- Phenothiazine Derivative as Organic Photocatalyst for Metal Free Atom Transfer Radical Polymerization
Hoan Minh Tran*, Lan Ngoc Tan Phan*, Thang Van Le**,***, Thuy Thuy Truong*, Tam Huu Nguyen*, Khuong Tung Truong*, Le-Thu T. Nguyen*,**, Mai Thanh Phong****, and Ha Tran Nguyen*,**,†

*National Key Laboratory of Polymer and Composite Materials, University of Technology, Vietnam National University – Ho Chi Minh City (VNU–HCM), 268 Ly Thuong Kiet, District 10, Ho Chi Minh City, Vietnam
**Faculty of Materials Technology, Ho Chi Minh City University of Technology, Vietnam National University, 268 Ly Thuong Kiet, District 10, Ho Chi Minh City, Vietnam
***Materials Technology Key Laboratory (Mtlab), Ho Chi Minh City University of Technology, Vietnam National University, 268 Ly Thuong Kiet, District 10, Ho Chi Minh City, Vietnam
****Faculty of Chemical Engineering, Ho Chi Minh City University of Technology (HCMUT), Vietnam National University, 268 Ly Thuong Kiet, District 10, Ho Chi Minh City, Vietnam- Phenothiazine 유래 유기광촉매을 이용한 Metal Free 원자이동 라디칼 중합
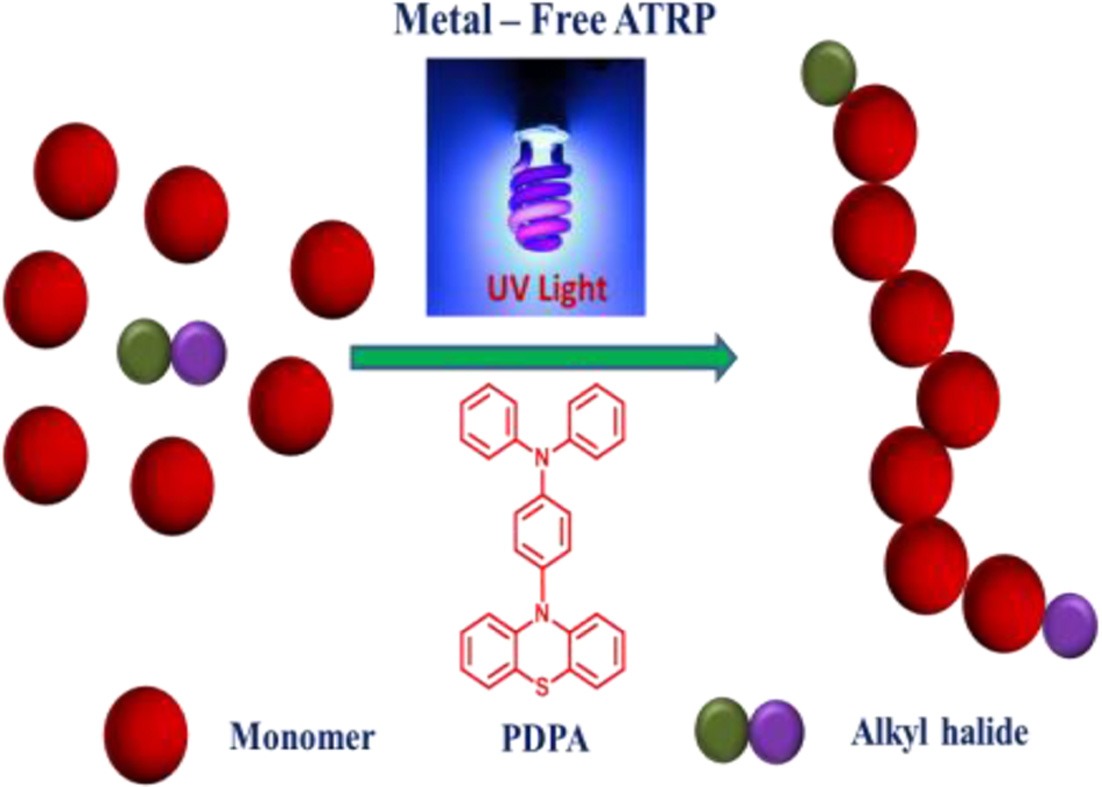
A novel organic photocatalyst, 4-(10H-phenothiazin-10-yl)-N,N-diphenylaniline (PDPA) has been successfully synthesized from triphenylamine and phenothiazine moiety via Buchwald-Hartwig C-N coupling. The chemical structure of catalyst was characterized via proton nuclear magnetic resonance (1H NMR) and the optical properties were investigated via UV-vis spectroscopy. The PDPA has been applied as an organic photocatalyst for metal free atom transfer radical polymerization (O-ATRP). The well-controlled molecular weights of polymethacrylates have been obtained with high yield of 95% and narrow polydispersity index (Đ).
A novel organic photocatalyst, 4-(10H-phenothiazin-10-yl)-N,N-diphenylaniline (PDPA) has been synthesized and used for the metal free atom transfer radical polymerization of metha(acrylate) monomers.
Keywords: phenothiazine, organic photocatalyst, metal-free atom transfer radical polymerization, Buchwald-Hartwig C-N coupling, methacrylate monomers
This research was supported by project “NV2018-20-03/HĐ-KHCN” from Ho Chi Minh City University of Technology - Vietnam National University – Ho Chi Minh City, 268 Ly Thuong Kiet, District 10, Ho Chi Minh City, Viet Nam
In the field of polymer synthesis, controlled free radical polymerization (CRP) is considered one of the most attractive methods since it is able to produce a variety of functionalized polymers and diblock copolymers having controlled molar mass, narrow polydispersity as well as desired end-groups of polymer chains.1-3 Among CRP techniques, ATRP is used widely for the polymerization of vinyl monomers incuding methacrylate.4-7 In traditional ATRP, the transition metal such as Ru, Fe, Mo and Cu have been used for controlled polymerization process via a redox equilibrium process mediated by a ligated metal catalyst. However, for several applications related to microelectronics, biomaterials, and photo-electronic materials, the metal contamination is one of the key limiting factors which is negative effect for such materials.8-10 Therefore, a significant research for ATRP field has focused on decrease in the amount of transition metal catalyst loading or removal of residual metals after polymerization process.11-14 Although, catalyst loadings have been reduced to parts per million (ppm), we expected that an ambitious method to this challenge would be development of a metal-free catalyst system for ATRP which lead to the complete removal of the trace metal catalyst in final products. Following this trend, one of the strategy to reduce the metal catalyst in ATRP is used the supporting catalyst in a solid and solid phase ATRP via electrochemical ATRP technique.15,16
In few years ago, metal-free ATRP (O-ATRP) was discovered by using photoredox catalysts (PC) to mediate the exchange between active and dormant species under light irradiation. A proposed general photoredox O-ATRP mechanism is related to photoexcitation of the PC to an excited state PC (PC*) which is capable of reducing alkyl bromide via an oxidative quenching pathway to generate the active radical for polymerization propagation, simultaneous the ion pair complex of radical cation (PC+) and Br- was formed. Miyake and co-workers focused on organic photoredox catalysts using perylene aromatic conjugated compound for O-ATRP polymerization of methyl methacrylate under visible light. Phenylphenothiazine has been reported by Hawker and co-workers as an organic photocatalyst for metal-free ATRP for methyl methacrylate (MMA) polymerization under UV irradiation. The catalyst has been expanded for polymerization of acrylonitrile by Matyjaszewski.17-21 More recently, numerous organic photocatalysts such as pyrene, perylene, diaryl dihydrophenazines have been discovered for the controlled polymerization of methacrylate monomers in both UV and visible light irradiation. Among organic photocatalysts, phenolthiazine is one of the potential compounds for metal-free ATRP.
Herein, the novel phenolthiazine derivative of 4-(10H-phenothiazin-10-yl)-N,N-diphenylaniline (PDPA)) has been synthesized via direct amination then it was used as an organic photocatalyst for O-ATRP of methacrylate monomers, N,N-dimethylamino-2-ethyl methacrylate (DMAEMA) and 2-hydroxyethyl methacrylate (HEMA) under UV irradiation. Addition, we have investigated the efficiency of PDPA organic photocatalyst for O-ATRP process comparing with other PCs based on phenolthiazine derivative reported in the previous articles.
Materials. N-bromosuccinimide (NBS) and triphenylamine (99%) were purchased from Acros Organics (Bridgewater, NJ, USA). Phenyl 2-bromo-2-methylpropionate (PhBMP), phenothiazine (98%), sodium tert-butoxide (NaOtBu, 99.9%), palladium(II) acetate (Pd(OAc)2, 98%), N,N-dimethylformamide (DMF, 99.8%) and tri-tert-butylphosphine ligand (P(t-Bu)3, 98%) were purchased from Sigma Aldrich and used as received. Chloroform (CHCl3, 99.5%), toluene (99.5%), and tetrahydrofuran (THF, 99%) were purchased from Fisher/Acros and dried using molecular sieves under N2. Potassium carbonate (K2CO3), n-hexane (99%), methanol (99.8%), 1,4-dioxane (99%) and ethyl acetate (EtOAc, 99%) were purchased from Fisher/Acros and used as received. Methyl methacrylate (MMA), N,N-dimethylamino-2-ethyl methacrylate (DMAEMA) and 2-hydroxyethyl methacrylate (HEMA) monomers were purchased from Aldrich and purified by passing through a column of basic alumina to remove the inhibitor. Hydrochloric acid (HCl, 37%) was purchased from Merck. All reactions were carried out in oven-dried flask under purified nitrogen.
Methods. 1H NMR spectra was recorded in deuterated chloroform (CDCl3) with tetramethylsilane (TMS) as an internal reference on a Bruker Avance 500 MHz spectrometer. Size exclusion chromatography (SEC) measurements were performed on a Polymer PL-GPC 50 gel permeation chromatography (GPC) system equipped with an RI detector and using THF as the eluent at a flow rate of 1.0 mL min-1. Molecular weight and molecular weight distributions were calculated with reference to polystyrene standards. The ultraviolet-visible (UV–Vis) spectra were recorded via a Shimadzu UV-2450 spectrophotometer over the wavelength range 300–900 nm. The O-ATRP was performed under UV irradiation at room temperature using a 365 nm UV light (12×9 watt bulbs, intensity of 2.2 mW/cm2 determined by a VLX365 radiometer).
Synthesis of 4-bromo-N,N-diphenylaniline (Compound 1) (BrDPhA). N-bromosuccinimide (415 mg, 2.32 mmol) and triphenylamine (570 mg, 2.32 mmol) were added to anhydrous DMF (6 mL) at 0 ℃ under nitrogen. The mixture was stirred at 50 ℃ for 1.5 h. After completion of the reaction, 10 mL of distilled water was added to the reaction mixture, which was extracted with dichloromethane. The organic layer was washed with 10% solution of Na2S2O3 and 10% solution of KOH, dried over anhydrous K2CO3 and concentrated. The product was precipitated in cold n-hexane and dried under vacuum to give a white powder (732 mg; Yield: 97%, Rf=0.7).
1H NMR (500 MHz, CDCl3), δ (ppm): 7.31 (d, 2H), 7.24 (t, 4H), 7.07 (d, 4H), 7.02 (t, 2H), 6.93 (m, 2H).
Synthesis of 4-(10H-phenothiazin-10-yl)-N,N-diphenyl-aniline (Compound 3) (PDPA). A 25 mL round-bottom flask was charged with a magnetic stir bar, flamed under vacuum and back filled with nitrogen three times. The flask was then charged with phenothiazine (160 mg, 0.81 mmol), NaOtBu (116 mg, 1.20 mmol), Pd(OAc)2 (3.61 mg, 0.016 mmol), P(t-Bu)3 (6.50 mg, 0.032 mmol) and anhydrous toluene (8 mL). The flask was evacuated and back-filled three times with nitrogen before 4-bromo-N,N-diphenylaniline (312 mg, 0.96 mmol) was added. The flask was then placed in an oil bath at 110 ℃ while stirring for 4 h. The flask was then cooled to room temperature, diluted with CHCl3, washed with water, brine, dried with K3CO3, and purified using column chromatography (5% EtOAc/n-hexane). The product was dried under reduced pressure to obtain white solid (257 mg, 72%).
1H NMR (500 MHz, CDCl3), δ (ppm): 7.31 (t, 4H), 7.19 (m, 8H), 7.08 (m, 2H), 7.00 (m, 2H), 6.88 (m, 2H), 6.79 (t, 2H), 6.31 (d, 2H). 13C NMR (75.5 MHz, CDCl3), δ (ppm): 146.0, 143.8, 140.3, 135.0, 129.6, 128.3, 127.2, 126.0, 122.5, 117.0. Anal. Calcd. for C30H22N2S: C, 81.41; H, 5.01; N, 6.33; S, 7.25. Found: C, 80.92; H, 5.10; N, 5.95; S: 8.03.
General Synthesis of Polymers. PMMA was synthesized via UV light-induced metal-free ATRP using the PhBMP-initiator and PDPA as oganic photocatalyst. In a typical experiment, 11.43 mg (47 µmol) of PhBMP initiator was placed in a 25 mL flask, to the solution 1 mL of degassed THF was added by a syringe. The solution was stirred until it became homogeneous. Then, MMA monomer (0.5 mL, 4.7 mmol) and PDPA (2.1 mg, 4.7 µmol) was added separately. The mixture was degassed by three freeze-pump-thaw cycles. The solution was continuously stirred until it became homogeneous and placed in a UV-box (wavelength of 365 nm) for 24 h at room temperature. Finally, the resulted polymer solution was precipitated in cold methanol, followed drying under vacuum to give the desired product.
The synthesis of organic photocatalyst 4-(10H-phenothiazin-10-yl)-N,N-diphenylaniline (PDPA) was illustrated in Scheme 1. 4-bromo-N,N-diphenylaniline (BrDPhA) was synthesized through bromination of electrophilic substitution (triphenylamine) using NBS in DMF, and the yield of reaction was obtained as 97%. Then BrDPhA was reacted with phenothiazine via Buchwald-Hartwig C-N coupling in the presence of Pd(OAc)2 and P(t-Bu)3 as catalyst and ligand, respectively (Yield: 72%).
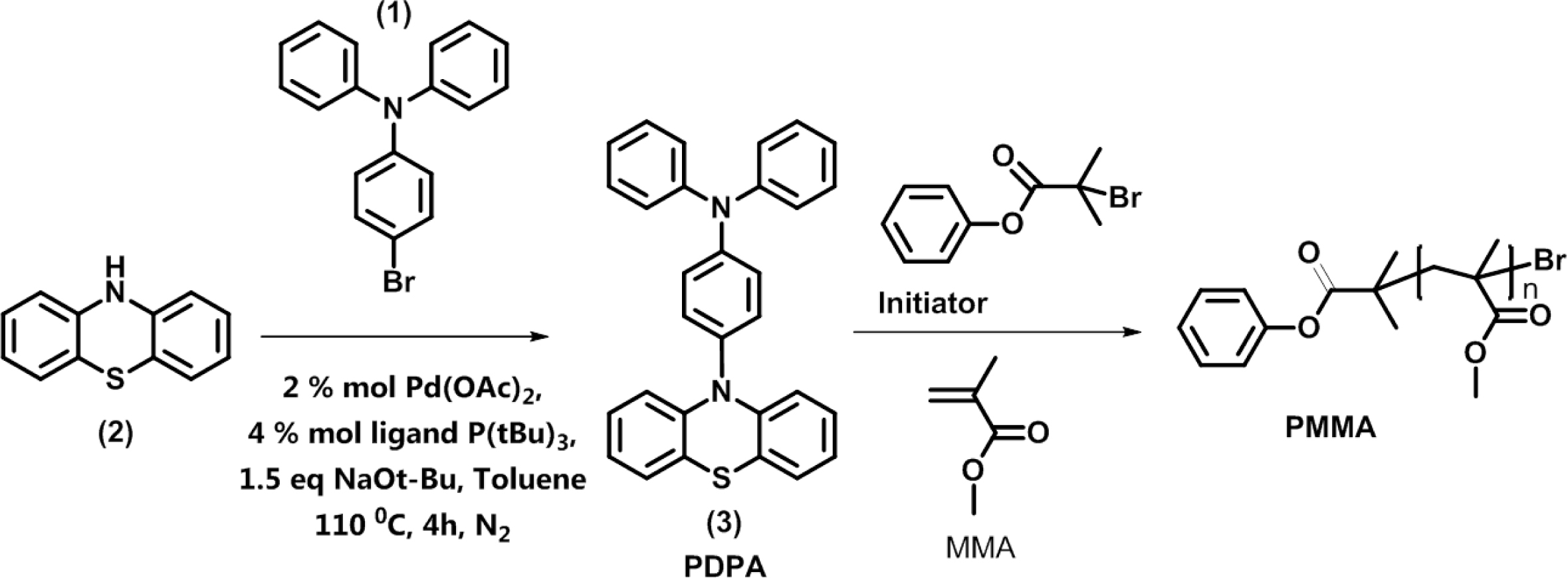
Scheme 1. Synthesis of PDPA organic photocatalyst and PMMA.
The chemical structure of PDPA was analyzed via 1H NMR spectrum in Figure 1. The data showed that 1H NMR spectrum of PDPA exhibited fully characteristic peaks of PDPA including peaks of phenolthiazine and triphenylamine. The peaks at 7.31 ppm (peak “f”, triplet), 7.19 ppm (peak “e”, quart) and 7.08 ppm (peak “g”, triplet) are corresponding to the protons of triphenylamine moieties. The peaks at 7.00 ppm (peak “d”, doublet), 6.88 ppm (peak “b”, triplet), 6.79 ppm (peak “c”, triplet) and 6.31 ppm (peak “a”, doublet) were assigned to the protons of phenolthiazine ring. Based on the characteristic peaks and reasonable their integration, the obtained product has been concluded that PDPA was synthesized successfully.
The UV-Vis spectrum of PDPA was measured in different concentrations of THF with different concentrations in Figure 2. The absorbed curves exhibited two distinct absorption peaks at 258 and 306 nm which corresponding to the absorption of triphenylamine moieties and phenolthiazine, respectively. Moreover, the spectra showed a linear correlation between concentration and absorbance, and the molar extinction coefficient was determined through the Lambert – Beer law. The first absorption peak at 259 nm presents an extinction coefficient value of e (33790 M−1cm−1) and the peak at 306 nm shows a extinction coefficient of e (27150 M−1cm−1). These results suggested that PDPA catalyst has the absorption maxima in the UV regime.
The mechanism of organic photocatalyst for O-ATRP was proposed in Scheme 2. According to the pioneer work of Hawker, Matyjaszewski, and Miyake, we carried out the following process for metal-free ATRP where PDPA is used as the organic photocatalyst. Under UV irradiation, PDPA is excited to form a reductant PDPA*, which activates phenyl 2-bromo-2-methylpropanoate (PBMP) initiator and generates radicals. The generated radical can be added to methacrylate monomers (MMA, HEMA and DMAEMA) to form alkyl radicals, which are deactivated by the oxidized radical cation PDPAˆ+ to regenerate the ground state of PDPA as insulated Scheme 2. In this typical O-ATRP, we also investigated the solvents, the molar ratio of PDPA catalyst with initiator, monomers which impact to the efficiency of polymerization.
To test the ability of PDPA to mediate the O-ATRP, MMA was polymerized under nitrogen environment using appropriate alkyl halide sources, such as PBMP. The results obtained with the PDPA/PBMP initiating system are summarized in Table 1.
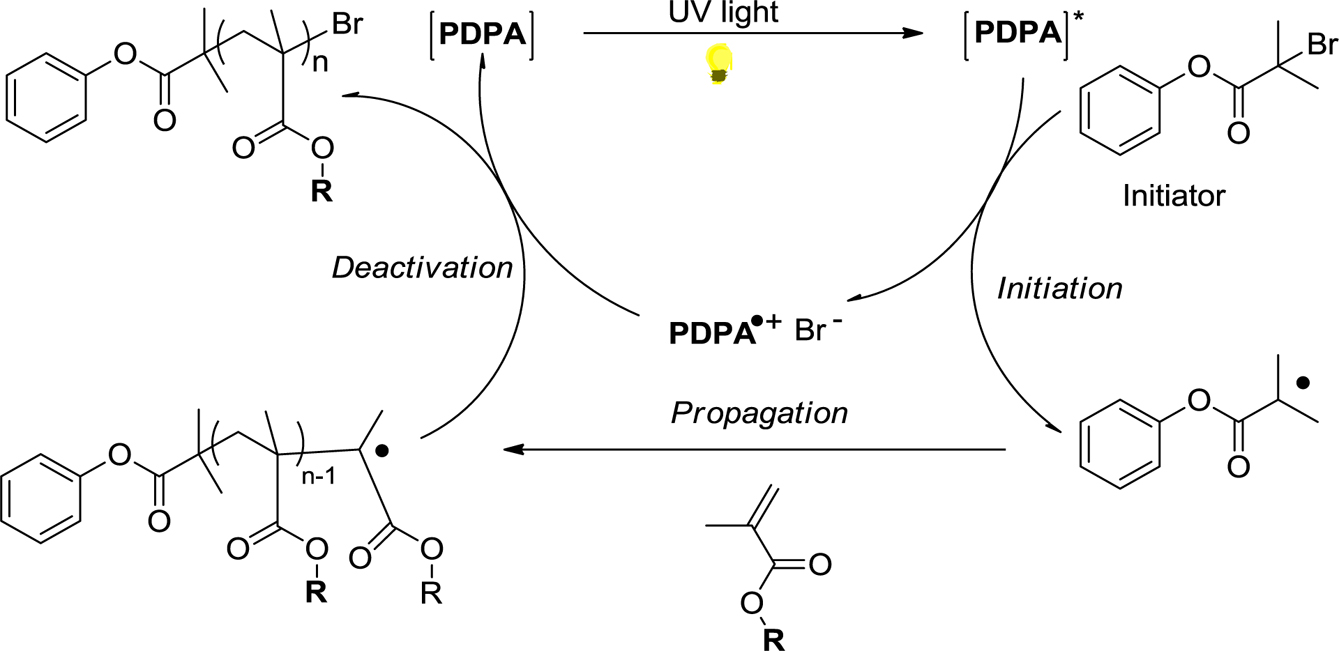
Scheme 2. Proposed mechanism for a O-ATRP of methacrylate monomers under UV irradiation.
The influence of the amount of PDPA catalyst on the O-ATRP polymerization of MMA was investigated by fixing of the molar ratio of [MMA]/[Initiator] = 100/1 with different content of PDPA catalyst including 1, 0.5, 0.1 and 0.05 equivalent (Table 1, Entry 1-4). The result showed that the amount of PDPA about 10% molar ratio with initiator has the high monomer conversion of 94.58%. On the other hand, the conversion of monomer in the polymerization was decreased if the amount of PDPA catalyst increased. It should be noted that the resulted PMMA exhibited the Mn of 11700 g/mol which is similar to the theoretical molecular weight, and the polydispersity index (Đ) of obtained PMMA exhibited the value of 1.46 which is reasonable for controlled polymerization (normally Đ is required below 1.5). On the contrary, the molar ratio PDPA/ PhBMP-initiator is higher or lower 0.1 that leads the decreasing of polymerization yield. Based on these results, the molar ratio of PDPA catalyst/initiator should be remained at 0.1 for high yield of polymerization, and THF is also reasonable solvent for this O-ATRP polymerization.
Consequently, we continued to investigate the influence of different solvents to O-ATRP (Table 2, entry 5-7). In this case, we select the polar solvents such as DMF, N,N-dimethylacetamide, and non-polar solvents including toluene. In the same condition of molar ratio based on monomer, initiator and PDPA catalyst where the molar ratio of PDPA/initiator was established 0.1, the O-ATRP of MMA in THF gives the high yield of 94.5% which is much higher the yield of polymerization performed in DMF, DMAc and toluene with 85%, 76% and 43%, respectively. In addition, the obtained PMMA was characterized via 1H NMR in Figure 3 to determine its structure as well as the absolute molecular weight. Based on the integration ratio of characteristic peaks of PMMA in 1H NMR spectrum, the molecular weight of PMMA was determined about 10800 g/mol.
In addition, the obtained PMMA was characterized via 1H NMR in Figure 3 to determine the structure of obtained PMMA as well as the absolute molecular weight. Based on the integration ratio of characteristic peaks of PMMA in 1H NMR spectrum, the molecular weight of PMMA was determined about 10800 g/mol.
In addition, the relationship between activated/deactivated of dormant species during polymerization has been performed via light on-off condition. The feed ratio of [MMA]/[I]/[PDA] was established as 100/1/0.1, MMA was polymerized according to the illuminated time. At each interval, an aliquot of mixture was removed and precipitated in cold methanol to determine the monomer conversion, the precipitated PMMA was collected and characterized by GPC method to determine the average molecular weight. As seen in Figure 4(A), the GPC traces of the resulted PMMA were increased with irradiation time, and after 24 h the average molecular weight of PMMA was 11700 g/mol which was determined via GPC.
The results showed that the polymerizations catalyzed by PDPA depends on the irradiation time. As can be seen in Figure 4(C), the linear plot of ln([M]0/[M]) versus irradiation time indicated that the growing radical concentration remained constant throughout irradiation process following the first-order kinetics during the polymerization. The polymer obtained at each time interval was precipitated in cold methanol and characterized by GPC to determine the average molecular weight.
To expand this polymerization system to other methacrylate monomers, the polymerizations of DMAEMA and HEMA were investigated (Table 3). All reactions using a ratio of [Monomer]/[I]/[PDPA] = 100/1/0.1 were performed in THF under UV light irradiation. As a result, the polymerization of DMAEMA monomer gave the high yield of 80.24% and low polydispersity index of 1.36. In the case of HEMA monomer, the yield of polymerization was determined 51% with polydispersity index of 1.48. It can be recognized that the polymerization performance is not only depend on the solvent but also the natural monomers.

|
Figure 1 1H NMR of PDPA organic photocatalyst. |

|
Figure 2 UV-vis spectra of PDPA organic photocatalyst. |

|
Figure 3 1H NMR of PMMA polymerized via O-ATRP. |

|
Figure 4 (A) GPC trace of obtained PMMA vs. time reaction. (B) Plot of MMA monomer conversion vs. time demonstrating the control over polymerization propagation through the “on/off” light irradiation experiment. (C) Semilogarithmic kinetic plots of polymerization. |
|
Table 1 Macromolecular Characteristic Features of PMMA Synthesized by O-ATRP Using PDPA Catalyst |

aReaction conditions: VMonomer:VSolvent = 1:2. Freeze-pump-thaw cycle three times to deoxygenize. Irradiation under 365 nm of UV light for 24 h with stirring at room temperature. bYield determined gravimetrically: Yield = (m–mI–mPDPA)/mM where m denotes the weight of product, and mI, mPDPA and mM the weights of the macroinitiator, PDPA catalyst and monomer, respectively. cNumber-average molecular weight (Mn,GPC) and polydispersity index (PDI) were determined by GPC. |
|
Table 2 Macromolecular Characteristic Features of PMMA Synthesized by O-ATRP Using PDPA Catalyst in Different Solvents |

aReaction conditions: VMonomer:VSolvent = 1:2. Freeze-pump-thaw cycle three times to deoxygenize. Irradiation under 365 nm UVA for 24 h with vigorous stirring at room temperature. bYield determined gravimetrically: Yield = (m–mI–mPDPA)/mM where m denotes the weight of product, and mI, mPDPA and mM the weights of the macroinitiator, PDPA catalyst and monomer, respectively. cNumber-average molecular weight (Mn,GPC) and polydispersity index (PDI) were determined by GPC. |
|
Table 3 Macromolecular Characteristic Features of PDMAEMA and PHEMA Synthesized by O-ATRP Using PDPA Catalyst |

aReaction conditions: VMonomer:VSolvent = 1:2. Freeze-pump-thaw cycle three times to deoxygenize. Irradiation under 365 nm UVA for 24 h with vigorous stirring at room temperature. bYield determined gravimetrically: Yield = (m–mI–mPDPA)/mM where m denotes the weight of product, and mI, mPDPA and mM the weights of the macroinitiator, PDPA catalyst and monomer, respectively. cNumber-average molecular weight (Mn,GPC) and polydispersity index (PDI) were determined by GPC. |
In summary, a novel organic catalyst PDPA was successfully prepared and has been proved to be an efficient metal-free catalyst for ATRP which produced polymethacrylates with controlled molecular weight of 11000 g/mol as well as narrow polydispersity of 1.46 by UV irradiation. Moreover, the methacrylate monomers such as DMAEMA and HEMA can be polymerized using organic photocatalyst PDPA that eliminate the trace metal element in final polymeric compound. This research enables the synthesis pathway for potential bio/electronic polymeric materials.
- 1. T. Fukuda, T. Terauchi , A. Goto, K. Ohno, K. Y. Tsujii, T. Miyamoto, S. Kobatake, and B. Yamada, Macromolecules, 29, 6393 (1996).
-

- 2. K. Matyjaszewsk and T. P. Davis, Editors, Handbook of radical polymerization, John Wiley & Sons, Hobo Ken, 2002.
-

- 3. J. Nicolas, C. Dire, L. Mueller, J. Belleney, and B. Charleue, Macromolecules, 39, 8274 (2006).
-

- 4. K. Matyjaszewski, Chem. Eur. J., 5, 3095 (1999).
-

- 5. K. Matyjaszewski, Macromolecules, 45, 4015 (2012).
-

- 6. K. Matyjaszewski and J. Xia, Chem. Rev., 101, 2921 (2001).
-

- 7. J. S. Wang and K. Matyjaszewski, J. Am. Chem. Soc., 117, 5614 (1995).
-

- 8. C. Cheng, E. Khoshdel, and K. L. Wooley, NanoLett., 6, 1741 (2006).
-

- 9. A. P. Vogt and B. S. Sumerlin, Soft Matter, 5, 2347 (2009).
-

- 10. K. Matyjaszewski and N. V. J. Tsarevsky, J. Am. Chem. Soc., 136, 6513 (2014).
-

- 11. K. Matyjaszewski, T. Pintauer, and S. Gaynor, Macromolecules, 33, 1476 (2000).
-

- 12. S. Munirasu, R. Aggarwal, and D. Baskaran, Chem. Commun., 4518 (2009).
-

- 13. Y. Shen and S. Zhu, Macromolecules, 34, 8603 (2001).
-

- 14. B. P. Fors and C. J. Hawker, Angew. Chem. Int. Ed., 51, 8850 (2012).
-

- 15. D. B. Francesco, F. M. Fantin, A. I. Abdirisak, and G. Armando, Polym. Chem., 9, 646 (2018).
-

- 16. F. Marco, P. Sangwoo, W. Yi, and K. Matyjaszewski, Macromolecules, 49, 8838 (2016).
-

- 17. J. C. Theriot, B. G. McCarthy, C. H. Lim, and G. M. Miyake, Macromol. Rapid Commun., 38, 1700040 (2017).
-

- 18. G. Yilmaz and Y. Yagci, Polym. Chem., 9, 1757 (2018).
-

- 19. G. M. Miyake and J. C. Theriot, Macromolecules, 47, 8255 (2014).
-

- 20. R. M. Pearson, C. H. Lim, B. G. McCarthy, C. B. Musgrave, and G. M. Miyake, J. Am. Chem. Soc., 138, 11399 (2016).
-

- 21. C. Kutahya, A. Allushi, R. Isci, J. Kreutzer, T. Ozturk, G. Yilmaz, and Y. Yagci, Macromolecules, 50, 6903 (2017).
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2019; 43(4): 496-502
Published online Jul 25, 2019
- 10.7317/pk.2019.43.4.496
- Received on Oct 1, 2018
- Revised on May 14, 2019
- Accepted on Jun 7, 2019
 Services
Services
- Full Text PDF
- Abstract
- ToC
- Acknowledgements
Introduction
Experimental
Result and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Ha Tran Nguyen
-
*National Key Laboratory of Polymer and Composite Materials, University of Technology, Vietnam National University – Ho Chi Minh City (VNU–HCM), 268 Ly Thuong Kiet, District 10, Ho Chi Minh City, Vietnam
**Faculty of Materials Technology, Ho Chi Minh City University of Technology, Vietnam National University, 268 Ly Thuong Kiet, District 10, Ho Chi Minh City, Vietnam - E-mail: nguyentranha@hcmut.edu.vn
- ORCID:
0000-0001-5201-201X









 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.