethanes were explored using a rubber processing analyzer (RPA) in the mode of strain sweep. And the mechanical properties (tensile strength, hardness value, resilience, abrasion resistance) were characterized by UTM, shore A tester, ball rebound and taber abrasion resistance tester. From the results above, the bio-polyurethane which synthesized in this research with bio-polyester polyol showed better abrasion resistance, elongation rate and viscoelastic properties compared to the general polyurethane material as elastomers.
바이오매스 유래의 azelaic acid, 1,3-propanediol(1,3-PD)를 사용하여 바이오 폴리에스터 폴리올을 합성하였다. 합성한 폴리에스터 폴리올에 MDI(4,4'-methylenebis (phenyl isocyanate)), H12MDI and IPDI(isophorone diisocyanate)와 사슬연장제로 1,4-butanediol(1,4-BD)을 넣고 바이오 폴리우레탄을 합성하였다. 그리고 사슬연장제 없이 poly(1,4-butylene adipate)를 폴리올로 사용하여 일반 폴리우레탄을 합성하였다. 고분자 가공분석기(RPA)의 변형 스윕(strain sweep) 기능을 사용하여 제조된 폴리우레탄의 점탄성을 조사하였다. UTM, shore A, ball rebound 및 taber기계를 사용하여 폴리우레탄의 인장강도, 경도, 반발탄성 및 내마모성 측정을 통하여 기계적 물성을 확인하였다. 제조된 바이오 폴리우레탄은 일반 폴리우레탄보다 더 좋은 점탄성, 내마모성 및 신장률(elongation rate)을 보였다.
Bio-based polyester polyol had been synthesized by the esterification method by azelaic acid and 1,3-PD. the bio-based polyurethanes were synthesized by polyester polyol above, MDI (4,4'-methylenebis (phenyl isocyanate)), H12MDI and IPDI (isophorone diisocyanate) were used as isocyanates, 1,4-BD was used as chain extender, the bio-TPU which synthesized in this research showed better mechanical and viscoelastic properties than the general PU material as elastomers.
Keywords: bio-polyurethane, bio-polyester polyol, viscoelastic properties, abrasion resistance, mechanical properties
The use of renewable resources in energy and materials has attracted great interest in both industrial and academic fields because of their environmental aspect and sustainability. Polyurethane (PU) has become one of the most widely used plastics for various applications such as insulation, automotive parts, seating materials,1 and artificial leather since its first synthesis in 1937 by Otto Bayer et al.2 because of its valuable properties, such as good flexibility, elasticity, and damping ability. However, the starting materials for preparation of PU are strongly dependent on petroleum as a feedstock,3 and this fact became problematic because of dramatic depletion of fossil oils, continuous fluctuations in the oil price, and environmental concerns.4 PUs are mainly synthesized from components which are derived from nonrenewable resources. One of the aims in research on PU is the application of polyol from biomass in their synthesis due to the face that the resources of petroleum oils are near exhaustion.5 In recently years, bio-based polymers have received much attention due to an increasing environmental concern.
The physical properties of PU can be adjusted by varying the types and amounts of polyols, isocyanates, and chain extenders in the precursor mixture. PU is segmented block copolymer composed of a soft domain (polyols) and a hard domain (isocyanate and the chain extender). The polyol in the soft domain gives the PU its elastic quality, while the hard domain (depending on its nature) confers hardness and rigidity. Thus, microphase separations and the contents of the soft and hard domains significantly affect the mechanical properties of PU.
Biodegradable elastomers are expected to be suited for any application requiring the use of a flexible, elastic material such as soft tissue engineering, the work reported herein involves the synthesis and characterization of a series of biodegradable linear PU elastomers.
In this research, the azelaic acid which is extracted from castor beans6 which was synthesized with 1,3-propanediol (1,3-PD) which prepared from fermentation process of biomass to make the bio-polyester polyols as the bio-based polyol. And polyols of that type offer lower viscosity than oligoesters derived from dicarboxylic acids and glycols,7 which could provide superior mechanical properties for PU materials as elastomers.
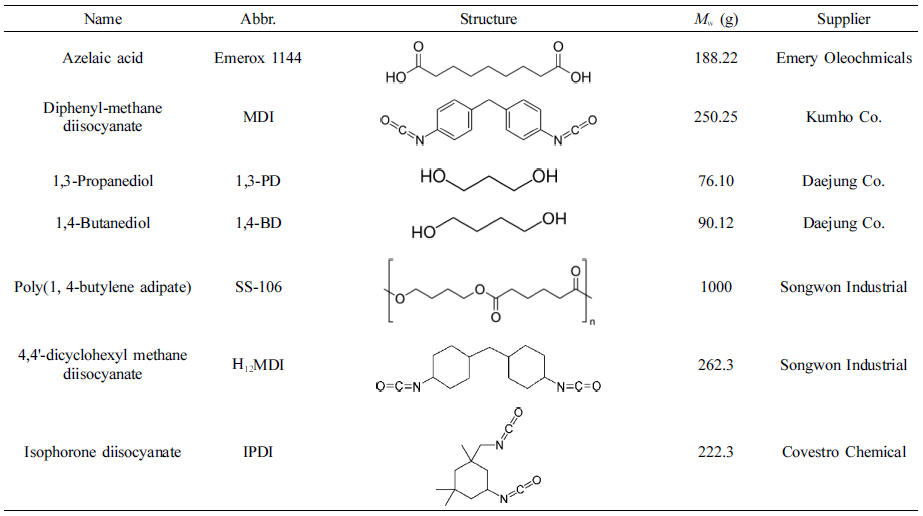
Raw Materials. The raw materials in this research were shown in Table 1. And diisocyanates were used with industrial class, other materials were used with chemical pure class. The nitrogen gas which used as protect gas in polyester synthesis process was from Daesung Co.
The Polymerization of Polyester Polyol: The mechanism for polymerization of polyester polyol was shown in Scheme 1. A typical polymerization reaction was carried out as following: azelaic acid powder and 1,3-PD were poured into the reaction system. The mixture was stirred by a magnetic stirrer at 400 rpm. And first 4 h, it was controlled below 180 ℃ with atmosphere of nitrogen, and next 4 h, raised the temperature of 200 ℃ and dropped 50 ppm stannous octoate8 as catalyst, and last 4 h, removed the nitrogen atmosphere and set the vacuum system (2 torr)9 with the temperature of 160 ℃, then cooled down the system temperature below 60 ℃, and the polyester polyol in this research had been synthesized.
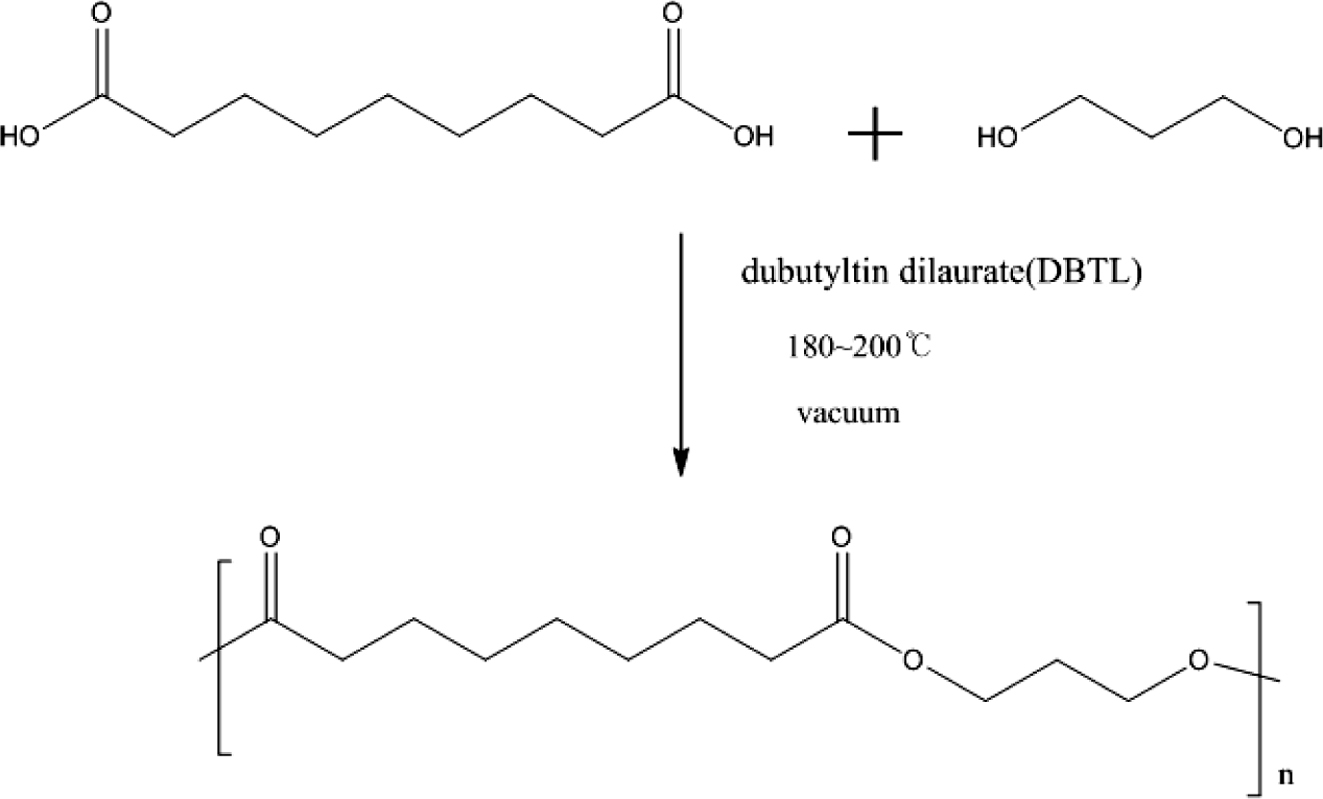
Scheme 1. The mechanism for polymerization of polyester polyol.
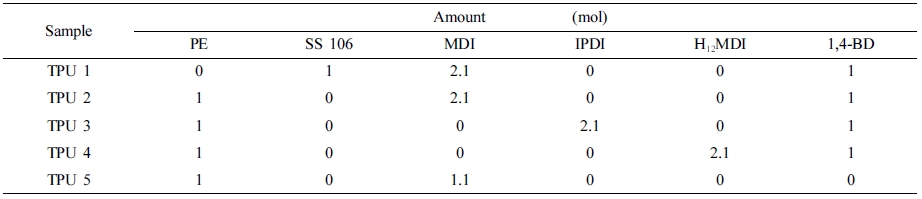
Polymerization of Polyurethane Materials: The PU polymerization raw materials ratios were shown in Table 2. Polyols, diisocyanates and chain extender had been poured into reaction system by one-step synthesis method, stirred with 200 rpm at room temperature, aged at 80 ℃ for 2 weeks.
Characterization. Acid Number Characterization of Polyester Polyol: In order to the determine the degree of esterification reaction, the acid number10 test had been characterized according to ASTM D7253 (Standard test method for PU raw materials). And the acid number of polyol could be calculated in milligrams of KOH/gram of samples with the formula below:11
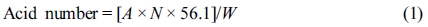
Where: A = volume of 0.1 N KOH solution for the titration of the sample in mL, N = the normality of the KOH solution, and W = the weight of the sample used in g.
Hydroxyl Number Characterization of Polyester Polyol: Determination the hydroxyl number of polyol could calculate the theoretical molecular weight of polyol,12 the hydroxyl value was characterized according to ASTM E220 (Standard test methods for hydroxyl groups using acetic anhydride acetylation). And the hydroxyl number of the hydroxyl-containing compound could be calculated as formula follows:13
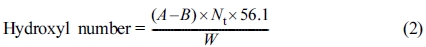
where A = 1 N NaOH solution, mL, required for titration of the sample, B = NaOH solution, mL, required for titration of the blank, Nt = meq/mL of the solution at the temperature during analysis, W = sample used, g.
The theoretical molecular weight of sample could be calculated by the formula follows14:
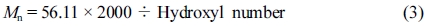
Viscoelasticity and Mechanical Properties of the Thermoplastic Polyurethane (TPU) Materials: In this part, the viscoelastic properties and the curing characteristic were determined with a rubber process analyzer (RPA-V1, U-Can Dynatex Inc., Taiwan. The strain sweep test from 0.01 degree to 20 degrees was operated at 60 ℃ and 1 Hz on RPA according to ASTM D 6204-97. The hardness of samples was obtained by a shore durometer type A following ASTM D22-40. The tensile strength test was measured three times on a Tinius Olsen H5KT-0401 testing machine at a speed of 500 mm/min according to ASTM D412. The samples were made of a dumb-bell shape with the dimensions of 25 mm × 6 mm × 1 mm after the specimens forming on the heat-press machine under 180 ℃ for 1h. The resilience test was performed by Ball Rebound machine which according to the ASTM D3574-17 and ISO 8307. And there were also Abrasion resistance test was performed by Taber Abrasion tester 5135 according to ASTM D1044.
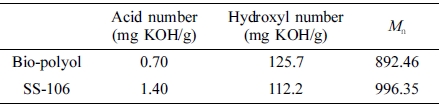
Acid Number and Hydroxyl Number. Table 3 showed the results of acid number, hydroxyl number and Mn of bio-polyol and SS-106, from this table, it can be found that the acid number of bio-polyol is small, which means the degree of esterification reaction is high. And compare to SS-106, the results of hydroxyl number and Mn of bio-polyol are almost similar to polyols from petrochemical industry, which means bio-polyol could be a good raw material for PU synthesis process.
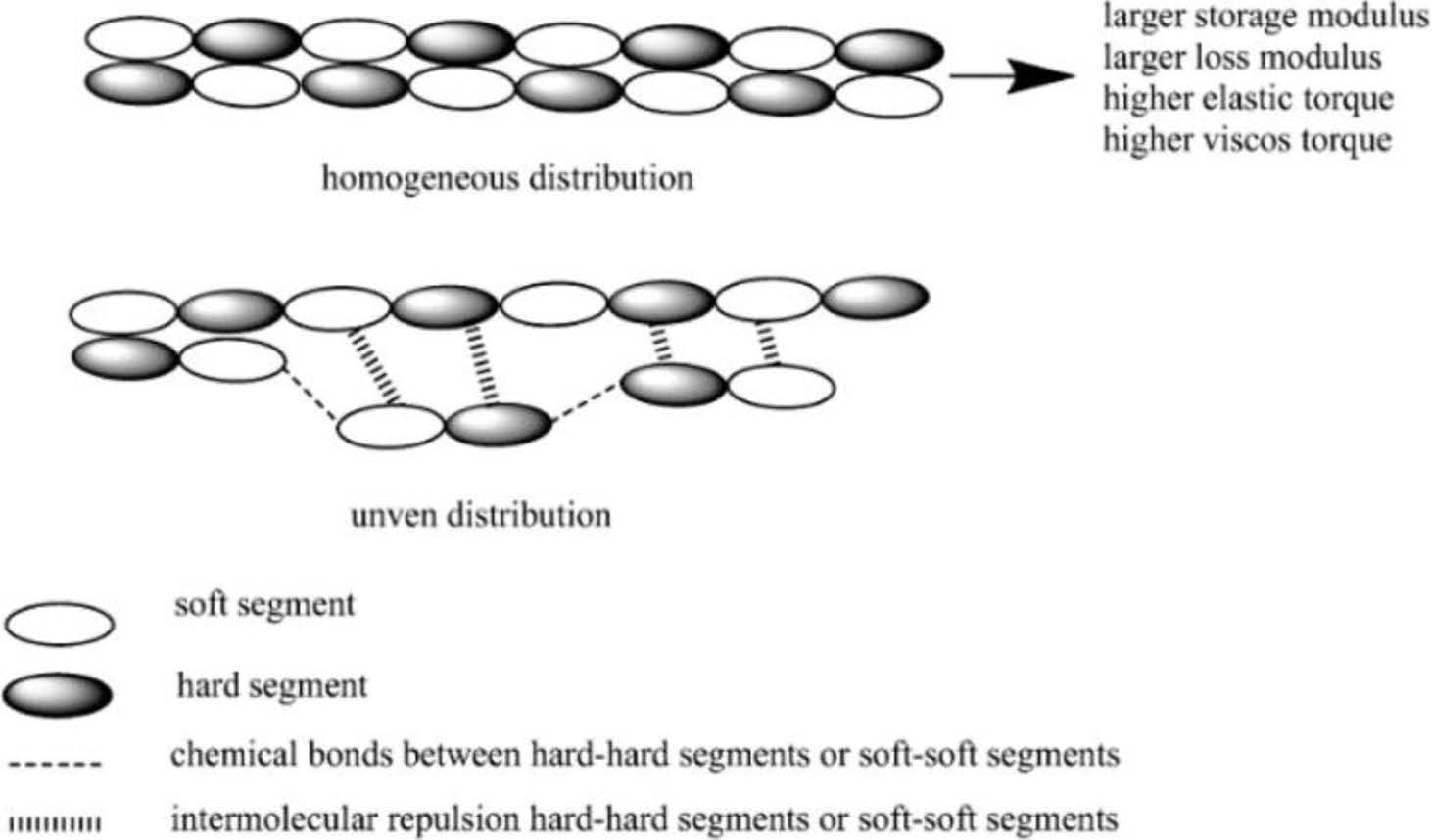
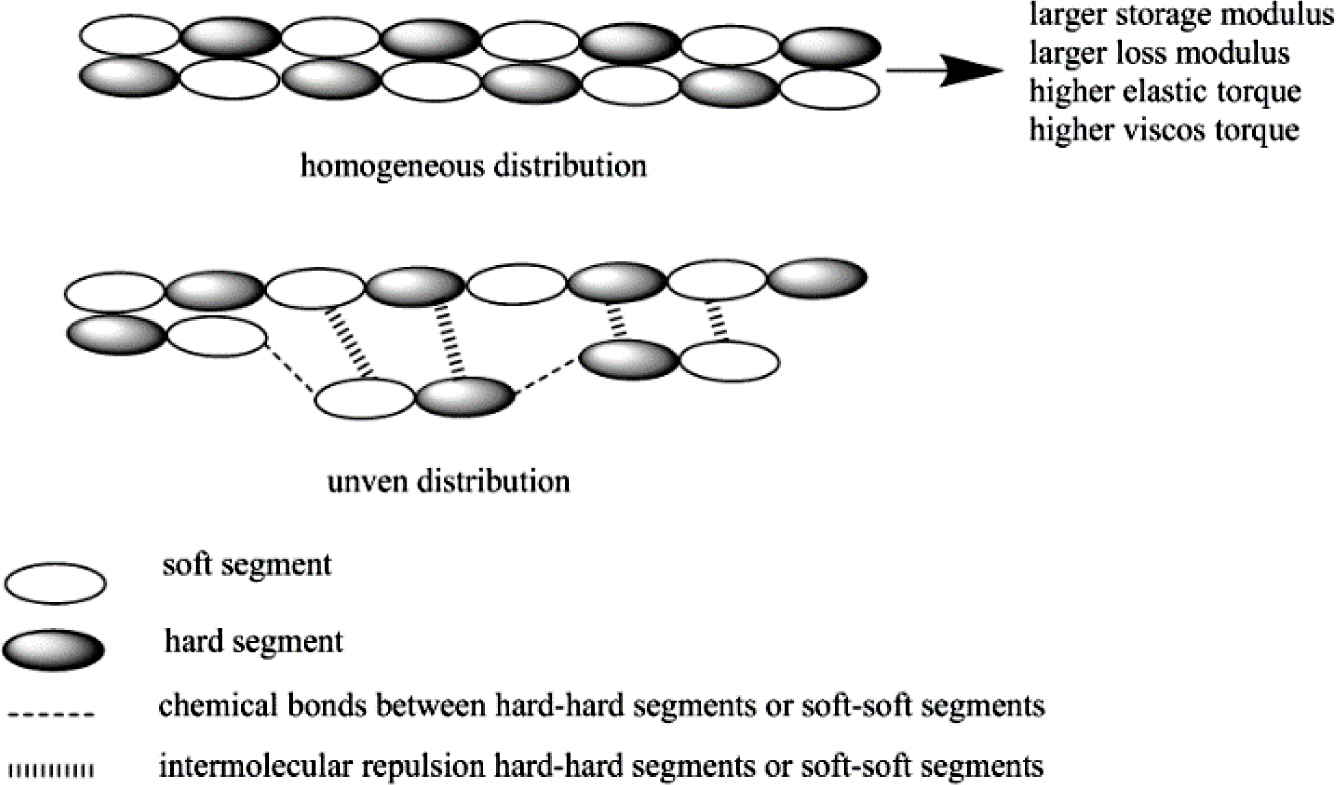
Viscoelastic Properties. As the dynamic strain sweep mode, it is always used for explaining Payne effect of rubber materials to describe the dispersion state of fillers. The more uniform dispersion state, the more homogeneous matrix, and then, the larger storage/loss modulus and also higher regular elastic/viscos torque of the materials. But as bio-TPUs in this research, due to without any other filler in matrix, so when discussed with “Payne Effect” of TPU matrix, it is mainly about the regularity of block (soft segments and hard segments) arrangement distribution (shown in Figure 3), thus, the more homogeneous block arrangement distribution, the larger storage/loss modulus and higher elastic/viscos torque of TPU materials. Otherwise, it also could be known whether the array distribution of TPU matrix is through the dynamic strain sweep mode results.
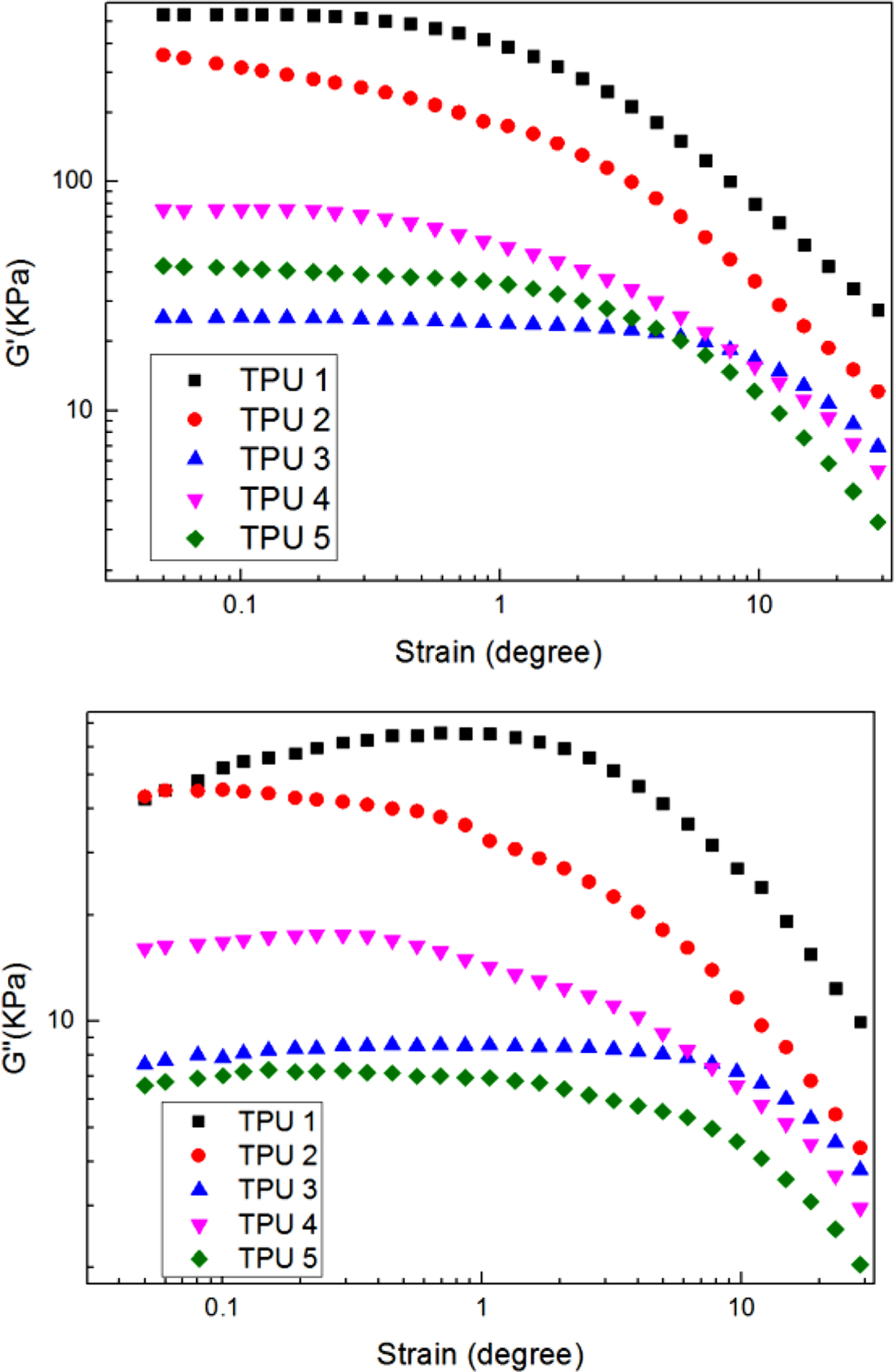
The viscoelastic results of PU samples were shown in Figure 1. From the figures, it can be found that compared to the SS-106 (the feedstock for petroleum cracking),15 although there is a disparity, the bio-polyester polyol also showed good performance for mechanical properties when reacted with MDI and 1,4-BD.
The higher storage modulus value, the better segments distribution, the higher loss modulus value, the more anti destructive property. In TPU 2, 3, and 4, it can be found that MDI could provide higher storage modulus and loss modulus compare to IPDI and H12MDI. And in TPU 2 and 5, it can be found when 1,4-BD had been added as chain extender, the viscoelastic property had increased with the segments distribution regularity.
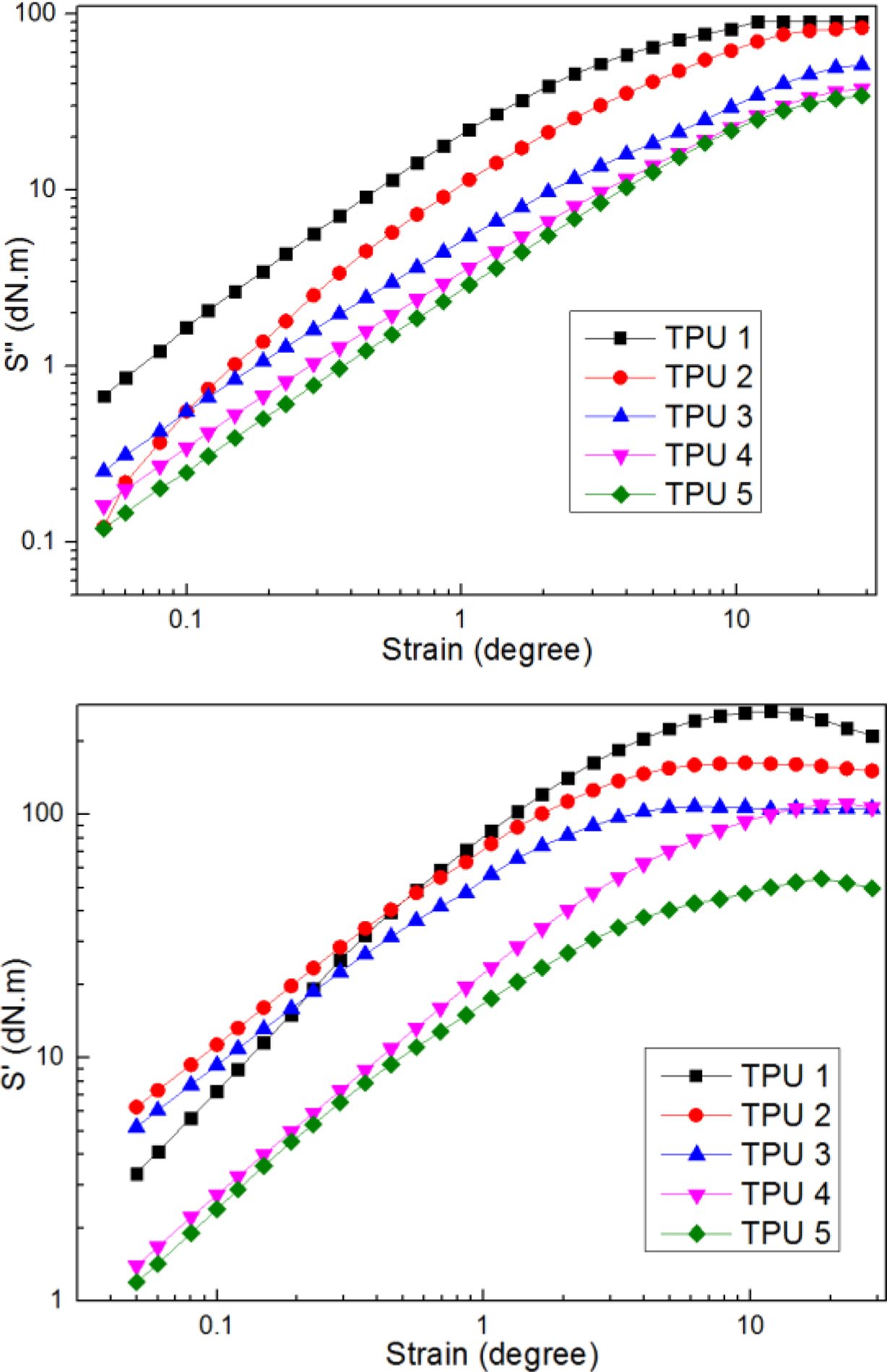
Figure 2 showed the results of elastic torque and viscos torque, from the curves of elastic toque, it could be found at low strain amplitudes, TPU 1 showed smaller value of S', but when at high strain amplitudes, TPU 1 presented a great increase and always on the top of others, which means TPU 1 had the best elastic restore performance in this research, as for TPU 2, it showed relatively stable trend compared to other samples during all the strain amplitudes stage, and also presented great elastic performance. And from the curves of viscos torque, it was found that TPU 1 and TPU 2 showed high strain amplitudes, which means these two TPU materials had great viscous property.
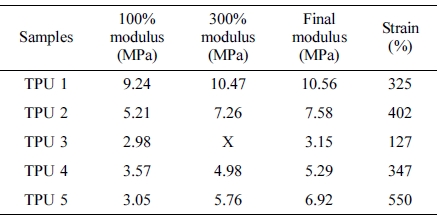
Mechanical Properties. The tensile strength and elongation rate results were shown in Table 4. From this table, it can be found that the SS-106(TPU 1) could provide the best tensile strength in this research, and in TPU 2, 3 and 4, with the same ratio of diisocyanate, MDI provided the best tensile strength in TPU synthesis process.
Also, compared with TPU 5, TPU 2 showed the better modulus, but as strain, which also said as elongation rate, the TPU without chain extender showed larger strain value than other TPU samples, the reason is chain extender could increase the reaction rate16 of PU synthesis, and also could reduce the ratio of carboxyl end group,17 which could improve the mechanical properties of TPU materials, such as hardness and tensile strength.
The resilience result (percentage rebound value R) can be calculated from the equation below:18

where h is the ball rebound height, expressed in mm; hmax is the height of the drop (500 mm).
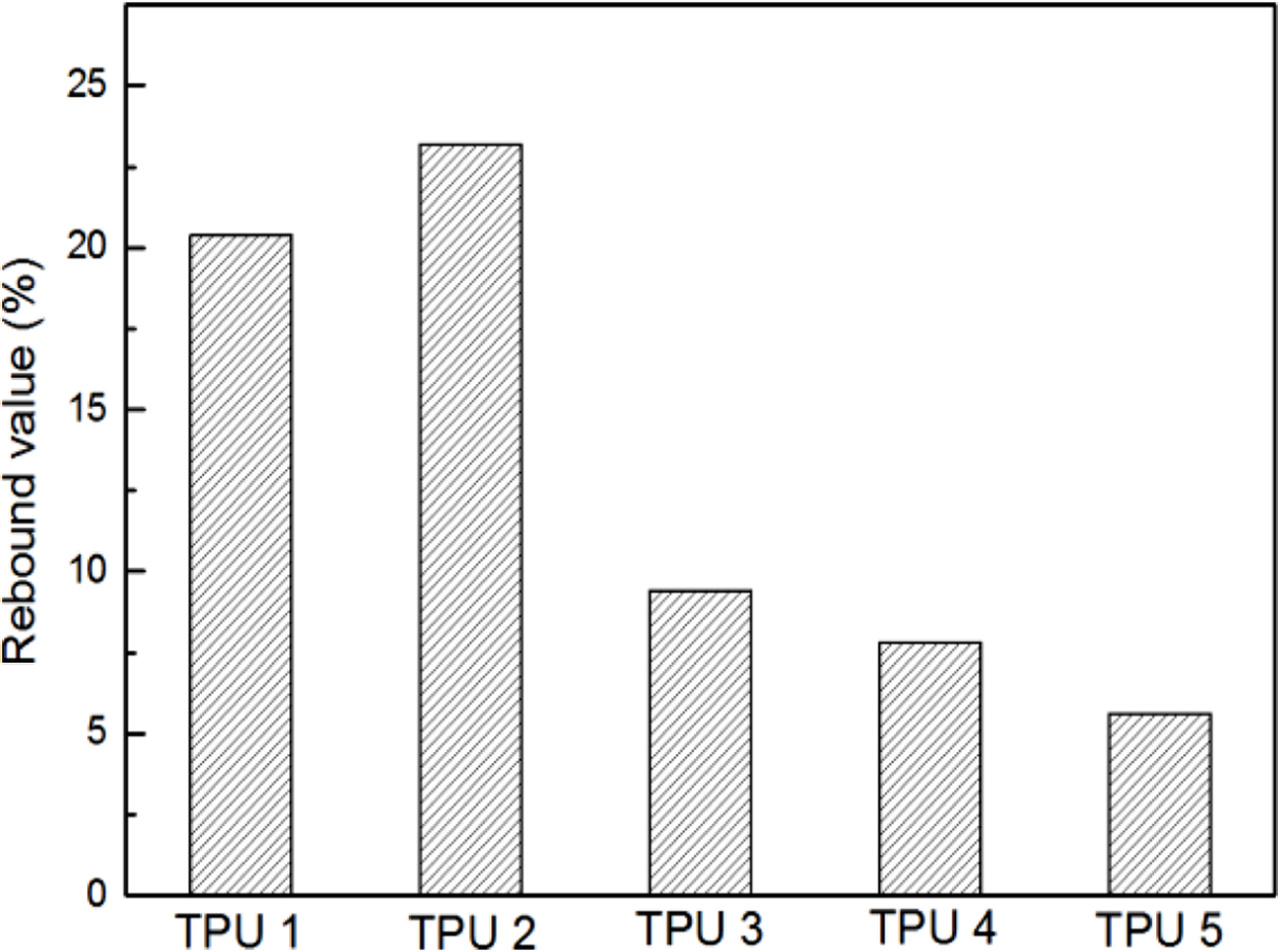
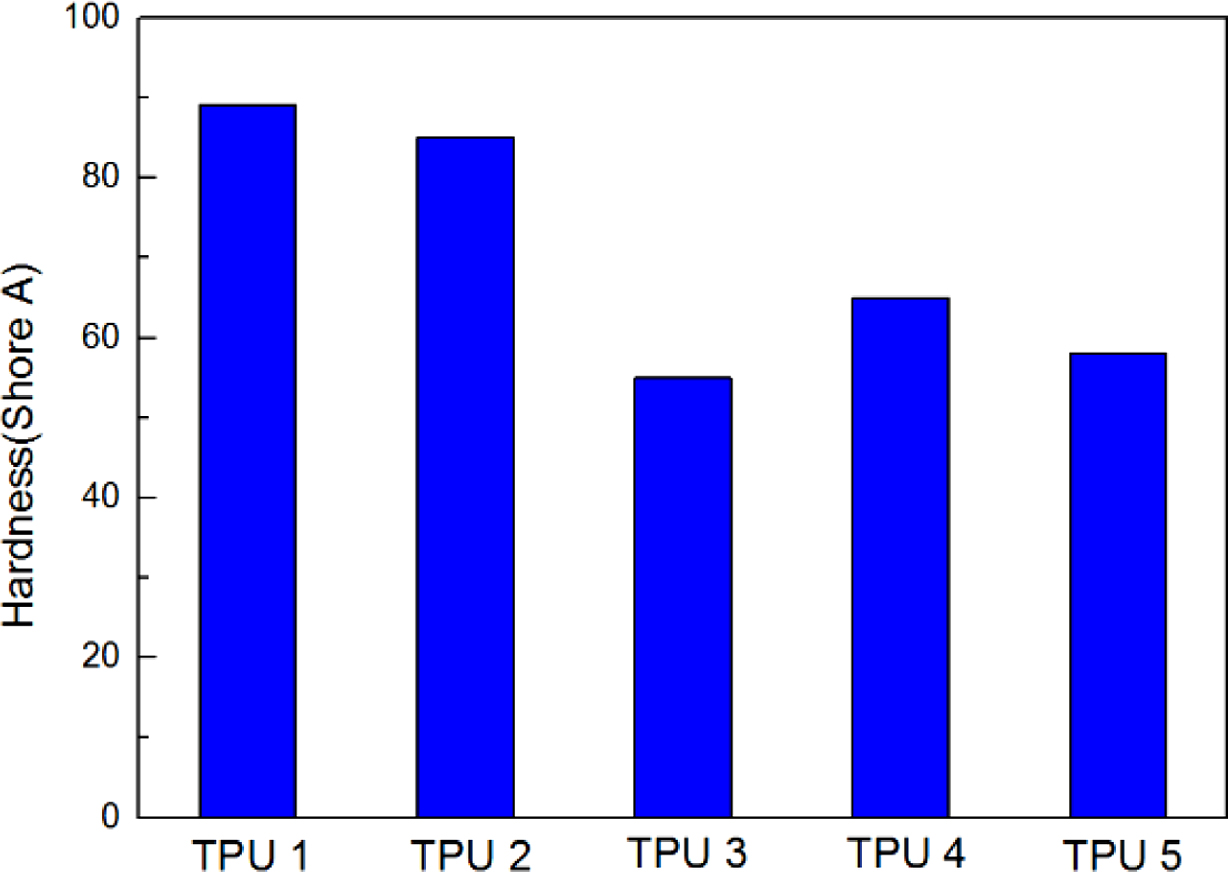
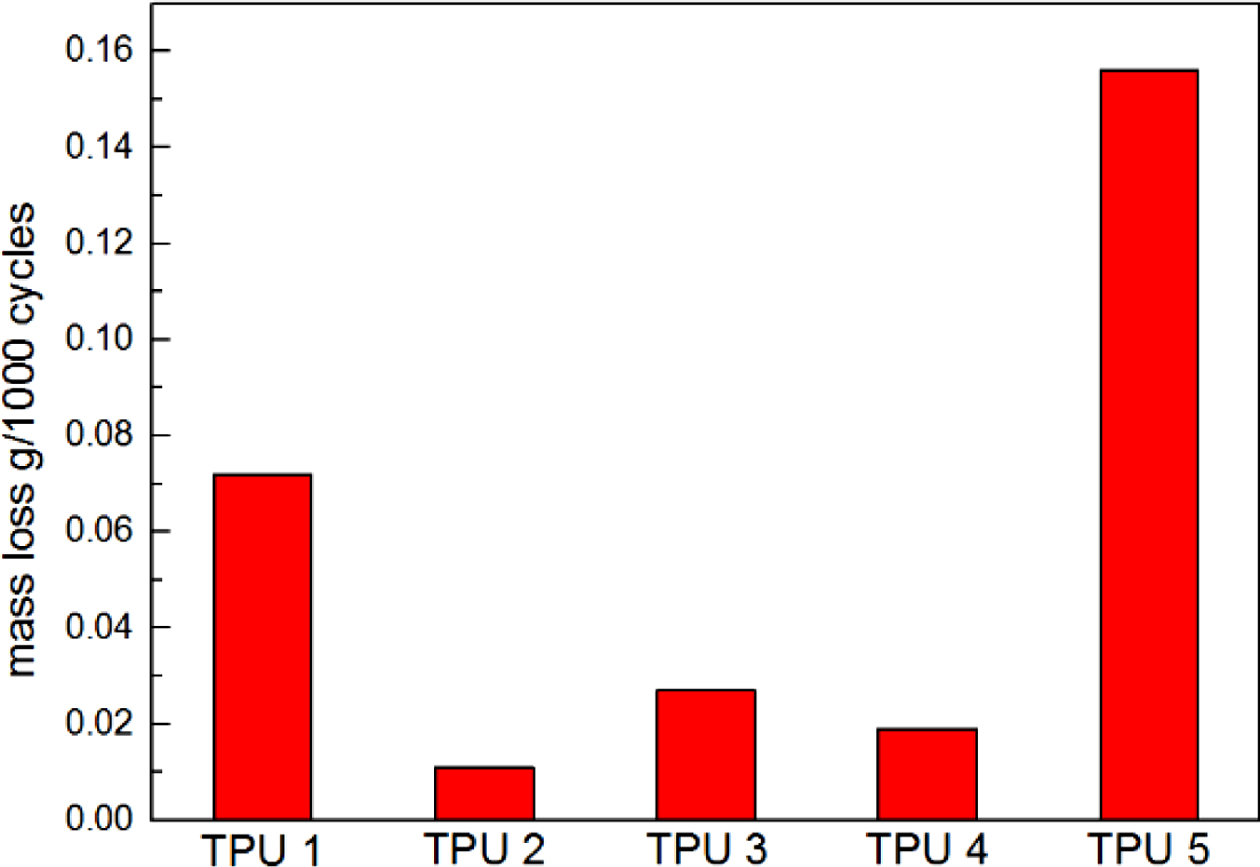
The results of resilience were shown in Figure 4, it could be found TPU 2 showed the largest rebound value, which means this material had the best resilience. It could be found TPU synthesized by bio-polyester polyol also showed the same hardness properties with traditional TPU material, which is an important mechanical property in TPU industry (Figure 5). Also, from the Figure 6, TPU 2 showed the smallest mass loss per 1000 cycles, which means this material has the best abrasion resistance in this research. The probable reason for the results is: the soft segment in bio-polyester polyol has longer carbon chain and lower glass state temperature,19 which could provide better molecular flexibility and increase the hysteresis characteristic of polymer,20 thus the bio-PU showed these great performance.
|
Figure 3 Viscoelastic illustration of TPUs. |
|
Figure 1 Dynamic strain sweep of G'; G'' for samples at a frequency of 60 cpm and at a temperature of 60 ℃. |
|
Figure 2 Dynamic strain sweep of S'; S'' for samples at a frequency of 60 cpm and at a temperature of 60 ℃. |
|
Figure 4 Resilience results of TPUs. |
|
Figure 5 Hardness results of all the samples. |
|
Figure 6 Abrasion resistance of TPUs. |
|
Table 3 Results of Acid Number, Hydroxyl Number and Mn of Nio-polyol and SS-106 |
In this research, the synthesis and properties analysis (mechanical and viscoelastic) of bio-PU materials had been studied. The bio-polyester polyol was prepared by azelaic acid and 1,3-PD with esterification synthesis method, and MDI, H12MDI and IPDI were used as isocyanates, 1,4-BD was used as chain extender, and from the results of characterization process, it can be found the bio-polyester polyol could provide good viscoelastic and mechanical properties when combined with diisocyanates, especially with MDI, such as storage modulus, loss modulus, tensile strength, elongation rate, resilience, hardness and abrasion resistance, the probable reason is due to the regularity of block (soft segments and hard segments) arrangement distribution. The more homogeneous block arrangement distribution, the larger storage/loss modulus and higher elastic/viscous torque of TPU materials. As an important elastomer material in polymer industry, the bio-PU materials will attract great attention in the near future.
- 1. K. H. Zia, H. N. Bhatti, and I. A. Bhatti, React. Funct. Polym., 67, 675 (2007).
-

- 2. S. H. Park, K. W. Oh, and S. H. Kim, Compos. Sci. Technol., 86, 82 (2013).
-

- 3. A. S. Carlsson, Biochimie, 91, 665 (2009).
-

- 4. K. Ermis, A. Midilli, I. Dincer, and M. A. Rosen, Energ. Policy, 35, 1731 (2007).
-

- 5. J. Datta and E. Glowinska, Ind. Crop. Prod., 61, 84 (2014).
-

- 6. D. Hutton and P. K. Stumpf, Arch. Biochem. Biophys., 142, 48 (1971).
-

- 7. R. A. Gross, M. Ganesh, and W. Lu, Trends Biothchnol., 28, 435 (2010).
-

- 8. K. H. Jin and U. R. Cho, Elastomers Compos., 49, 31 (2014).
-

- 9. K. H. Jin, M. S. Kim, and U. R. Cho, Elastomers Compos., 48, 3 (2013).
-

- 10. P. Felizardo, M. J. N. Correia, I. Raposo, J. F. Mendes, R. Berkemeier, and J. M. Bordado, Waste Manage., 26, 487 (2007).
-

- 11. L. L. Monteavaro, E. O. da Silva, A. P. O. Costa, D. Samios, A. E. Gerbase, and C. L. Perzhold, J. Am. Oil. Chem. Soc., 82, 365 (2005).
-

- 12. Z. S. Petrovic, W. Zhang, and I. Javin, Biomacromolecules, 6, 713 (2005).
-

- 13. S. Y. Choi and U. R. Cho, Elastomers Compos., 40, 249 (2005).
- 14. S. O. Hwang, B. H. Lee, and U. R. Cho, Elastomers Compos., 47, 238 (2012).
-

- 15. A. Molino, P. Lovane, A. Donatelli, G. Braccio, S. Chianese, and D. Musmarra, Chem. Eng. Trans., 32, 337 (2013).
-

- 16. Y. K. Jhon, I. W. Cheong, and J. H. Kim, Colloids Surf. A, 179, 71 (2001).
-

- 17. U. R. Vaidya and V. M. Nadkarni, J. Appl. Polym. Sci., 35, 775 (1988).
-

- 18. H. Pawlik and A. Prociak, J. Polym. Environ., 20, 438 (2012).
-

- 19. H. Koerner, G. Price, N. A. Pearce, M. Alexander, and R. A. Vaia, Nat. Mater., 3, 115 (2004).
-

- 20. K. Uemura, R. Matsuda, and S. Kitagawa, J. Solid State Chem., 178, 2420 (2005).
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2019; 43(5): 674-679
Published online Sep 25, 2019
- 10.7317/pk.2019.43.5.674
- Received on Mar 6, 2019
- Revised on May 20, 2019
- Accepted on Jul 10, 2019
 Services
Services
Shared
 Correspondence to
Correspondence to
- Ur Ryong Cho
-
*School of Energy, Materials and Chemical Engineering, Korea University of Technology and Education, Cheonan, Chungnam 31253, Korea
**Research Center of Eco-friendly & High Performance Chemical Materials, Cheonan, Chungnam 31253, Korea - E-mail: urcho@koreatech.ac.kr
- ORCID:
0000-0003-4866-8109










 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.