- Single-Step Solution Polymerization and Thermal Properties of Copolyesters Based on High Trans-1,4-Cyclohexanedimethanol, Terephthaloyl Dichloride, and 2,6-Naphthalene Dicarboxylic Chloride
Fiaz Hussain, Jaemin Jeong, Sangwon Park, Soo-Jung Kang, and Jinhwan Kim†

Department of Polymer Science and Engineering, Sungkyunkwan University, 300 Cheoncheon-dong, Jangan-gu, Suwon, Gyeonggi 16419, Korea
- Trans와 Cis-1,4-Cyclohexanedimethanol, Terephthaloyl Dichloride, 2,6-Naphthalenedicarboxylic Chloride로부터 합성된 폴리에스터 공중합체의 단일 단계 용액 중합과 열적 특성
성균관대학교 고분자공학과
In this work, two series of poly(1,4-cyclohexylenedimethylene terephthalate-co-1,4-cyclohexylenedimethylene 2,6-naphthalenedicarboxylate) (PC#TN#) copolyesters were synthesized by a simple one-step solution polymerization method at room temperature without the use of stabilizer and metallic catalyst. Chemical compositions of obtained PC#TN# copolyesters were confirmed by nuclear magnetic resonance (1H NMR) spectroscopy. Thermal properties of synthesized copolyesters were determined by differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA). Thermal properties of synthesized PC#TN# were controlled by varying the ratio of naphthalene units in diacid part and cis/trans-1,4-cyclohexanedimethanol (CHDM) isomers in diol part. An increased content of trans-CHDM can increase glass transition temperature (Tg) and melting temperature (Tm) profoundly. By increasing the content of naphthalene units, Tg increases linearly while Tm is first decreased then it starts to increase once the eutectic point (2,6-naphthalene dicarboxylic acid 36 mole%) is reached. Thermal degradation behavior of synthesized copolyesters was also significantly improved by increasing naphthalene and trans-CHDM units.
Poly(1,4-cyclohexylenedimethylene terephthalate-co-1,4-cyclohexylenedimethylene 2,6-naphthalenedicarboxylate) (PC#TN#) 폴리에스테르 공중합체를 안정제 및 금속 촉매를 넣지 않고 실온에서 단일 단계 용액 중합법으로 합성하였다. 얻어진 PC#TN# 폴리에스테르 공중합체의 화학 구조는 1H NMR로 규명하였고, 열특성은 TGA와 DSC로 확인하였다. 열적 특성은 diacid 부분의 나프탈렌 단위와 diol 부분의 cis/trans-1,4-cyclohexanedimethanol(CHDM) 이성질체의 비율을 변화시킴으로써 조절하였다. trans-CHDM의 증가된 함량은 유리 전이 온도(Tg) 및 용융 온도(Tm)를 증가시킬 수 있었다. 나프탈렌 단위의 함량을 증가시킴으로써 Tg는 선형적으로 증가하는 반면, Tm은 감소하다가 공융점(2,6-naphthalene dicarboxylic acid 36 mole%)에 도달하면 증가하였다. 합성된 폴리에스테르 공중합체의 열적 분해 거동은 나프탈렌 및 trans-CHDM 단위를 증가시킴으로써 상당히 개선되었다.
Keywords: 1,4-cyclohexanedimethanol, terephthalic acid, naphthalene units, solution polymerization, thermal properties
The authors would like to express
appreciations to BASF for the financial support.
Thermoplastic polyesters have gained the attention of academia and industrial researchers because of their wide range of technical applications.1-6 Synthesis of high molecular weight aliphatic polyesters was first reported by Carother and Hill.7 But, inherent poor hydrolytic stability, low glass transition temperature (Tg), and melting temperature (Tm) of aliphatic polyesters strictly abandoned their commercial applications. Whinfield and Dickson reported the synthesis of poly(ethylene terephthalate) (PET) exhibiting acceptable thermal properties in 1949.8 However, high crystallinity and low Tg of PET (80 oC) limits its commercial applications at elevated temperatures.
Performance properties of PET can be improved by controlling the chemical structure of its backbone chain. Kibler et al. disclosed the synthesis process and thermal properties of poly(cyclohexane 1,4-dimethylene terephthalate) (PCT) in 1964.9 When ethylene glycol of PET is replaced by 1,4-cyclohexanedimethanol (CHDM), the resulting PCT polyester have much higher Tg (88 vs 80 oC) and Tm (300 vs 260 oC).9 For general plastic applications, processability of PCT polymer must be improved by modifying it with diacid or diol components. CHDM-modified PET copolyester and glycol-modified PCT copolyesters are amorphous in nature and they have better heat distortion temperature, higher impact strength, and superior chemical resistance than PET. These copolyesters have variety of commercial applications as injection molded polymers for medical and electronics applications.4,10 In short, copolymerization depending upon different kinds of monomers and their content ratio directly influence thermal and crystallization behavior of synthesized copolyesters.10,11 However, both high crystallinity and high melting temperature of PCT homopolymer act as obstacles for its commercial applications as a film.
Performance properties can also be improved by incorporating the thermally stable and rigid naphthalene units as diacid moiety into the PET backbone itself. When terephthalic acid (TPA) is replaced by the naphthalene dicarboxylic acid (NDA), the resulting polyester, poly(ethylene naphthalate) (PEN) have superior Tg (about 120 vs 80 oC) and TmTm (270 vs 260 oC) than conventional PET. Soon after the discovery of PEN in 1969,12 this polymer rapidly finds its applications as a performance materials in versatile areas.13 PEN and its copolymers are considered to be strong candidates for high temperature applications.4,13,14 However, high cost of monomer (NDA) used for the synthesis of PEN, birefringence, and necking behavior of PEN film during stretching process limit the wide range applications of this polymer. Crystallinity and thermal properties of PCT can also be controlled by introducing second diacid component into the molecular structure, these copolyesters are called acid-modified copolyesters (PCTA). Isophthalic acid (IPA) and cyclohexanedicarboxylic acid (CHDA) are the most common diacid modifiers. Introduction of small amount of second diacid widens the processing window of PCT polyester by lowering its Tm. However, these copolyesters are not suitable for the applications where good thermal properties are required and the higher amount of second diacid results in high cost and processing issues.10,15,16
Not only diol and diacid moieties but their stereochemistry can also tune the thermal and mechanical properties of the copolyesters. Stereochemistry of CHDM (cis/trans isomers) can affect the comprehensive performance properties of CHDM based polymers. It is well established in the literatures that polymers based on trans-CHDM have superior barrier, thermal, mechanical, and chemical resistance when compared with their analogous polymers based on cis-CHDM.17-21 For the first time in 1964, Kibler et al. discovered that PCT based on trans-CHDM have superior thermal properties than analogous polyester containing cis-CHDM isomers (310 vs 251 oC).9 Berti et al. studied the effect of cis- and trans- isomers of diacid moieties and disclosed that performance properties, especially the thermal and barrier properties of copolyesters can be effectively improved by using monomers that contain high trans- isomers.22 Young and Won tuned the thermal properties and crystal structure of poly(cyclohexane 1,4-dimethylene 2,6-naphthalate) (PCN) polymer by controlling the cis- and trans- configuration of CHDM.23 It is suggested that molecular arrangements of copolyesters containing trans-CHDM isomers are more regular, stable, and symmetric than copolyesters based on high cis-CHDM isomers.18,21,23-25
Currently, almost all high temperature polyesters are synthesized by a complex two-step melt polymerization. First step involves the formation of pre-polymer by esterification reaction and the polymer of high molecular weight is obtained in the second step called polycondensation reaction. For the synthesis, prepolymer reacts with diol at relatively high temperature and pressure. By product removal set up is also attached at both steps of polymerization.11,15,26 Due to harsh condition applied during melt polymerization a stoichiometric imbalance is observed due to degradation and sublimation of monomers and this phenomenon leads to the increment in side reactions and the reaction efficacy is reduced.27,28 Some of the problems associated with melt polymerization are addressed by using suitable metallic catalysts.29,30 However, titanium based catalysts, which are considered to be most effective catalysts among all metallic catalysts, induce yellow color into the synthesized product while antimony based catalysts are associated with some toxicity issues.31,32 Generally, an additional thermal stabilizer is also used with metallic catalyst to prevent polymer degradation during the polycondensation and subsequent process which results in increased cost.33 It is worthy to note that neither polymer degradation nor discoloration of the product is observed during solution polymerization reaction.34,35 The major concern about the solution polymerization is the selection of pure solvent which facilitates the solubility of monomers and easy recovery of synthesized polymer product. It is well established that pure and well defined polymers are synthesized by a reproducible one-step solution polycondensation process.
It would be a great effort to introduce a synthesis of novel thermally stable copolyesters by using the advantageous features of trans-isomers of diol (CHDM) and second dicarboxylic acid (NDA), simultaneously. To the best of our knowledge, only one industrial patent36 is reported in which thermal and mechanical properties of copolyester film are controlled by utilizing the effect of stereochemistry of CHDM and second dicarboxylic acid (NDA). However, very limited information was provided in this patent. For the first time, we are reporting a detailed academia research showing the effect of both NDA (second diacid) and stereochemistry of diol (cis-/trans-CHDM) on the thermal properties of synthesized two series of copolymers, PC70TN# and PC100TN# containing 70 and 100% trans-CHDM isomers, respectively. They were synthesized by a short, simple, controlled, and straightforward one-step solution polymerization method. It is important to note that any kind of metallic catalyst or additional stabilizer is not used during the whole synthesis, so eliminating any kind of toxicity related issues. This study mainly focusses three parts: (1) introduction of a novel solution polymerization approach for the synthesis of two series of copolymers, at room temperature in the absence of toxic metallic catalysts and stabilizer; (2) characterization of synthesized copolyesters and (3) study the effect of stereochemistry of CHDM and naphthalene units on the thermal properties of synthesized copolyesters. So, in this study, the crystal structure and thermal properties of PCT homopolymers were controlled by the ratio of cis-/trans-CHDM isomers and the second dicarboxylic acid.
Materials. Reagent grade terephthaloyl dichloride (TPC, C8H4Cl2O2), trans-1,4-cyclohexanedimethanol (100 mole% trans- isomer) (C8H16O2), and 4-dimethylaminopyridine (DMAP, C7H10N2, 99% purity) were obtained from TCI (Japan). Extra pure (99.5%) tetrahydrofuran (THF, C4H8O), sodium hydroxide (NaOH), benzene (C6H6), heptane (C7H14), methyl alcohol (CH3OH), and thionyl chloride (SOCl2) were purchased from Samchun Chemical Co. Ltd (Korea). Phenolphthalein was purchased from Sigma Aldrich (China). 1,4-cyclohexanedimethanol (1,4-CHDM, 99.8 mole% purity) with 70 mole% trans-isomers was supplied by SK Chemicals (Korea). 2,6-naphthalenedicarboxylic acid (NDA, C12H8O4) was supplied by BASF (Germany). Dimethylformamide (DMF, C3H7NO) was purchased from Duksan Chemicals (Korea). 2-Chlorophenol (OCP) was purchased from Junsei Chemical Co. Ltd (Japan). All chemical reagents were stored in the desiccator before use. Extra pure deionized water (DI H2O) was used during the whole synthesis process.
Preparation of 2,6-Naphthalenedicarboxylic Chloride (NDC). In order to improve the reactivity of NDA during the solution polymerization of two series of copolymers, it was converted into NDC (C12H6Cl2O2) by following the procedure reported in the literature.37 Schematic diagram for the modification of NDA into NDC is shown in Scheme 1.
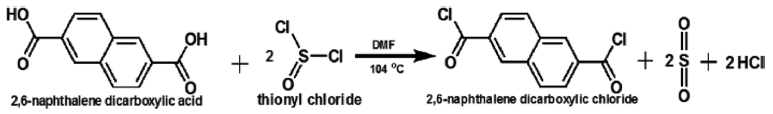
Scheme 1. Modification of NDA into NDC by thionyl chloride.
Thionyl chloride (4 mole) and 1.7175 g DMF (0.07618 mole) which was used as a catalyst were added into the solution of NDA (1 mole) in 375 mL benzene. The mixture of all reactants was heated slowly under gentle reflex up to 104 oC and these conditions were maintained for 24 h. Solution became clear upon the completion of reaction. It was cooled down to room temperature and the product in the form of yellow crystals was appeared. Obtained crude NDC crystals were washed with a solution of benzene/heptane (60/40) under sonication for 10 min. Pure NDC crystals were collected from the solution by vacuum filtration and dried by storing in vacuum oven at 40 oC for 24 h.
Solution Polymerization of Copolyesters. Homogeneous mixture of DMAP (0.04 mole) and CHDM (0.012 mole) in 150 mL of THF was poured into a 250 mL three-necked round-bottom flask, equipped with a magnetic stirrer, a dropping funnel, and a reflux condenser. Transparent solution of equimolar amounts (0.01 mole) of TPC and NDC mixture with the designated ratio was dissolved in 50 mL of THF solvent and added dropwise into the flask through the dropping funnel.
Homogeneous mixtures of all chemicals were mixed by sonication process for 10 min. Diol (CHDM), diacid chloride (mixture of NDC and TPC), and catalyst (DMAP) were used with a molar ratio of 1.2:1.0:4.0. DMAP played a dual role as a catalyst and as an absorbent of by product (HCl). Diacid chloride solution was added dropwise into the flask. A milky white polymer was appeared gradually, indicating that polymer was successfully synthesized. Solution polymerization of polymer took place in 90 min in which the 30 min dropping time of diacid chloride components was included. All the chemical reactions were carried out at room temperature. After completion of reaction, synthesized polymer was precipitated in 600 mL methanol with constant stirring for 30 min. Polymer was purified by washing with methanol and water and it was placed in the vacuum oven for overnight at 50 oC. The schematic diagram of polymer synthesis by solution polymerization reaction is shown in Scheme 2.
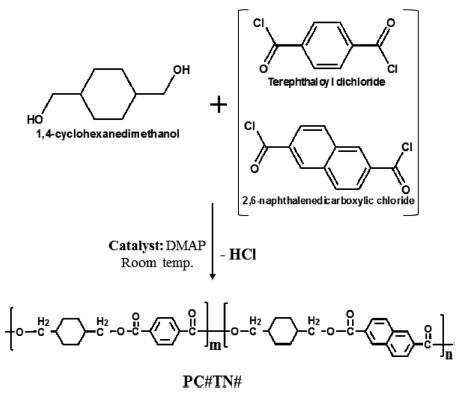
Scheme 2. Schematic diagram for the solution polymerization of PC#TN# series.
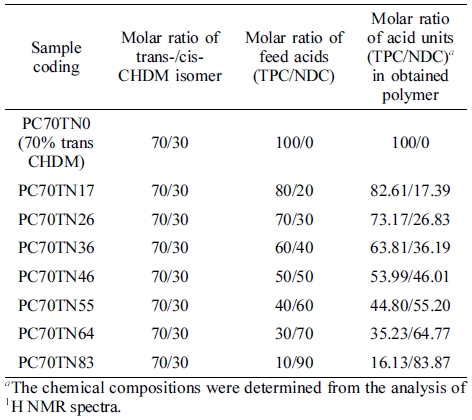
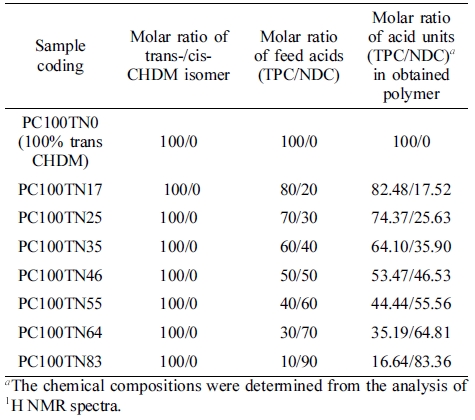
Two series of copolyesters based on CHDM and TPC/NDC mixture, PC70TN# and PC100TN# (C = CHDM, numbers after C = mole% of trans content of trans/cis isomer in CHDM, T = TPC, N = NDC, and number after N = mole% of NDC content in diacid unit (TPC/NDC)) copolyesters, were synthesized by the procedures described above. Both series of copolyesters were prepared with different composition of NDC and TPC as summarized in Tables 1 and 2, respectively.
Characterization. The actual chemical compositions of the synthesized copolymers were determined by proton nuclear magnetic resonance (1H NMR) spectroscopy. 1H NMR experiments were performed by a Unity Inova 500NB High Resolution 500 MHz NMR Console at 25 oC. Polymer samples were dissolved in a mixture of deuterated chloroform (CDCl3) and trifluoroacetic acid (TFA-d) (1:1) and tetramethylsilane (TMS) was used as an internal standard.
Intrinsic viscosity (IV) of all synthesized copolyesters was measured in OCP at 25 oC by an automated ubheload-1C viscometer. Molecular weights (Mv) of synthesized copolyesters were determined using Mark-Houwink equation by following American polymer standards.38
Thermal properties like glass transition temperature (Tg), melting temperature (Tm), and the melting enthalpy (ΔHm in J/g) of synthesized copolymers were determined by a differential scanning calorimeter (DSC Q20, TA instruments). About 4-6 mg of the sample was loaded in the DSC sample pan and heated from 40 to 300 oC at a heating rate of 10 oC min-1 under nitrogen with a purge flow of 50 mL min-1. Samples were first heated and cooled down in order to eliminate the thermal history of polymer then values were determined from the second heating cycle.
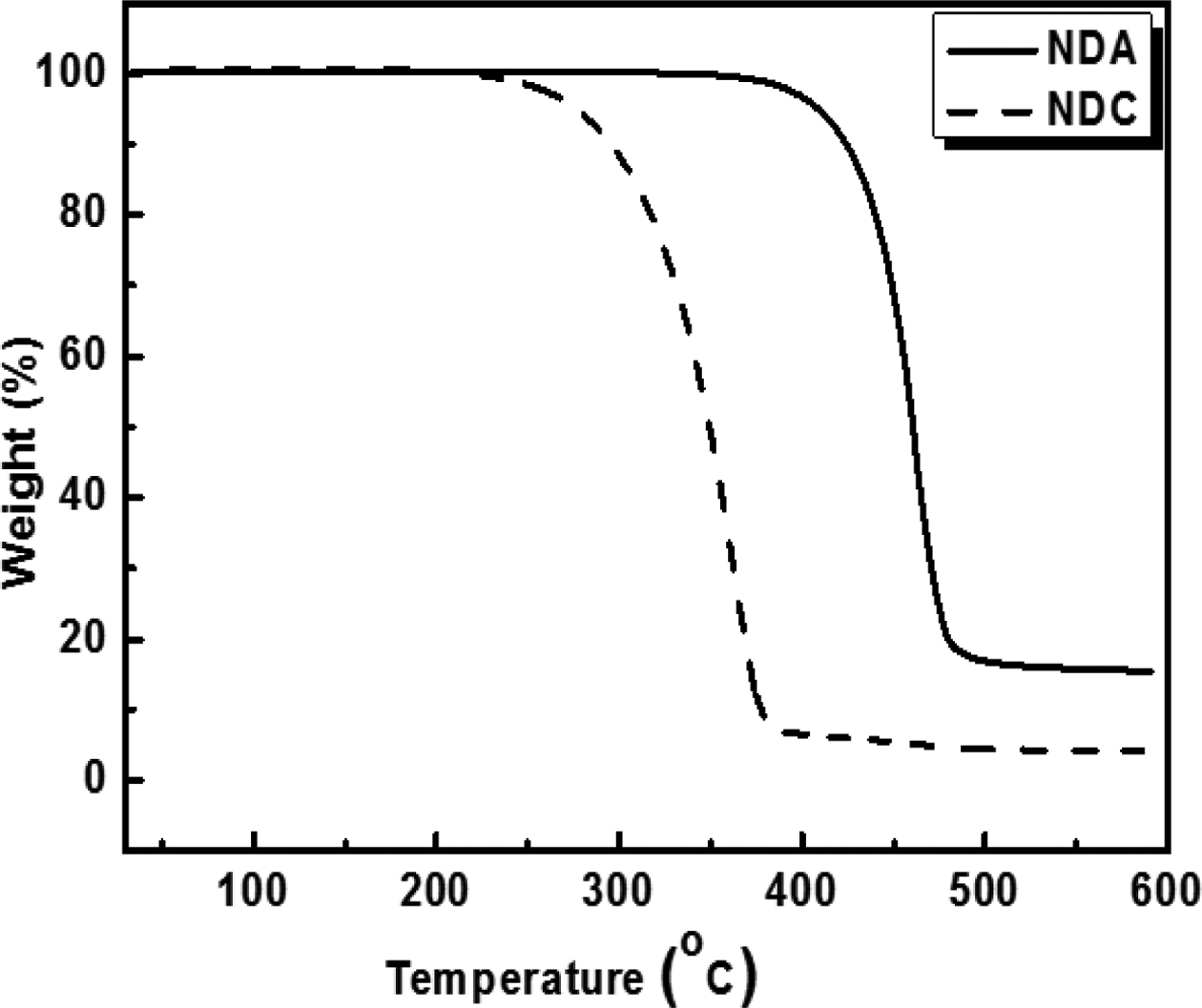
The thermal degradation behaviors of the synthesized NDC and copolymers were analyzed by a thermo-gravimetric analyzer (TGA Q50, TA instruments). For this, 8-10 mg of the sample was used for the measurement and heated from 40 to 600 oC under nitrogen atmosphere at a heating rate of 10 oC min-1 with a nitrogen purge flow of 50 mL min-1. The temperatures corresponding to 5% and 50% weight loss and the amount of residue (%) at 600 oC were determined from TGA analysis.
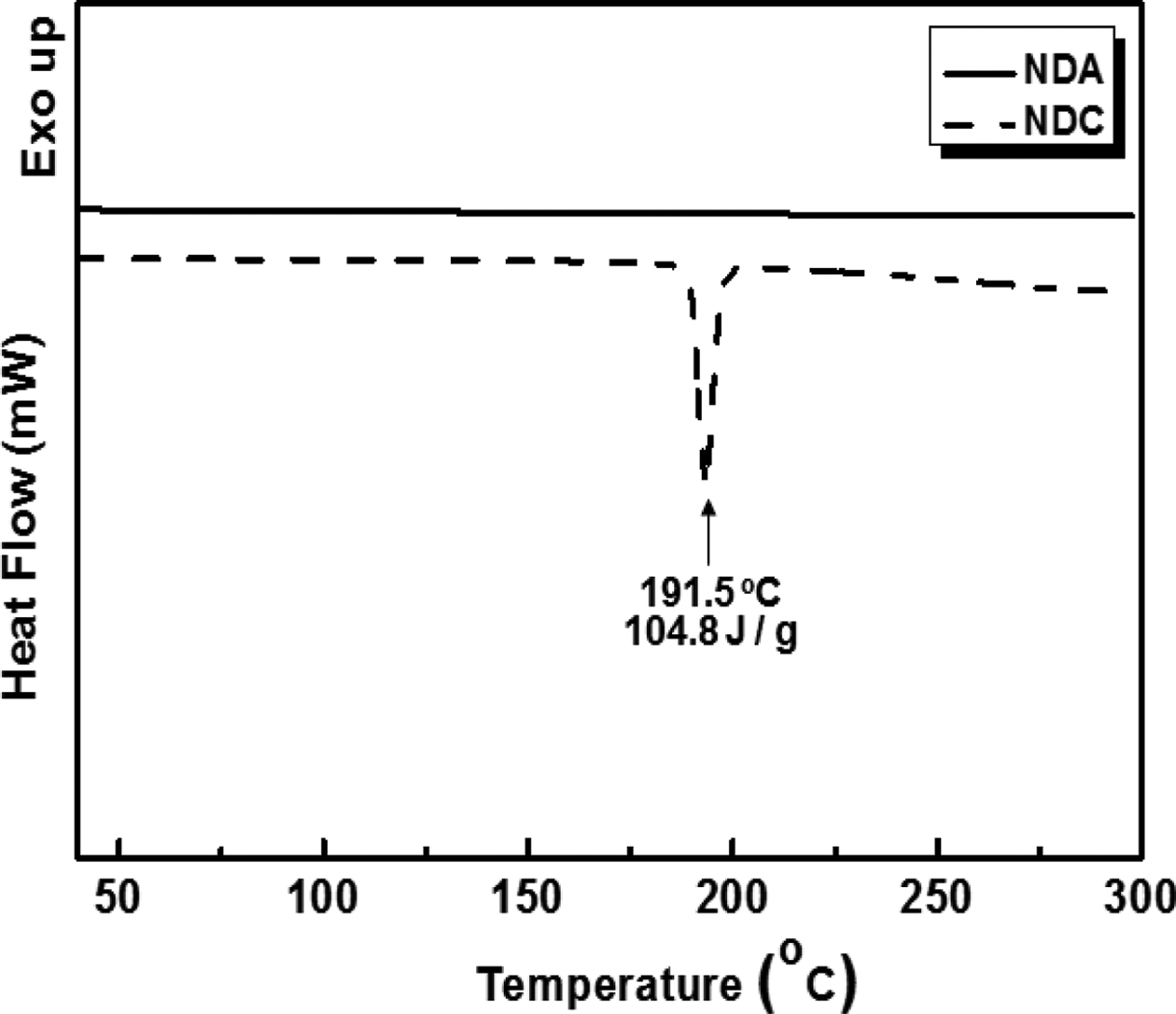
Confirmation of Synthesis of NDC from NDA. In an effort to improve the reactivity of NDA during solution polymerization, modification of NDA into NDC was attempted and the success of the synthesis was confirmed by DSC and TGA whose results are shown in Figures 1 and 2, respectively.
Melting behavior of resulting NDC was analyzed and compared with starting NDA. Clear melting point at 191.5 oC was observed for NDC while no melting was detected up to 300 oC for NDA. Our results are compatible with the previous reported other researchers data found in the literatures.37,39 It was found that NDA have superior thermal stability than NDC (Figure 2). From these results, it is concluded that NDA was successfully modified into NDC with a highly efficient reaction (97.7%).
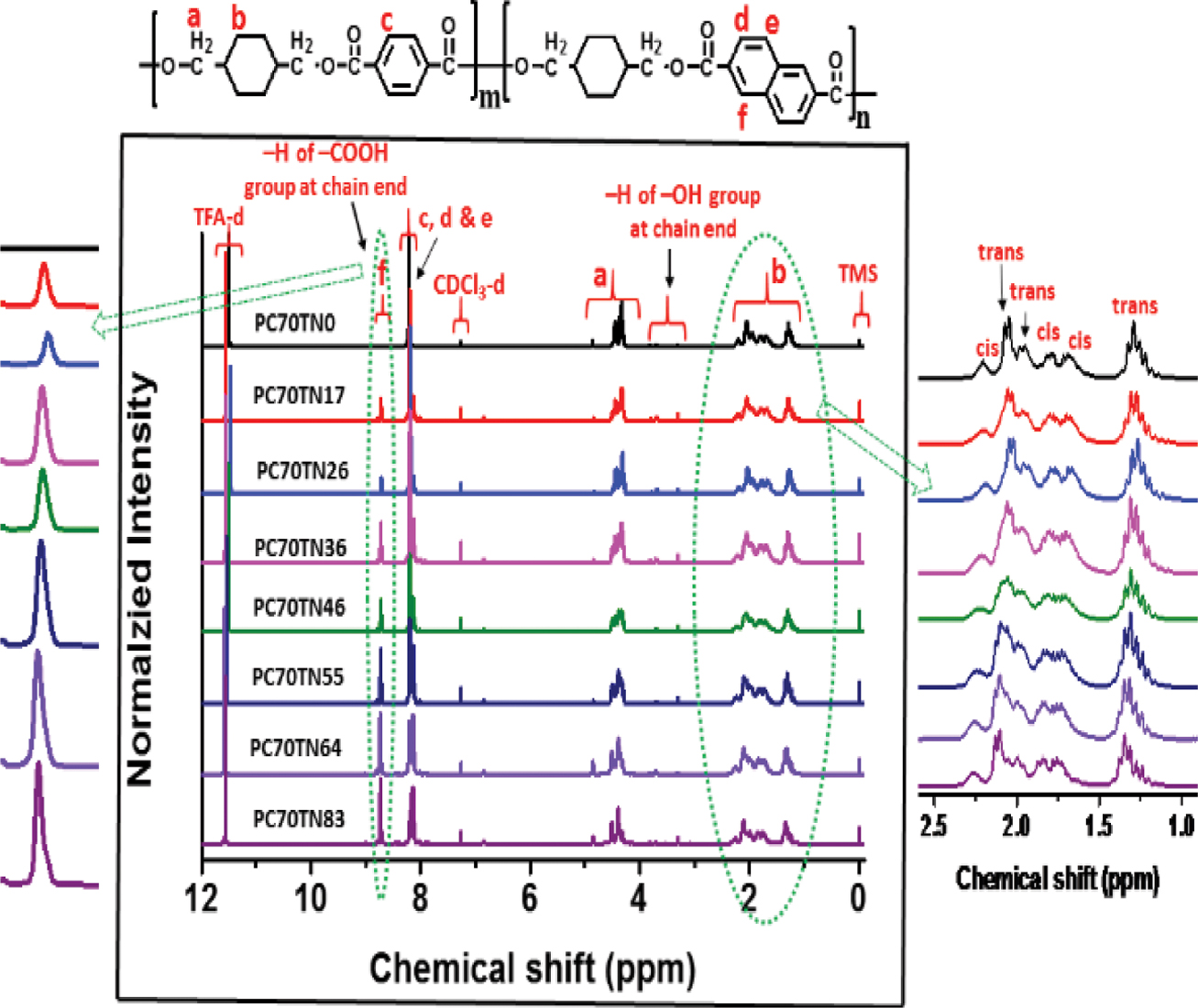
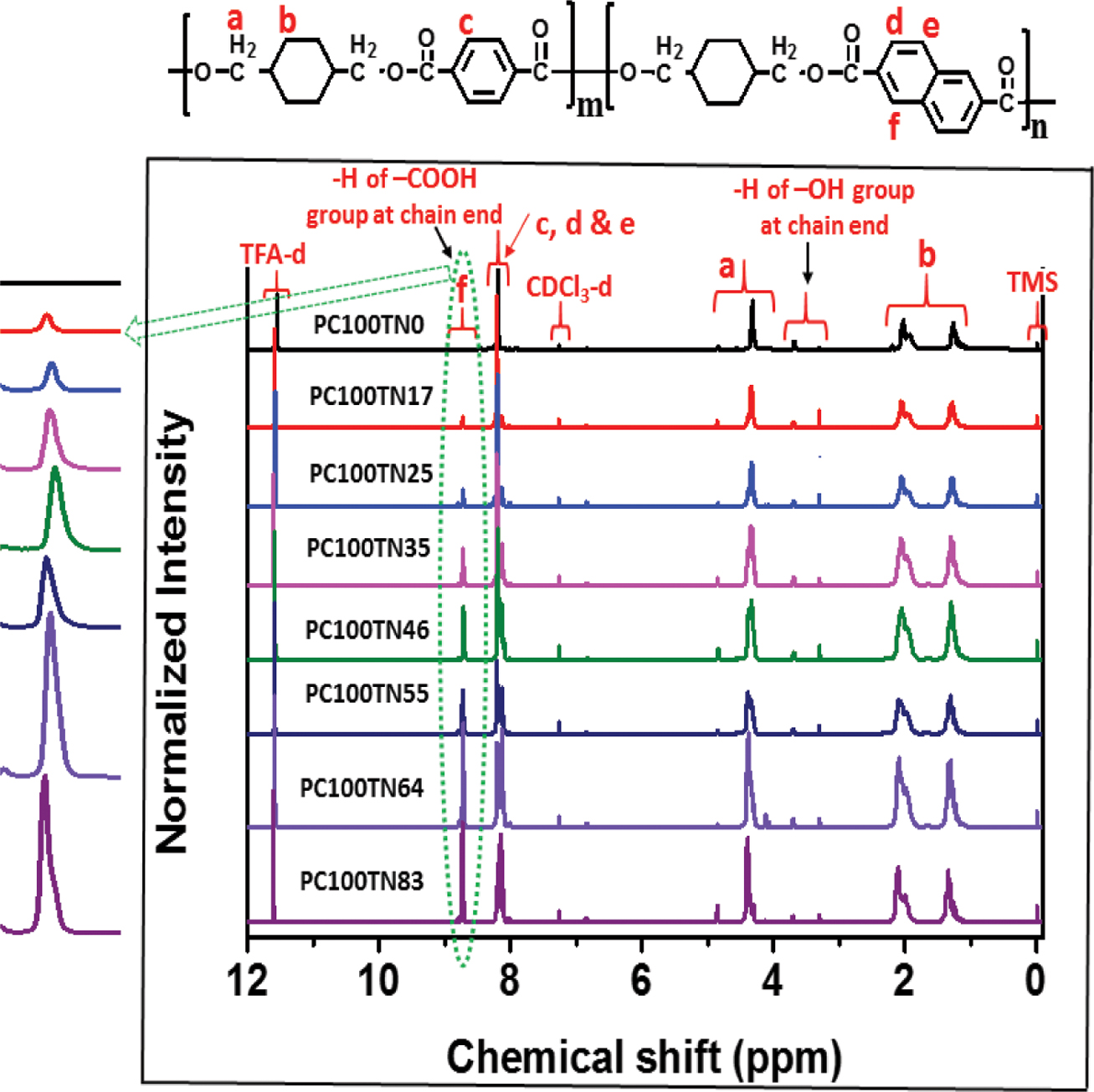
Determination of Chemical Compositions of Synthesized Copolymers. Chemical compositions of synthesized PC70TN# and PC100TN# copolyesters were analyzed by 1H NMR and the results are shown in Figures 3 and 4, respectively. Since obtained copolyesters contain rigid aromatic structures, they were not completely soluble in CHCl3 so a mixture of TFA-d and CDCl3-d (1:1) was used to dissolve the polymer samples. The characteristic chemical shifts (δ) (ppm) peaks at 1.21-2.30, 4.28-4.49, and 4.82 ppm are assigned to hydrogen atoms of 1,4-CHDM diol component of copolymers. The characteristic d peaks at 3.37-3.81 are assigned to hydrogen atoms of -CH2OH at chain end of polymer chain. The characteristic δ peaks at 8.10-8.18 ppm are assigned to hydrogen atoms of di-acid components of copolymers coming from TPC (c) and NDC (d and e), however, the δ peak at 8.71 ppm represented by (f) in the figures is assigned to hydrogen atom of NDA at chain end, which enables us to calculate actual chemical compositions of all copolymers. It is evident from the figures that intensity of d peak at 8.71 ppm which represents the naphthalene content in the synthesized copolyesters is increased linearly with increasing the content of NDC in the feeding diacid mixture. In the case of PC70TN0 and PC100TN0, no d peak was observed at 8.71 ppm, which confirms that these copolyesters were not containing NDC. In addition, in the case of PC70TN# series, characteristic δ peaks of cis-CHDM observed at 1.5-1.7, 2.1, and 4.7 ppm were clearly detected while, in the case of PC100TN# series, no peaks were observed. The results of chemical composition analysis for PC70TN# and PC100TN# copolymers series are summarized in Tables 1 and 2, respectively. 1H NMR analysis of synthesized copolymers clearly indicates that higher amount of TPA and lower amount of NDA were present in the synthesized PC70TN# and PC100TN# copolymers when compared with feeding amount of monomers. This result suggests that the reactivity of NDC is slightly lower than TPC. Our results are compatible with the results of other researchers reported in the literatures.11,15,23,40
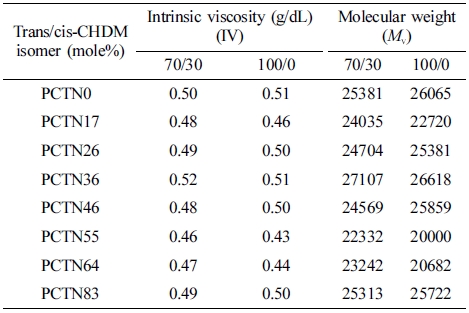
Molecular Weights (Mv) and Intrinsic Viscosity (IV). Intrinsic viscosity (IV) and molecular weights (Mv) of obtained copolyesters recorded using Mark-Houwink equation are summarized in Table 3. It important to note that PCTN copolyesters have high enough molecular weights which make them suitable to be used in the form of films, nanofibers or any other desired shape for the practical applications in versatile areas.
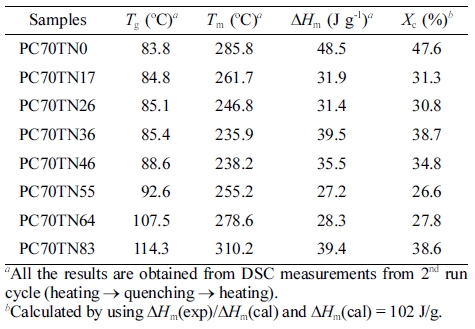
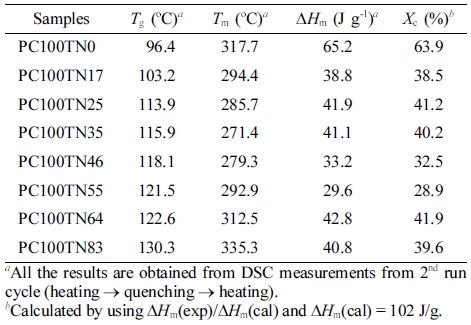
Thermal Properties Examined by DSC. Solid state polycondensation (SSP) which is normally performed between the Tg and Tm has numerous potential advantages to mechanical properties of finally obtained product and it effectively improves the performance properties of polymer. In industry, molecular weight of synthesized polymers is improved by SSP which effectively enhance the mechanical, thermal, and barrier properties of polymer, suitable for wide range of commercial applications.41,42 In our study, all the results of synthesized PC#TN# copolyesters are analyzed after SSP. Thermal properties of synthesized PC70TN# and PC100TN# copolyesters were analyzed by DSC and the results for Tg, Tm, ΔHm, and degree of crystallinity are summarized in Tables 4 and 5, respectively.
The degree of crystallinity (Xc) of samples was calculated as follows:
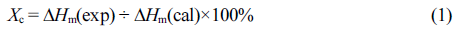
where ΔHm(exp) is the melting enthalpy determined by DSC experiment and ΔHm(cal) is the melting enthalpy of 100% crystalline PCT which is calculated by adopting a group contribution theory.43,44 Inherent thermal properties of synthesized copolyesters were determined after eliminating the thermal history by applying heating-cooling cycle. All the DSC results were determined during the second heating cycle.
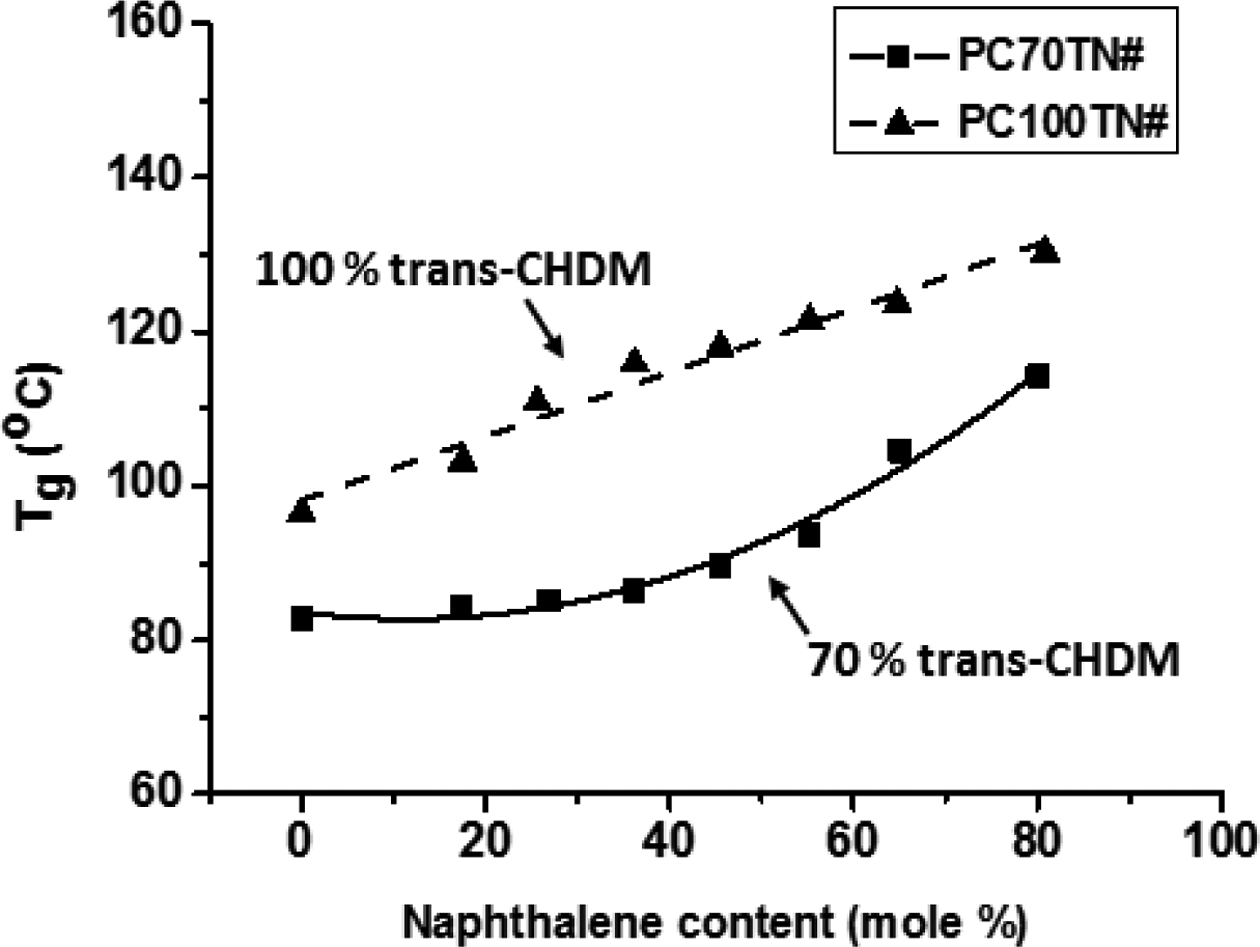
To demonstrate the effect of naphthalene content (mole%) on the Tg and Tm of PC70TN# and PC100TN# copolymers more clearly, the data shown in Tables 3, 4 are plotted and the results are shown in Figures 5 and 6, respectively. It is evident from the graph that Tg of both series, PC70TN# and PC100TN#, is increased linearly by increasing the naphthalene units. It is also clear that Tg of synthesized PC100TN# copolymers prepared with high trans-CHDM are higher than their analogous PC70TN# copolymers prepared with 70% trans-CHDM. This behavior can be resulted from the increased symmetry and rigidity of PC100TN# copolymers that contain 100% trans-CHDM isomer compared to PC70TN# series.
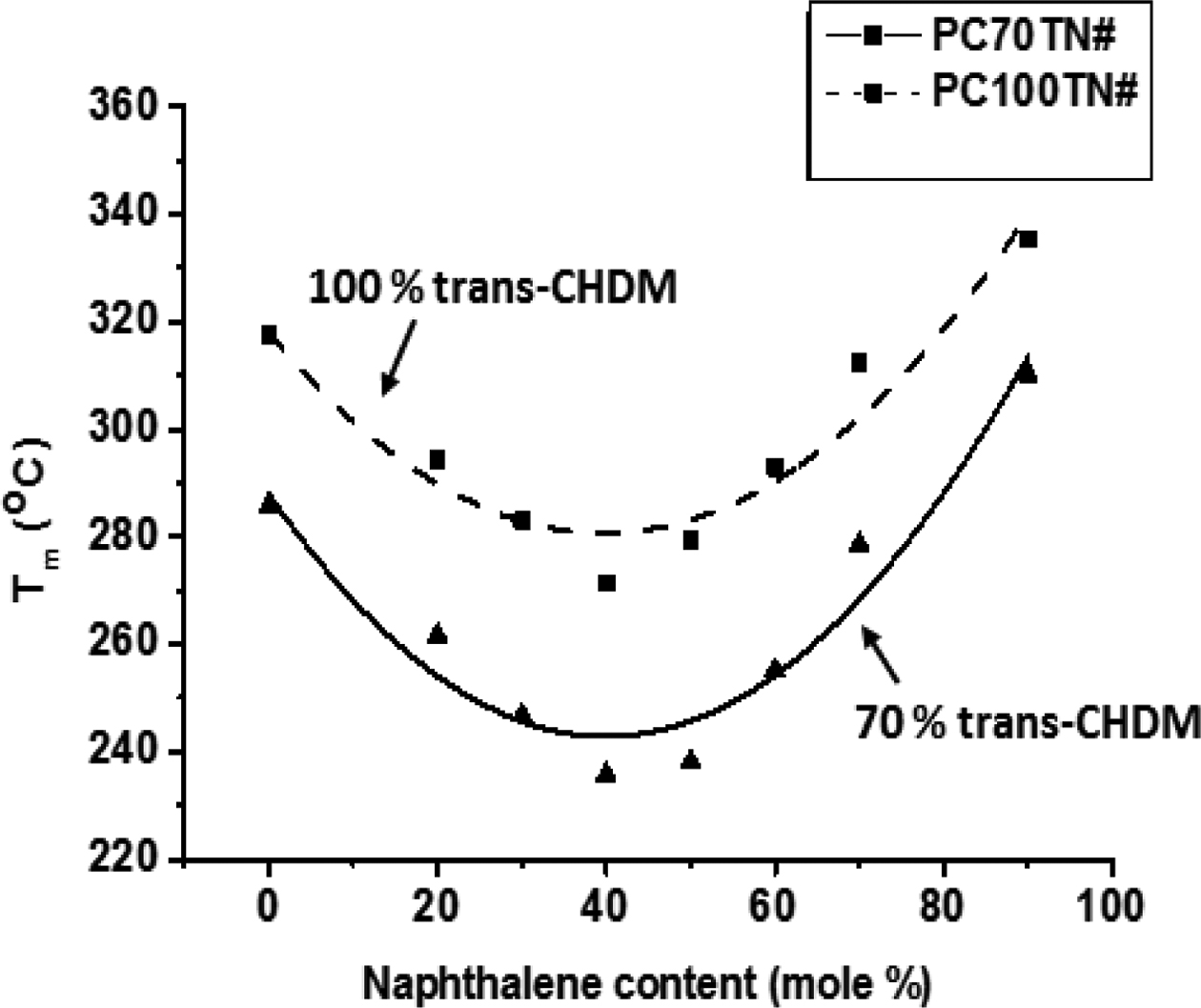
Melting temperatures of synthesized two series, PC70TN# and PC100TN# copolyesters were also determined by DSC and the results are given in Figure 6 and summarized in Tables 3 and 4, respectively. It is found that Tm of naphthalene modified PC70TN# and PC100TN# copolymers decreases until the content of naphthalene unit reaches 36 mole% (eutectic point) and then it starts to increase rapidly by increasing naphthalene content furthermore.
At eutectic point, poly(cyclohexane 1,4-dimethylene naphthalate) (PCT) and poly(cyclohexane 1,4-dimethylene terephthalate) (PCN) crystals coexist in the copolymers and after this point the main crystal structure is dominated by the PCN-type crystals that enhance the thermal and physical properties of copolymers. Similar trends have been reported previously for naphthalene units containing copolyesters which were synthesized by melt polymerization.15,45 Copolymers synthesized from aromatic diacids and cycloaliphatic trans-CHDM diol have symmetry and rigidity in their molecular structure. It is considered that copolymers based on trans-CHDM have superior thermal properties than their analogous copolymers having benzene based diols components, like 1,4-benzenedimethanol.46
It is evident from these data that naphthalene units effectively improve the heat stability of synthesized copolyesters. Not only naphthalene unit but stereochemistry of 1,4-CHDM (trans/cis content) also improve the comprehensive properties of copolyesters.17-21 Most important finding of this is that PC100TN# copolyesters have superior thermal properties when compared with PC70TN# copolyesters. This behavior can be attributed to trans-CHDM isomers which impart more symmetry and rigidity to their molecular structure.
It can be seen from the Figures 5 and 6 that PC70TN# copolyesters have Tg ranging between 83.7-114.3 oC and Tm ranging between 285.7-310.2 oC while PC100TN# copolyesters have relatively superior Tg and Tm ranging between 96.4-130.3 oC and 317-335 oC, respectively. Unexpectedly high heat resistance, superior Tg, and Tm of PC100TN# copolyesters containing 100% trans-CHDM content can be attributed to the thermally stable and symmetric structure of cycloaliphatic trans-CHDM isomers which facilitate the formation of stable crystal structure.18,21-25 Similar trends have already been reported for melt polymerized PCT and PCN homopolymers containing high trans-CHDM isomers.9,23 Therefore, it is concluded that not only the naphthalate content but also the trans-1,4-CHDM isomer impart rigidity and symmetry to copolyester backbone.
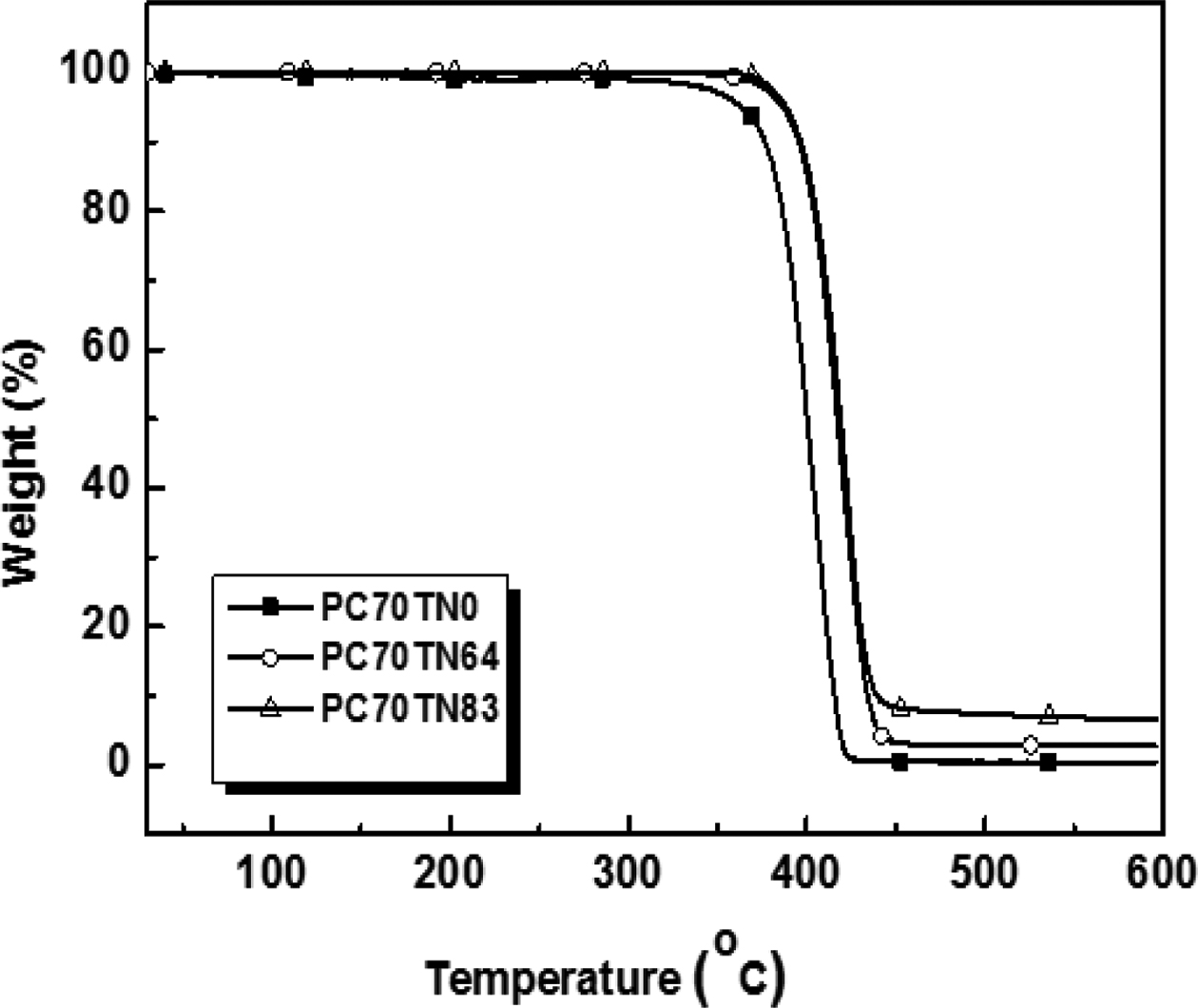
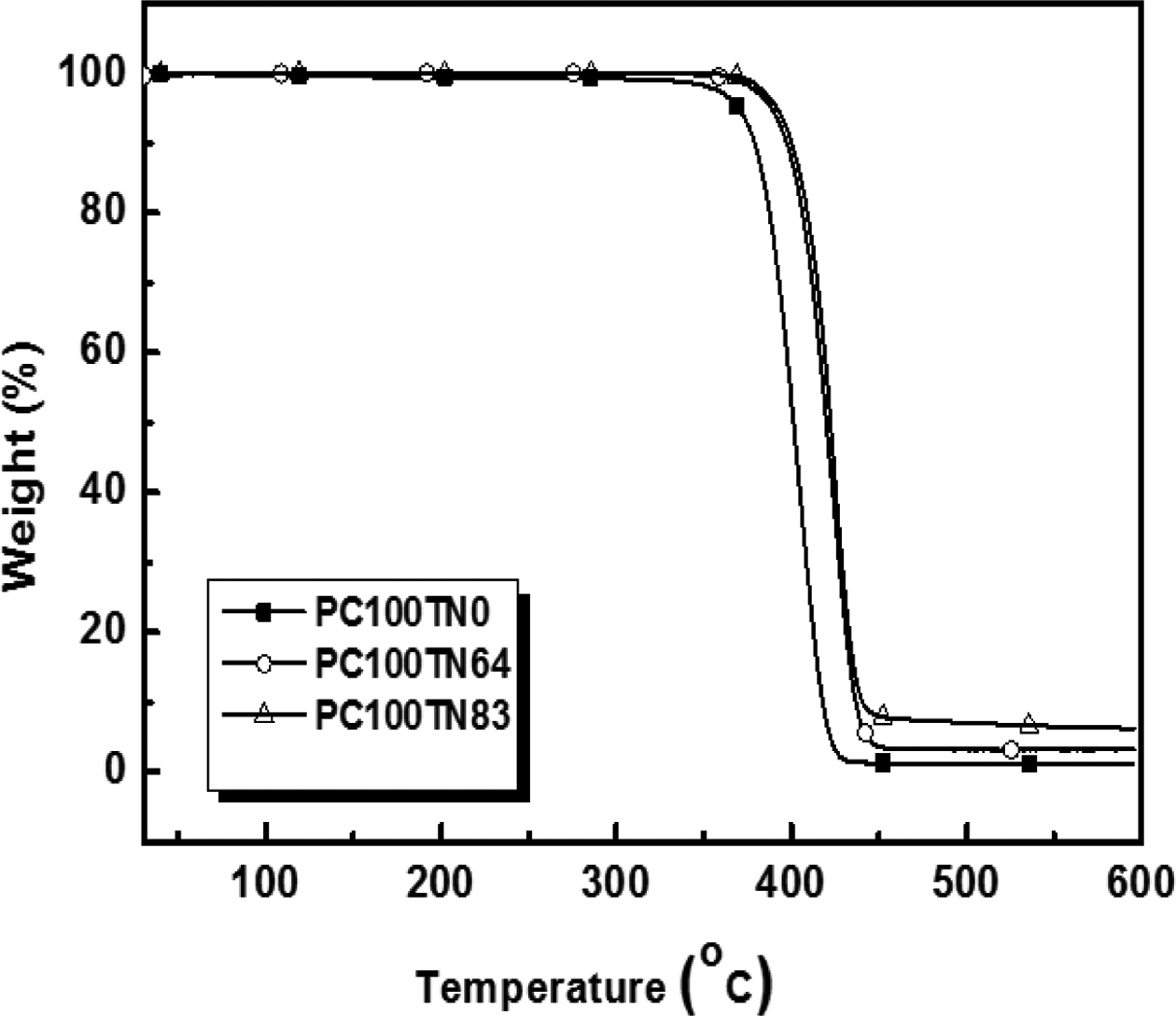
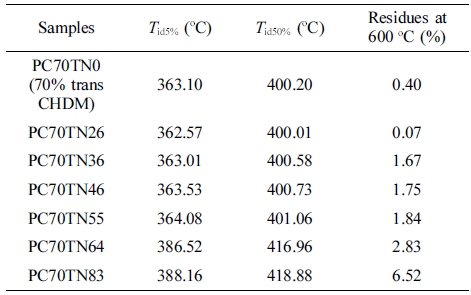
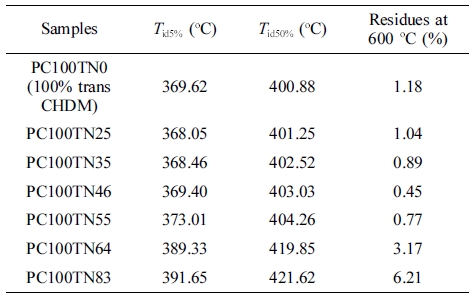
Thermal Stability of Synthesized Copolyesters. Thermal stability of synthesized copolyesters was analyzed by TGA and the results are given in Figures 7 and 8. Thermograms of only three different compositions of synthesized copolyesters are shown for the clarity of interpretation because the data overlap each other too much when drawn together. However, the detailed results are summarized in Tables 6 and 7. It was found that series of PC70TN# and PC100TN# copolyesters were stable up to 360 oC. Initial degradation temperature at which 5 wt% loss (Tid5%) and temperature at which 50 wt% loss (Tid50%) of initial weight observed are summarized in Tables 6 and 7 along with the amount of residue at 600 oC. All the copolyesters showed one-stage decomposition process (Figures 7 and 8), indicating that all the synthesized polymers were random copolyesters. From these figures it is clear that naphthalene units improve the thermal stability of synthesized copolyesters and this effect becomes more prominent when naphthalene content is higher than 46 mole%. Naphthalene units incorporated in copolyesters are thermally more stable than their analogous polyesters containing terephthalate units only. It was found that more residues were observed when naphthalene contents were increased. This finding is comparable to the results reported for the melt polymerized polyesters.47 Our results show that thermally stable copolyesters can be synthesized by a straightforward one-step solution polymerization process at room temperature in relatively short reaction time.
|
Figure 1 DSC thermograms of NDA and NDC. |
|
Figure 2 Thermal degradation behaviors of NDA and NDC. |
|
Figure 3 1H NMR spectra of solution polymerized PC70TN# copolyesters with peak assignments. |
|
Figure 4 1H NMR spectra of solution polymerized PC100TN# copolyesters with peak assignments. |
|
Figure 5 Effect of naphthalene content (mole%), defined as n/(m + n) of Scheme 1, on glass transition temperature (Tg) of PC70TN# and PC100TN# copolymers |
|
Figure 6 Effect of naphthalene content (mole%), defined as n/(m + n) of Scheme 1, on melting temperature (Tm) of PC70TN# and PC100TN# copolymers. |
|
Figure 7 TGA thermograms of synthesized PC70TN# copolyesters. |
|
Figure 8 TGA thermograms of synthesized PC100TN# copolyesters. |
|
Table 3 Molecular Weights and Intrinsic Viscosities of Synthesized PC#TN Copolymers |
Two series of copolyester, PC70TN# and PC100TN# were successfully synthesized by an efficient one-step solution polymerization and their structure-property relationship was studied. CHDM was used as a diol part and various amounts of NDC and TPC were used as diacid part for the synthesis. In order to study the effect of stereochemistry of CHDM unit, CHDM containing 70% trans- and 100% trans-isomers were employed. The determination of chemical compositions of synthesized copolyesters confirmed that NDC is less reactive than TPC. Thermal analysis showed that obtained copolyesters were semi-crystalline in nature and both the content of trans-CHDM isomers and the naphthalene units’ amount imparted thermal stability into obtained copolyesters. DSC results revealed that Tg of both series of copolyesters was increased by increasing the thermally stable naphthalene unit in a linear trend while Tm was first decreased until eutectic point (36 mole% naphthalene unit) then started to increase rapidly by increasing naphthalene units. It is worthy to note that Tg, Tm, and thermal degradation behavior of PC100TN# copolyesters, containing 100% trans-CHDM isomers were better that their analogues PC70TN# copolyesters that contain 70% trans-CHDM isomers.
To the best of our knowledge, it is the first time that one-step solution polymerization of aromatic PCTN copolyesters containing CHDM, terephthalate, and naphthalate units is conducted at room temperature in the absence of metallic catalyst and stabilizer. Our results also indicate that aromatic homopolyesters (PCT and PCN whose synthesis are very difficult by conventional melt polymerization) can be successfully synthesized by solution polymerization in a relatively short time.
- 1. C. S. Boland, U. Khan, G. Ryan, S. Barwich, R. Charifou, A. Harvey, C. Backes, Z. Li, M. S. Ferreira, M. E. Mobius, R. J. Young, and J. N. Coleman, Science, 354, 1257 (2016).
-

- 2. B. K. Behera and H. Arora, J. Ind. Text., 38, 205 (2009).
- 3. W. Shen, X. Zhang, Q. Huang, Q. Xu, and W. Song, Nanoscale, 6, 1622 (2014).
-

- 4. S. R. Turner, J. Polym. Sci., Part A: Polym. Chem., 42, 5847 (2004).
-

- 5. J. E. McIntyre, in Modern Polyesters: Chemistry and Technology of Polyesters and Copolyesters, J. Scheirs and T. E. Long, Eds., Wiley Susse, England, pp 1-28 (2004).
- 6. C. Bastioli, M. Foa, G. Floridi, F. Farachi, and T. Milizia, US Patent 6,727,342 B1 (2004).
- 7. W. H. Carothers and J. W. Hill, J. Am. Chem. Soc., 54, 1559 (1932).
-

- 8. J. R. Whinfiled and J. T. Dickson, US Patent 2,465,319 (1949).
- 9. C. J. Kibler, A. Bell, and J. G. Smith, J. Polym. Sci., Part A: Gen. Pap., 2, 2115 (1964).
- 10. S. R. Turner and R. W. Seymour, in Modern Polyesters: Chemistry and Technology of Polyesters and Copolyesters, J. Scheirs and T. E. Long, Eds., Wiley Susse, England, pp 267-288 (2004).
- 11. J. M. Koo, S. Y. Hwang, W. J. Yoon, Y. G. Lee, S. H. Kim, and S. S. Im, Polym. Chem., 6, 6973 (2015).
-

- 12. I. N. Duling, US Patent 2,547,730 (1969).
- 13. B. Hu and R. M. Ottenbrite, in Modern Polyesters: Chemistry and Technology of Polyesters and Copolyesters, J. Scheirs and T. E. Long, Eds., Wiley Susse, England, pp 335-359 (2004).
- 14. Y. U. Shi and S. A. Jabarin, J. Appl. Polym. Sci., 81, 11 (2001).
- 15. S. Park, F. Hussain, S. Kang, J. Jeong, and J. Kim, Polym. Korea, 42, 662 (2018).
-

- 16. H. Kliesh, M. O. Klein, and B. Kuhmann, US Patent 2012/0196111 A1 (2012).
- 17. F. Liu, J. Zhang, J. Wang, X. Liu, R. Zhang, G. Hu, H. Na, and J. Zhu, J. Mater. Chem. A, 3, 13637 (2015).
-

- 18. A. Celli, P. Marchese, L. Sisti, D. Dumand, S. Sullalti, and G. Totaro, Polym. Int., 62, 1210 (2013).
-

- 19. C. J. Kiber, A. Bell, and J. G. Smith, US Patent 2,901,466 (1959).
- 20. J. Wang, X. Liu, Z. Jia, L. Sun, Y. Zhang, and J. Zhu, Polymer, 137, 173 (2018).
-

- 21. A. Celli, P. Marchese, S. Sullalti, C. Berti, and G. Barbiroli, Macromol. Chem. Phys., 212, 1524 (2011).
-

- 22. C. Berti, A. Celli, P. Marchese, E. Marianucci, G. Barbiroli, and F. D. Credico, Macromol. Chem. Phys., 209, 1333 (2008).
-

- 23. Y. G. Jeong, W. H. Jo, and S. C. Lee, Macromolecules, 36, 5201 (2003).
-

- 24. B. Vanhaecht, M. N. Teerenstra, D. R. Suwier, R. Willem, M. Biesemans, and C. E. Koning, J. Polym. Sci., Part A: Polym. Chem., 39, 833 (2001).
-

- 25. D. J. Lyman, J. Polym. Sci., 55, 507 (1961).
-

- 26. Y. G. Jeong, W. H. Jo, and S. C. Lee, J. Polym. Sci., Part B: Polym. Phys., 42, 177 (2004).
-

- 27. L. Hu, L. Wu, F. Song, and B. G. Li, Macromol. React. Eng., 4, 621 (2010).
- 28. A. Lodha, R. S. Ghadage, and S. Ponrathnam, Polymer, 38, 6167 (1997).
-

- 29. T. H. Shah, J. I. Bhatty, G. A. Gamlen, and D. Dollimore, Polymer, 25, 1333 (1984).
-

- 30. G. P. Karayannidis, C. P. Roupakias, D. N. Bikiaris, and D. S. Achilias, Polymer, 44, 931 (2003).
-

- 31. W. Shotyk and M. Krachler, Environ. Sci. Technol., 41, 1560 (2007).
- 32. K. Pang, R. Kotek, and A. Tonelli, Prog. Polym. Sci., 31, 1009 (2006).
-

- 33. R. Muller, Trans. R. Soc. Trop. Med. Hyg., 97, 124 (2003).
- 34. S. L. Kwolek and P. W. Morgan, J. Polym. Sci., Part A: Gen. Pap., 2, 2693 (1964).
-

- 35. E. D. Giol, N. V. den Brande, B. V. Mele, S. V. Vlierberghe, and P. Dubruel, Polym. Int., 67, 292 (2018).
-

- 36. B. J. Sublett, US Patent 5,616,404 (1997).
- 37. L. Starr, J. Polym. Sci., Part A-1, 4, 3041 (1966).
-

- 38. American Polymer Standards Co., Polymer Standards Catalog (1991).
- 39. A. Paul, D. A. Young, G. E. Kulhman, W. Partenheimer, and W. P. Scbammel, US Patent 4,950,786 (1990).
- 40. C. A. Boye, J. Polym. Sci., 55, 275 (1961).
-

- 41. I. S. Rogulska and G. Rokicki, Polimery, 58, 85 (2013).
- 42. N. Kasmi, G. Z. Papageorgiou, D. S. Achilias, and D. N. Bikiaris, Polymers, 10, 1 (2018).
- 43. M. Ayyoob, D. H. Lee, J. H. Kim, S. W. Nam, and Y. J. Kim, Fibers Polym., 18, 407 (2017).
-

- 44. Y. C. Feng, H. Zhao, T. H. Hao, G. H. Hu, T. Jiang, and Q. C. Zhang, Materials, 10, 694 (2017).
-

- 45. Y. G. Jeong, W. H. Jo, and S. C. Lee, Macromolecules, 36, 4051 (2003).
-

- 46. E. V. MaTic and C. J. Kibler, in Man-made Fibres: Science and Technology, H. F. Mark, S. M. Atlas, and E. Cernia, Eds., Interscience, New York, pp 83-134 (1967).
- 47. I. C. Mcneill and M. Bounekhel, Polym. Degrad. Stab., 34, 187 (1991).
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2019; 43(3): 475-484
Published online May 25, 2019
- 10.7317/pk.2019.43.3.475
- Received on Feb 26, 2019
- Revised on Mar 15, 2019
- Accepted on Mar 15, 2019
 Services
Services
- Full Text PDF
- Abstract
- Acknowledgements
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Jinhwan Kim
-
Department of Polymer Science and Engineering, Sungkyunkwan University, 300 Cheoncheon-dong, Jangan-gu, Suwon, Gyeonggi 16419, Korea
- E-mail: jhkim@skku.edu
- ORCID:
0000-0002-8126-4221









 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.