- Effects of Thermal Treatment on Electrical Conductivity and Hardness of Polyaniline-Silica Composites
Young Been Lee, Sung Kyu Jang, Chung Hyoi Park, and Felix Sunjoo Kim†

School of Chemical Engineering and Materials Science, Chung-Ang University, Seoul 06974, Korea
- 폴리아닐린-실리카 복합체의 전기전도도와 경도에 대한 열처리 효과
중앙대학교 화학신소재공학부
Physical stability of conducting polymers is considerably important for their applications in electronics. Here, we studied the thermal annealing effects of conducting polyaniline (PANI) composites with a sol-gel silica on their mechanical and electrical properties. Films were prepared onto a substrate by simple drop-casting method using solutions including aniline, tetraethyl orthosilicate (TEOS), and ammonium persulfate. Spectroscopic analyses confirm the emeraldine-salt phase of PANI and the presence of silica network in the film. Nanoindentation measurement showed that, although the improvement was observed in the PANI/TEOS hybrid films, the annealing temperature was not a significant factor for the hardness. The electrical conductivity of the composite decreased from 5.5 to 1.9 S/cm after annealing, due to the slight dedoping of PANI by heat-induced removal of the HCl dopant.
전도성 고분자의 물리적 특성은 전자소자 응용에 큰 영향을 끼친다. 본 고에서는 열처리에 의한 폴리아닐린과 실리카 복합체의 기계적 특성과 전기전도도의 영향에 대해 분석하였다. 아닐린과 실리카 전구체의 혼합 수용액과 산화제 수용액을 순차적으로 떨어뜨려 전도성 복합체 박막을 만들고 열처리를 진행하였다. 분광분석 결과를 통해 에메랄딘염 상태의 폴리아닐린과 실리카 네트워크가 형성되었음을 확인하였다. 또한 나노인덴테이션을 통해 복합체의 경도가 순수 폴리아닐린보다 높은 것을 확인했으나, 열처리 온도에 큰 영향을 받지 않았다. 전기전도도는 5.5 S/cm에서 1.9 S/cm로 감소하는 경향을 확인했는데, 이는 열처리에 의한 디도핑의 결과로 분석된다.
In this work, the effects of thermal annealing on the electrical conductivity and nanoindentation force of the polyaniline/silica composite films were studied. Upon heat treatment, slight dedoping and a decrease in the conductivity were observed, while the mechanical hardness was not affected.
Keywords: polyaniline, tetraethyl orthosilicate, conducting polymer, composite, heat treatment
This work was supported by the National Research Foundation Program funded by the Ministry of Science and ICT (NRF-2014M3A7B4051749). This research was also supported by the Chung-Ang University Graduate Research Scholarship in 2017.
Polyaniline (PANI) is one of the most-widely studied conducting polymers for reasons of easy synthesis and doping.1-11 However, similar to most polymers, the mechanical properties of PANI are not sufficiently robust to withstand certain applications because of the non-covalent intermolecular interactions. In order to harden the PANI films, polymer/inorganic hybrid composites can be formed with sol-gel precursors.12-14 Early studies on the composites of PANI and inorganic components mainly used silica particles or silane precursors to form PANI/silica hybrid films. However, it is difficult to make homogeneous composites due to the limited compatibility of them. To resolve this issue, colloidal dispersion of silica nanoparticle15,16 and surface-modified silica particles17 have been studied for PANI composites. For sol-gel derived polymer composites, thermal annealing is one of the simplest processing variations. With sufficient thermal energy, the degree of reactions and the morphology of the organic/inorganic hybrid films can be controlled.
In this paper, we investigated the effects of thermal annealing on mechanical hardness and electrical conductivity of PANI/silica composite films deposited by a simple one-step process. As heat treatment of sol-gel film is a common step for formation of covalent linkage between silica particles, we varied the annealing temperature to study the changes induced by the heat treatment in the TEOS-reinforced PANI composite films. We observed that the hardness was enhanced by TEOS, but the annealing temperature was not a significant factor. The electrical conductivity, however, was decreased through dedoping of PANI in the course of annealing process.
Polyaniline (PANI) and PANI/silica films were fabricated by using a sequential solution-dropping method,6 which was carried out in the following procedure. Aniline (167 mg) in 3 mL of 1 M hydrochloric acid (HCl) was prepared. For preparation of composite films, 167 mg of aniline and 334 mg of tetraethyl orthosilicate (TEOS) were mixed in a solution of 1 M HCl, and then stirred for 24 h at room temperature for hydrolysis. As an oxidant, 410 mg of ammonium peroxydisulfate in the 3 mL of 1 M HCl was used. Polymerization started by sequential dropping of aniline or aniline/TEOS solution and the oxidant solution onto the 2 cm×2 cm glass substrate. After 10 min of oxidative polymerization, the PANI and PANI/TEOS films were washed with acetone several times to remove oxidant and unreacted chemicals. The films were then dried in vacuum for 1 h and annealed on a hot plate for 10 min at 80, 100 ℃, or 120 ℃ in air.
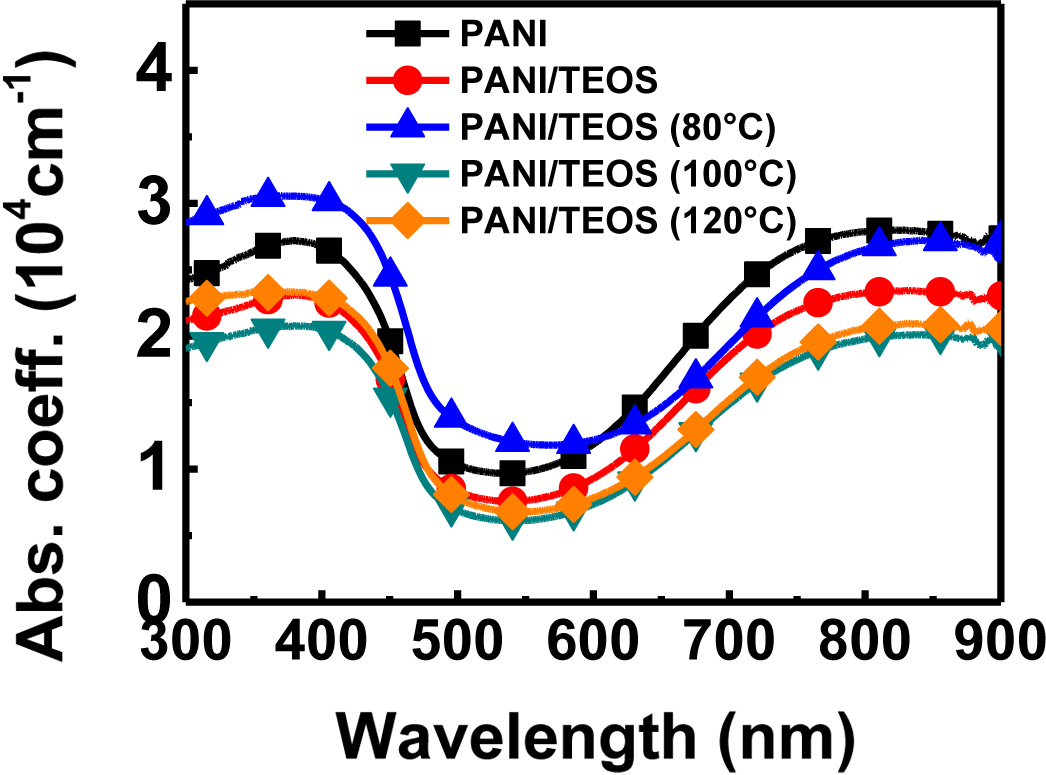
Figure 1 shows the spectroscopic features of polyaniline (PANI) and PANI/TEOS composites with heat treatment. The UV-vis spectra can provide the information on the phase of PANI. PANI is known for having various phases including leucoemeraldine base, pernigraniline base, emeraldine base, and emeraldine salt. A polaron state is formed in emeraldine salt when emeraldine base undergoes protonation with acid.4 Before this protonation, the bands for the base phase can be assigned at about 600 nm. In our PANI and composite films, we can observe typical bands for PANI in the emeraldine salt form at 325-360 nm, 400-430 nm, and 800 nm.18,19 As shown in Figure 1, there was no distinct changes in peak intensity or wavelength. Every peak at PANI emeraldine salt described above can be identified in all annealing conditions, suggesting that the electronic state of PANI is not significantly altered by the heat treatment up to 120 ℃.
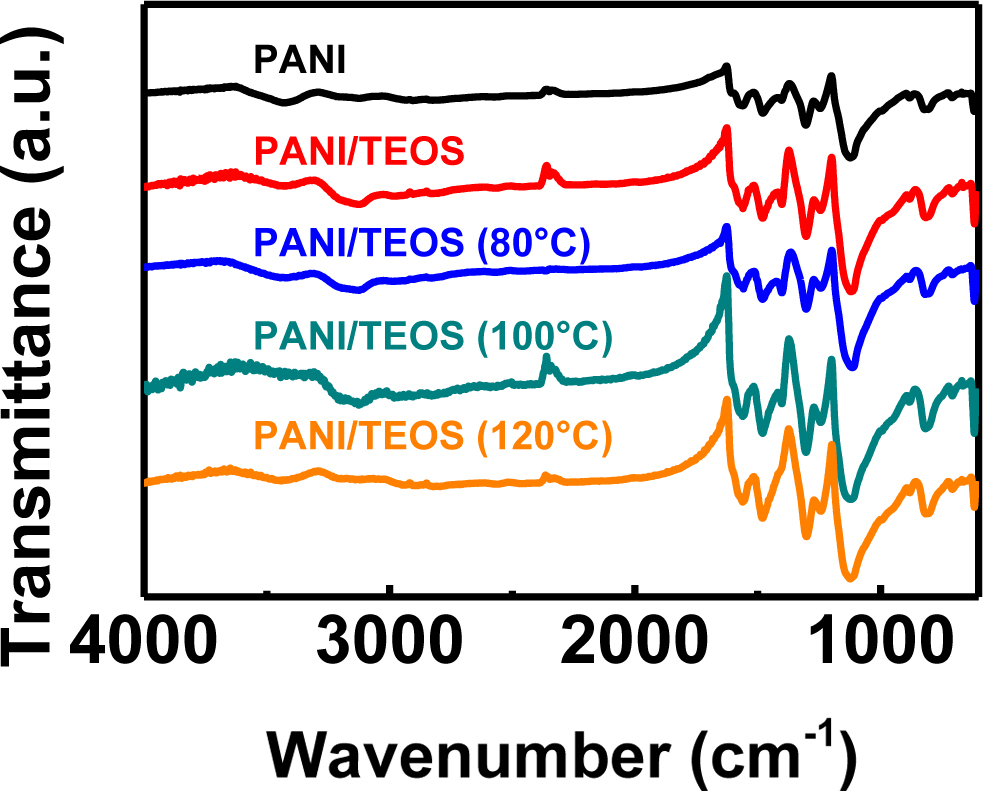
Fourier-transform infrared (FTIR) spectra were collected using KBr pellet to characterize the bonds in PANI and PANI/TEOS composites (Figure 2). The main peaks of PANI are observed at 1140 and 1307 cm-1. Stretching of six-membered carbon rings can be assigned at 1480 and 1580 cm-1.20,21 We note that the bands for cyclic species including Si frequently appear at 1100-1250 cm-1, which overlap with the main PANI peak at 1140 cm-1. Although it is challenging to quantify the amount of species in IR, the intensity of bands near 1100 cm-1 is slightly enhanced when TEOS is incorporated. Additional evidence for siloxane ring of sol-gel films appeared at 620 cm-1, which indicated residual cyclic structures in the silica network.22 This band suggests that an interpenetrated PANI/silica network is formed through the sol-gel process.
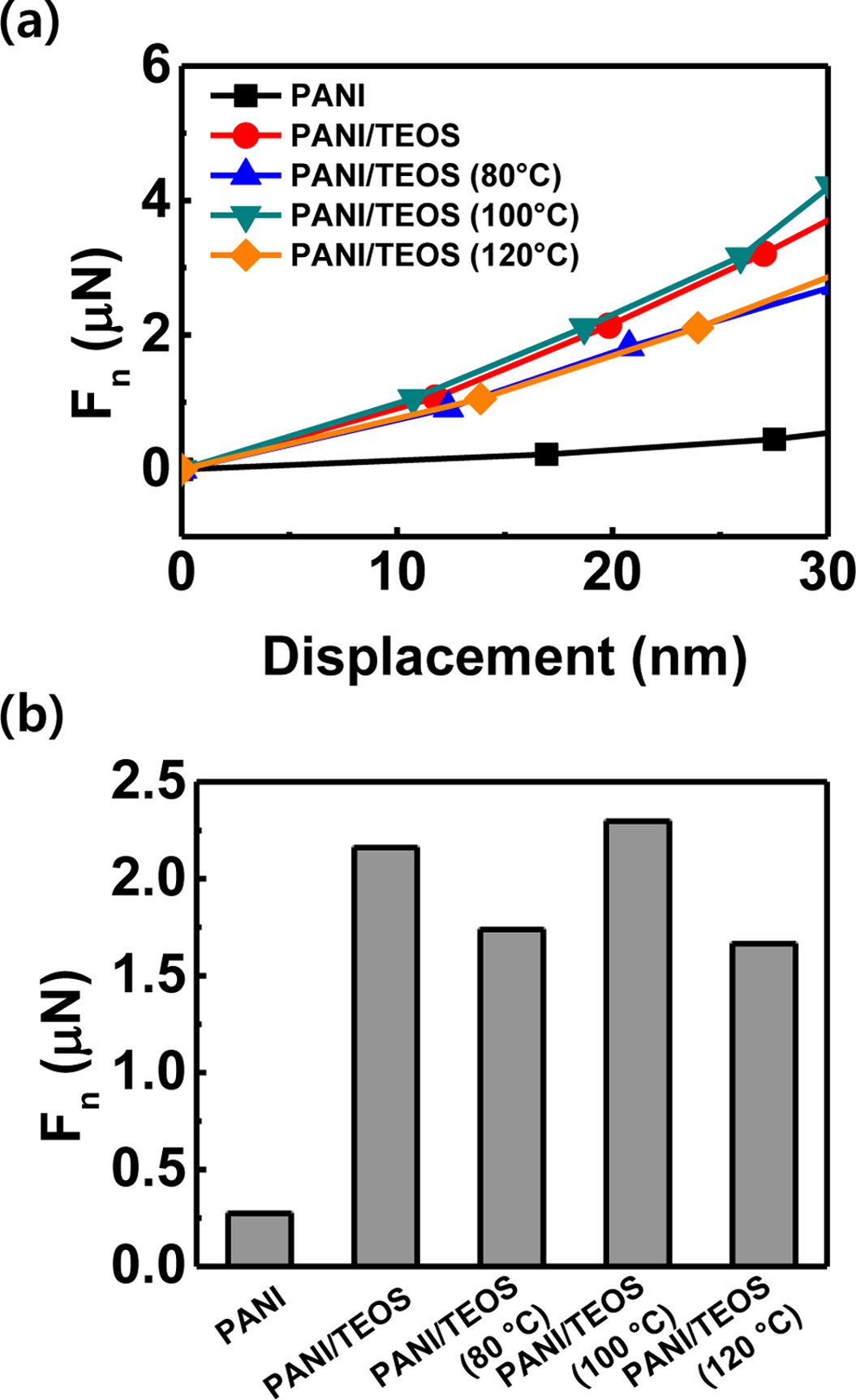
We performed the nano-indentation measurement on the conducting polymer composites. Figure 3(a) shows the indentation force-displacement curves of PANI and PANI/TEOS films. The composites show a sharp increase in the force as indentation depth increased, compared to pure PANI film. For a quantitative comparison, indentation forces at the displacement of 20 nm are plotted in Figure 3(b). The numerical values of indentation force at the 20 nm were extracted by fitting the first four points with a 3rd-order polynomial equation. The PANI/TEOS film showed the indentation force of 2.16 μN, which is eight-fold higher than that of the pure PANI without reinforcement (0.27 μN). This enhancement of hardness suggests successful hydrolysis and condensation of inorganic precursors during PANI/TEOS composite formation. We note that, although the highest force value was 2.30 μN appeared in the PANI/TEOS after annealing at 100 ℃, all the indentation forces are in the range of 1.7-2.3 μN without noticeable trends with or without thermal annealing. This result suggests that the annealing temperature is not a critical factor in hardness enhancement in the PANI/TEOS composites.
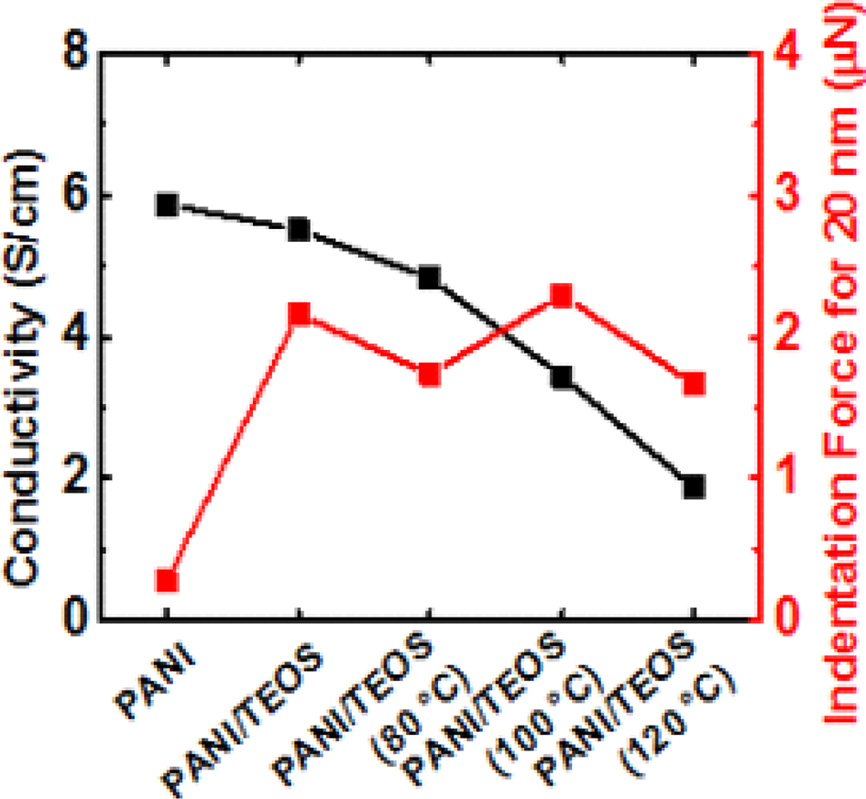
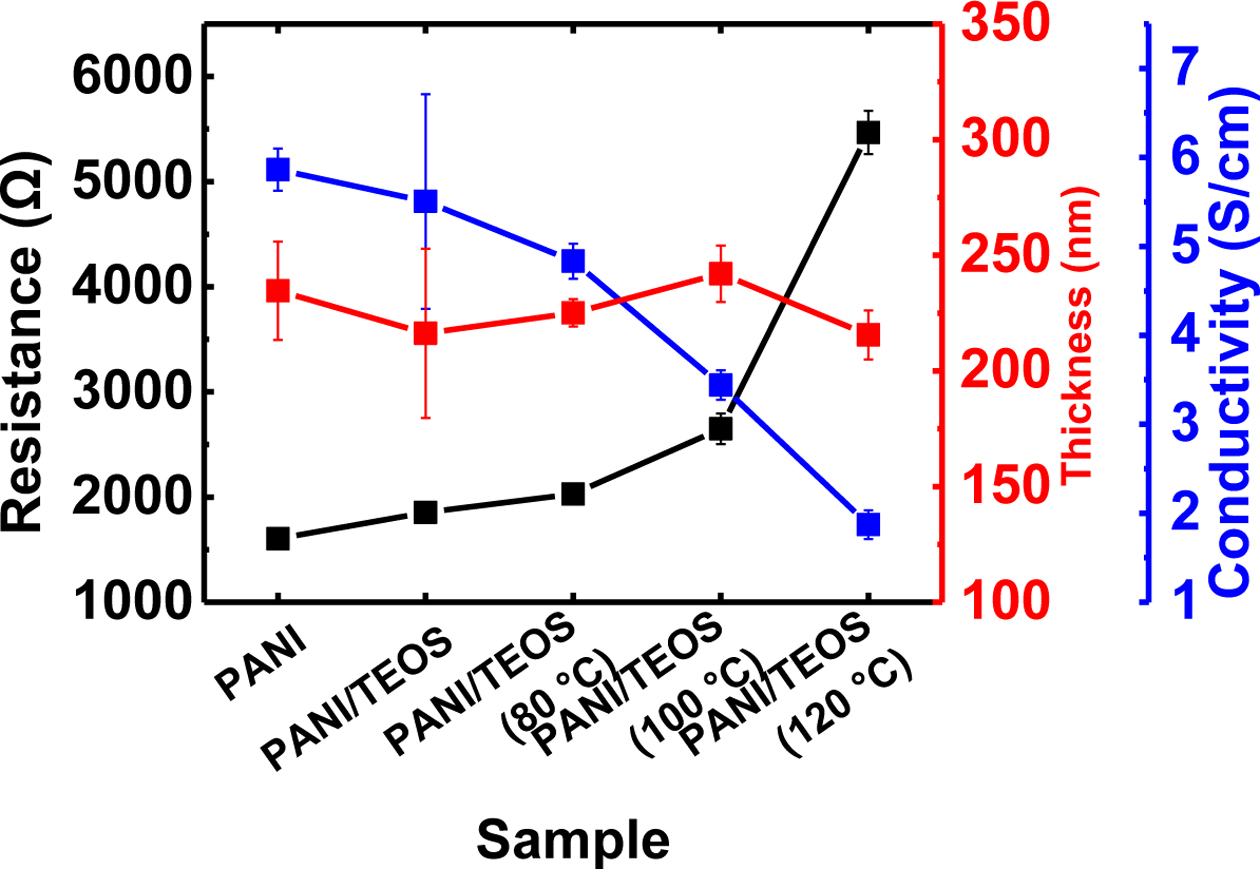
The electrical conductivity of PANI and PANI/TEOS films was investigated by measuring the resistance from four-point probe method and the thickness (Figure 4). The thickness was in a range of 215-242 nm for both pure PANI and PANI/TEOS films. Heat treatment does not affect the film thickness. In case of electrical resistance, slight increase was observed from 1.60 kΩ in PANI to 1.85 kΩ in PANI/TEOS without annealing. The presence of non-conducting silica is responsible for this increase in resistance. However, the resistance increased further to 2.03, to 2.65, and to 5.47 kΩ, when the PANI/TEOS film was annealed at 80, 100 ℃, and 120 ℃, respectively. Such an increase in resistance originates from the loss of acidic dopants.23 This loss of dopant can convert the composite films to partially deprotonated form, resulting in a low electrical conductivity. As a result, the electrical conductivity of PANI/TEOS films gradually decreased by 66%, from 5.5 S/cm without annealing to 1.9 S/cm after aging at elevated temperature of 120 ℃.
|
Figure 1 Absorption spectra of PANI/TEOS films upon thermal annealing. |
|
Figure 2 Fourier-transform infra-red (FTIR) spectra of PANI/TEOS films upon thermal annealing. |
|
Figure 3 (a) Force-displacement curves of the PANI/TEOS hybrid films; (b) Indentation force at 20 nm. |
|
Figure 4 Resistance, thickness, and electrical conductivity of conducting PANI/TEOS hybrid films as a function of annealing temperature. |
We investigated the effects of heat treatment on mechanical hardness and electrical conductivity of polyaniline (PANI) composites with sol-gel-derived silica. Formation of covalent siloxane bonds provided a better mechanical stability. UV-vis spectra showed that there is no significant influence on phase of PANI by thermal annealing. Distinctive IR spectra of PANI and siloxane rings appeared in the hybrid sol-gel films. To get a quantitative measure of mechanical stability, we performed nano-indentation measurement on the films. Although nearly eight-fold enhancement of indentation force was obtained in the hybrid films compared to the pure PANI films, the annealing temperature was not a significant factor. The PANI/TEOS annealed at 100 ℃ showed the indentation force of 2.3 μN at the displacement of 20 nm. This study suggests that the TEOS precursor can serve as robust support by undergoing siloxane bond formation in polymer composites. Annealing temperature has little effect on the hardness enhancement, although the conductivity is decreased by 66%.
- 1. J. Huang, S. Virji, B. H. Weiller, and R. B. Kaner, J. Am. Chem. Soc., 125, 314 (2003).
-

- 2. Y. Xia, A. G. MacDiarmid, and A. J. Epstein, Macromolecules, 27, 7212 (1994).
-

- 3. A. G. MacDiarmid, Synth. Met., 84, 27 (1997).
-

- 4. A. G. Macdiarmid, J.-C. Chiang, M. Halpern, W.-S. Huang, S.-L. Mu, L. D. Nanaxakkara, S. W. Wu, and S. I. Yaniger, Mol. Cryst. Liquid Cryst., 121, 173 (1985).
-

- 5. D. Li and R. B. Kaner, J. Am. Chem. Soc., 128, 968 (2006).
-

- 6. C. H. Park, S. K. Jang, and F. S. Kim, Appl. Surf. Sci., 429, 121 (2018).
-

- 7. S. K. Jang, J. Choi, and F. S. Kim, J. Nanosci. Nanotechnol., 17, 7793 (2017).
-

- 8. J. Choi, S. K. Jang, and F. S. Kim, Phys. Stat. Sol. A, 215, 1701019 (2018).
-

- 9. S. W. Kang and J. Bae, Macromol. Res., 26, 226 (2018).
-

- 10. L. Li, Y. Guo, C. Zhao, and L. Song, Macromol. Res., 26, 592 (2018).
-

- 11. U. Male and B. K. Shin, Macromol. Res., 25, 1121 (2017).
-

- 12. R. Roy, Science, 238, 1664 (1987).
-

- 13. U. Schubert, N. Huesing, and A. Lorenz, Chem. Mater., 7, 2010 (1995).
-

- 14. C. J. Brinker and G. W. Scherer, Sol-gel science: the physics and chemistry of sol-gel processing, Academic Press, 2013.
- 15. H. Xia and Q. Wang, J. Appl. Polym. Sci., 87, 1811 (2003).
-

- 16. N. Roosz, M. Euvard, B. Lakard, C. C. Buron, N. Martin, and L. Viau, J. Colloid Interface Sci., 502, 184 (2017).
-

- 17. C. F. Lee, H. H. Tsai, L. Y. Wang, C. F. Chen, and W. Y. Chiu, J. Polym. Sci.; Part A: Polym. Chem., 43, 342 (2005).
-

- 18. J. Stejskal, P. Kratochvil, and N. Radhakrishnan, Synth. Met., 61, 225 (1993).
-

- 19. T. Abdiryim, Z. Xiao-Gang, and R. Jamal, Mater. Chem. Phys., 90, 367 (2005).
-

- 20. J. Tang, X. Jing, B. Wang, and F. Wang, Synth. Met., 24, 231 (1988).
-

- 21. Z. Tang, S. Liu, Z. Wang, S. Dong, and E. Wang, Electrochem. Commun., 2, 32 (2000).
-

- 22. P. Innocenzi, J. Non-Cryst. Solids, 316, 309 (2003).
-

- 23. M. Trchova, I. Seděnkova, E. Tobolkova, and J. Stejskal, Polym. Degrad. Stabil., 86, 179 (2004).
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2019; 43(5): 661-664
Published online Sep 25, 2019
- 10.7317/pk.2019.43.5.661
- Received on Jun 14, 2019
- Revised on Jul 18, 2019
- Accepted on Jul 18, 2019
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Felix Sunjoo Kim
-
School of Chemical Engineering and Materials Science, Chung-Ang University, Seoul 06974, Korea
- E-mail: fskim@cau.ac.kr
- ORCID:
0000-0002-3286-6146









 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.