- Preparation of Hydroxyethyl Cellulose-Bamboo Charcoal (HxBy) Hybrid and Its Application to Reinforcement of Natural Rubber
*School of Energy, Materials and Chemical Engineering, Korea University of Technology and Education, Cheonan, Chungnam 31253, Korea
**Research Center of Eco-friendly & High performance Chemical Materials, Cheonan, Chungnam 31253, Korea- 하이드록시에틸 셀룰로오스-뱀부차콜 혼성체 제조와 천연고무 강화 응용
*한국기술교육대학교 에너지, 신소재, 화학공학부, **친환경고성능화학소재연구소
Hydroxyethyl cellulose-bamboo charcoal (HxBy) hybrids with different content ratios were applied to develop a biochar hybrid filler for natural rubber (NR) reinforcement. The hybrids were conducted with hydroxyethyl cellulose and bamboo charcoal by a hydrogel-adsorption method, and the NR composites filled with hybrid fillers were synthesized by latex-filler dispersion mixing method. The curing properties of the NR composites were tested by a rubber processing analyzer (RPA-V1), the morphological structure of the composites were characterized by SEM. And the dispersion characterization of the composites were characterized by dispersion tester. Tensile strength, hardness value, abrasion resistance and swelling ratio were investigated to verify the property improvement of the NR composites. From the results of all the tests, it could be found the hybrid H8B2 (hydroxyethyl cellulose: bamboo charcoal = 8:2) showed the best reinforcement. The probable reason is the hybrid filler with ratio of H8B2 could provide the best interpenetrating network structure.
비율을 달리한 하이드록시에틸 셀룰로오스-뱀부차콜 혼성체는 천연고무(NR) 보강을 위한 바이오 차콜 혼성충전제 개발에 쓰인다. 하이드록시에틸 셀룰로오스 및 뱀부차콜은 하이드로젤-흡착(hydrogel-adsorption)법으로 제조되었다. NR 복합체는 latex-filler 분산 혼합법으로 제조하였다. 제조된 NR 복합체의 물성은 고무 가공 분석기로 측정되었고, 형태학적 구조는 SEM으로 확인되었고, 분산 특징는 분산 분석기로 측정되었다, 인장강도, 경도, 마찰계수 및 반탄성은 NR 복합체의 물성을 확인하기 위하여 조사되었다. 모든 실험 결과에서, H8B2(hydroxyethyl cellulose: bamboo charcoal = 8:2) 혼성체가 NR 복합체에 가장 좋은 보강 효과를 보였다. 그 이유는 H8B2 혼성충전제로 가장 침투성이 좋은 망상 구조를 형성할 수 있기 때문이다.
Keywords: hydroxyethyl cellulose, bamboo charcoal, natural rubber, hybrid, reinforcement
Hydroxyethyl cellulose (HEC) as a cellulose derivative is susceptible to degradation by cellulases, in activated compost and under natural conditions in soil without releasing toxic products. This material was researched as a filler material in polymer reinforcement, due to its improvement on moisture adsorption, glass transition and tensile mechanical properties of polymer.6 It also could improve the properties of rubber as a green chemistry raw material.
Latex-filler dispersion mixing method is a synthesis method of polymer that makes composites consist of two or more networks, in interpenetrating polymer networks. When physical crosslinking and chemical crosslinking occur simultaneously in polymer materials, it is assumed that it could provide better viscoelastic properties.7 Therefore, it has attracted the attention of researchers, especially in the rubber field.
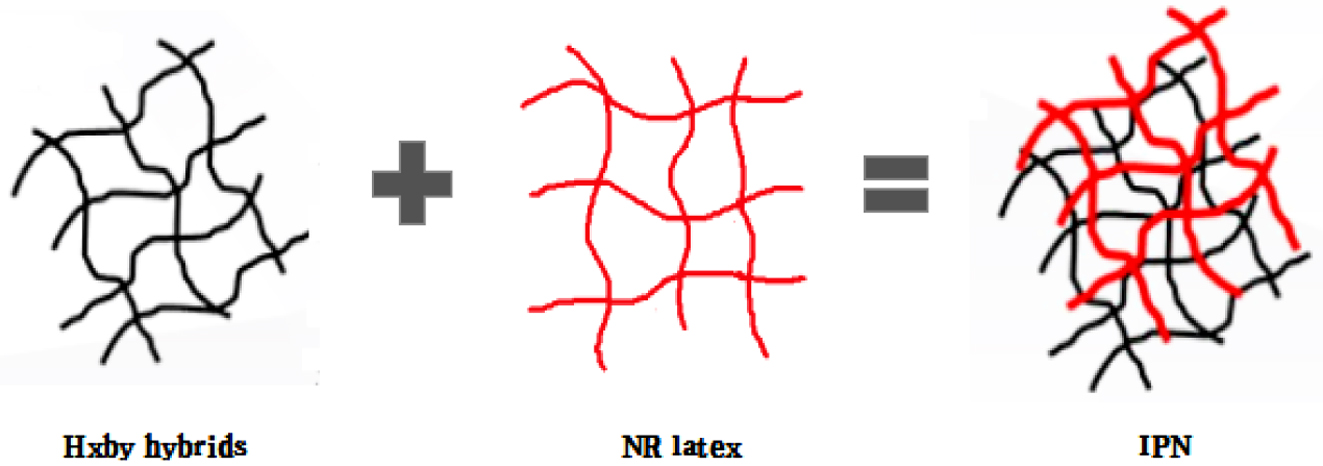
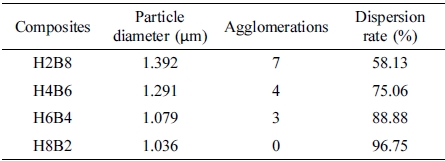
In this study, as shown in Figure 1. Rubber composites were synthesized by interpenetrating polymer networks method with natural rubber latex, which was used as main material, and HxBy hybrids in different content ratios, which used as fillers. As the fillers, HEC-BC hybrids were set into 4 ratios, which were H2B8 (HEC:BC = 2:8); H4B6 (HEC:BC = 4:6); H6B4 (HEC:BC = 6:4); and H8B2 (HEC:BC = 8:2), and fillers were filled with NR on a two roll mill. Neat NR was set as control group. After filling process, the vulcanizates were vulcanized during the curing process. Finally, the surface state, tensile strength, dispersion, hardness, abrasion resistance and swelling ratio had been characterized.
|
Figure 1 NR composites were synthesized by latex-filler dispersion mixing method. |
Materials. Natural rubber latex (Jungwoo company, Korea, effective mass 61±1%), sulfur (powder, Daejung, 99%), stearic acid (SA, Samchun chemical, 95% EP), N-cyclohexyl-2-ben-zothiazole sulfonamide (CBS, Tokyo Chemical Industry Company, Japan, 95%), 2,2' dibenzothiazolyl disulfide (DD, Tokyo Chemical Industry Company, Japan, 95%), zinc oxide (ZnO, Samchun chemical, 99%), hydroxyethyl cellulose (HEC, Daejung Chemicals & Metals Co., Ltd) and bamboo charcoal (BC, Quzhou Minxin Charcoal Company, Zhejiang Province, China).
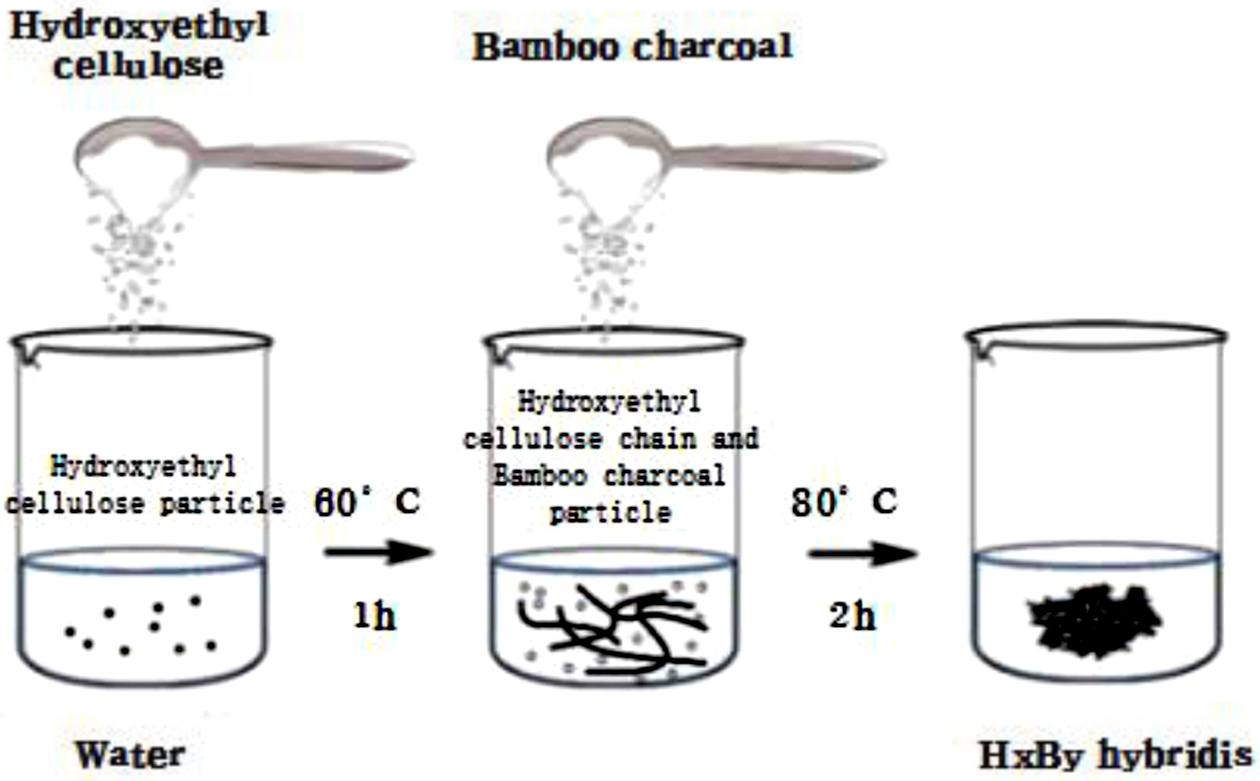
Synthesis of HxBy Hybrids. The formulation was shown in Table 1 and the process was shown in Figure 2. HEC hydrogel was prepared by mixing a certain amount of HEC in distilled water at a constant temperature of 60 ℃ in a water bath at 150 rpm stirring for 1 h to make hydrogel system.8 And BC was mixed into the hydrogel system, and stirred with a magnetic stirrer (about 300 rpm) for about 2 h at the temperature of 80 ℃. And made them better adsorbed together. Then dried the product in an oven with 60 ℃.
Compounding and Curing. HxBy hybrids and NR latex were mixed drying, the formulation was shown in Table 2. The compounding process was conducted on a two-roll mill. Note the sulfur and vulcanization promoters were added at the last step for avoiding the pre-vulcanization. After that, samples were vulcanized under 10 MPa for t90 at 160 ℃ in a heating press machine. The thickness of the samples was set as 1 mm.
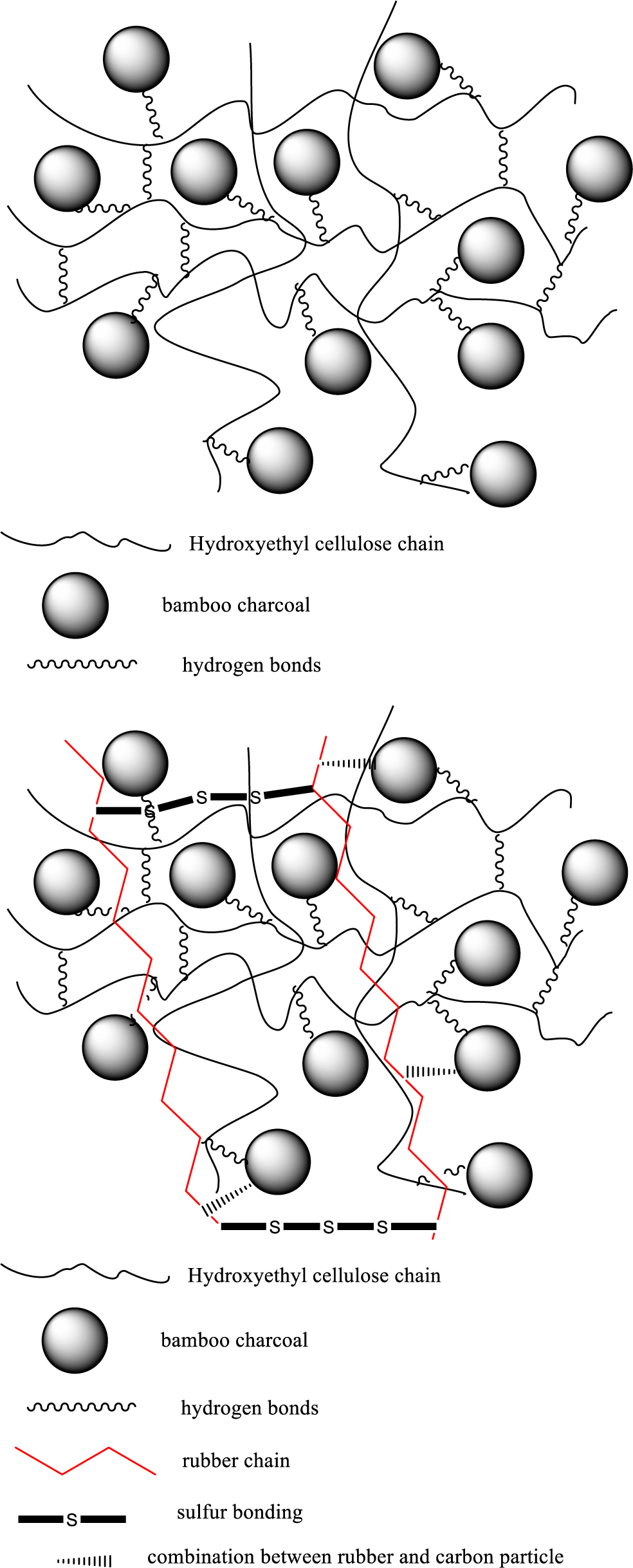
From the Figure 3, first of all, when the HEC was poured into hot water, it could get hydrogelation, and become into a network crosslinking state with high adsorption force due to the gelation effect. And at the same time, added the BC into the system which also exist some polar-functional groups, and also could form the hydrogen bond with HEC gel, due to the particle size and better hardness reinforcement of BC, the network crosslinking state become more compact. Before the gel solidification forming, pour the latex into the system, to make the rubber chain cross with the gel chain, and due to the carbon particle (BC) could also get good combination effect with rubber chain, so it could form better matrix with larger combination force. After the drying process, added the sulfur to form the vulcanization state, due to the strong force of -S-S- bonding between the rubber chains, the stronger matrix had been formed during this step.
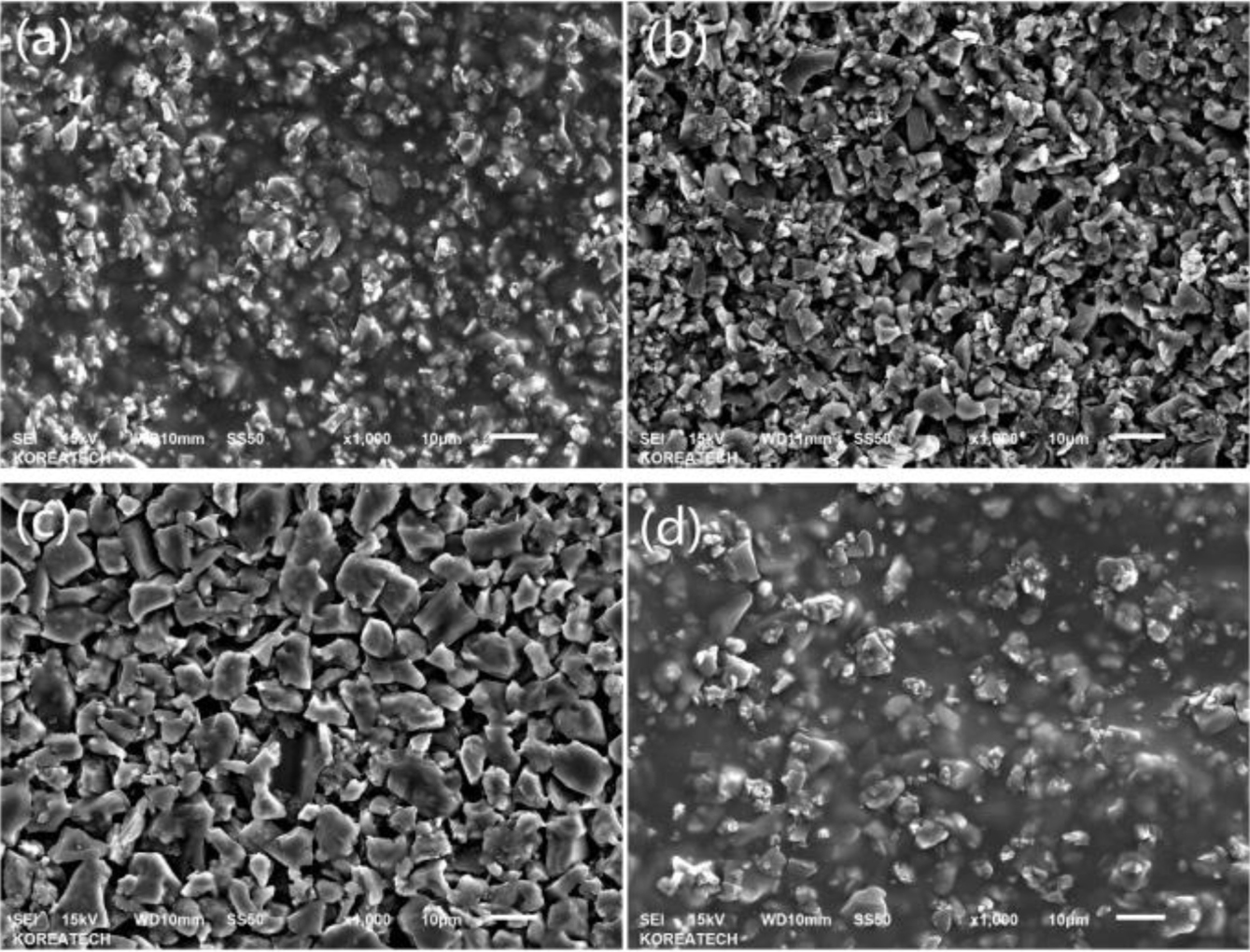
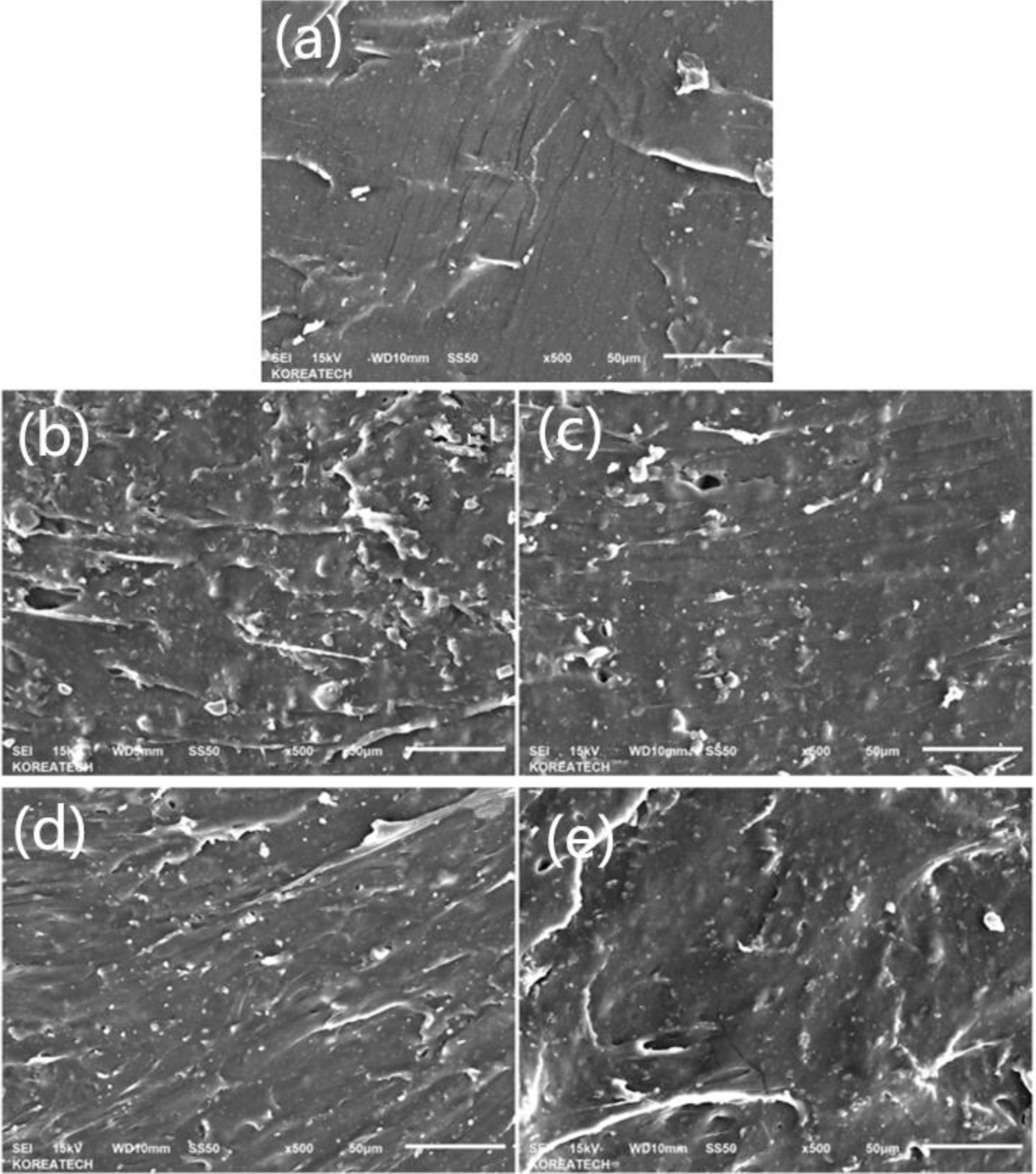
In the Figure 4 of SEM, (a) is for H2B8, (b) is for H4B6, (c) is for H6B4, and (d) is for H8B2, from the picture, it could be found obviously that (a) and (d) showed better gelation effect, which could form the compact matrix, but as (b) and (c), they presented block and plate state, but not the whole gel state, and with the ratio of HEC increasing, the particle size of hybrid filler also increased, especially in (c), it could be found some small size gels are wrapped on BC particles, but not form the whole state, and in (d), due to the more HEC ratio, the gelation could be more completed, and provide more network structure and adsorption force to form the compact hybrid. So during the characterization of fillers, it also could be found that H2B8 and H8B2 might provide better mechanical reinforcement effect during the mixing process with NR latex.
Characterization. The morphology of the samples after the tensile test was carried out on a FE-SEM (JSM-7500F, JEOL Ltd. Japan). The dispersion characterization was performed by U-CAN UD-3500 C.B. dispersion tester (U-CAN DYNATEX INC). The cure/vulcanization characteristic of samples were measured by a rubber process analyzer (RPA-V1, UCAN DYNATEX INC.). The minimum torque (ML), maximum torque (MH), scorch time (ts2), and optimum cure time (t90) were determined by the RPA. The cure rate index (CRI) was used to evaluate the cure rate of the rubber, and it was calculated by the following eq. (1):9
CRI = 100/(t90 − ts2) (1)
Mechanical Properties. Tensile strength of samples was performed on a Tinius Olsen H5KT- 0401 testing machine at a speed of 500 mm min-1 according to ASTM D412 with the average of three measurements.
Specimens on standard dumb-bell shape were cut from the vulcanizate sheets with dimensions 25 mm×6 mm×1 mm (length×width×thickness).
Shore A hardness of the specimens had been obtained with Shore Durometer Type A according to ASTM D22-40.
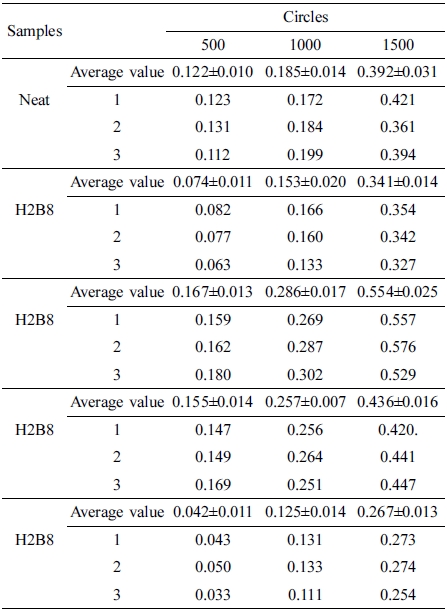
Abrasion resistance test was performed using Taber Abrasion tester 5135 at 500, 1000 and 1500 cycles of abrasion with a rotate speed of 80 r/min according to ASTM D1044.
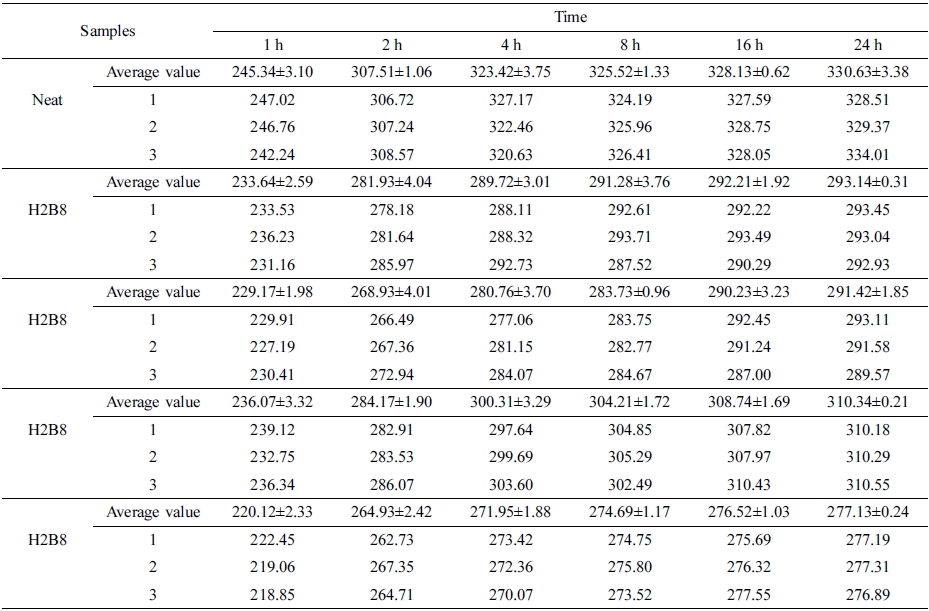
And swelling ratio tests were carried out in toluene for 1, 2, 4, 8, 16 and 24 h according to ASTM D71-79.
|
Figure 2 Synthesis of HxBy hybrids. |
|
Figure 3 Hybrids were conducted by a hydrogel-adsorption method. |
|
Figure 4 SEM picture of HxBy hybrids: (a) H2B8; (b) H4B6; (c) H6B4; (d) H8B2. |
|
Table 1 Formulation of HxBy Hybrids |
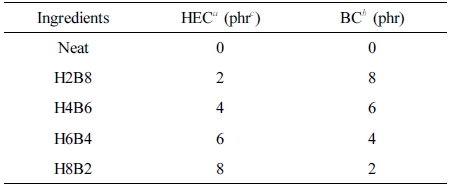
aHEC: Hydroxyethyl cellulose. bBC: Bamboo charcoal. cphr: part per hundreds of rubber. |
|
Table 2 Formulation of Test Sample Compounds |
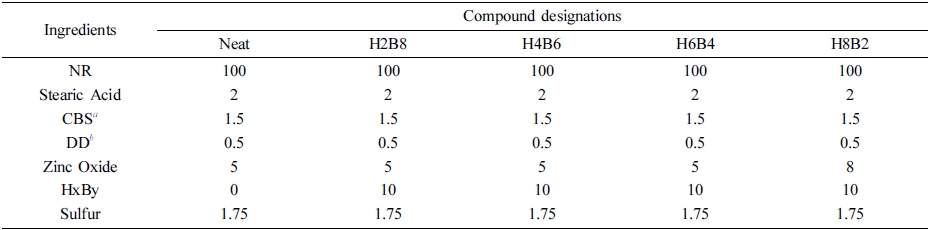
aCBS: N-Cyclohexyl-2-benzothiazole-sulfonamide. bDD: 2,2-Dibenzothiazolyl disulfide. |
The results of morphology were shown in Figure 5. Which were the pictures of samples after tensile strength testing, compared with Figure 5(a), the composites Figure 5(b)-(e) showed the layer structures of the matrix, and it also could be found with the filling ratio of BC decreased, the particle size of fillers decreased, the layer structure and gully-like structure became more and more compact. It was due to the interpenetrating network between filler and matrix, which could increase the density of crosslinking, and make the matrix more compact in morphology.12 And due to the self-reinforcing effect of natural rubber, the neat rubber had excellent tensile properties, it also could be found in Figure 5(a), which showed fine cracks during the tensile test. And from Figure 5(b) and 5(e), it could be found the larger layers in these two composites, which means there were strong interaction forces in these two matrix. But in Figure 5(c) and 5(d), the layer and gully-like structure were not obvious, which also meant the interaction between filler and matrix was weak. Thus, after the tensile test, the sliding of layer structure of rubber is easier than Figure 5(b) and 5(e), and turned into the relatively flat surface structures.
The results of dispersion characterization were shown in Table 4. It could be found with the filling content of HEC increasing, the agglomerations in matrix decreased, but dispersion rate of hybrid fillers increased, which presented the more compact layer structure and gully-like structure of matrix. It was due to the more hydrogen bonding effect could make contribution of combination, which could improve the dispersion effect in matrix, and reduce the agglomerations. Also, due to the more HEC content could provide the more hydrogel to cover and connect the BC particle in the processing of gelation step, which could make the hybrid filler more compact, thus showed the smaller particle size, and when the hybrid fillers filled into NR matrix, due to the polarity of double sulfur (-S-S-) bonds which were provided in vulcanization step and the polarity of hybrid fillers, the more HEC content, the stronger polarity, thus they could form the stronger combination matrix due to the theory of similar dissolve mutually.13
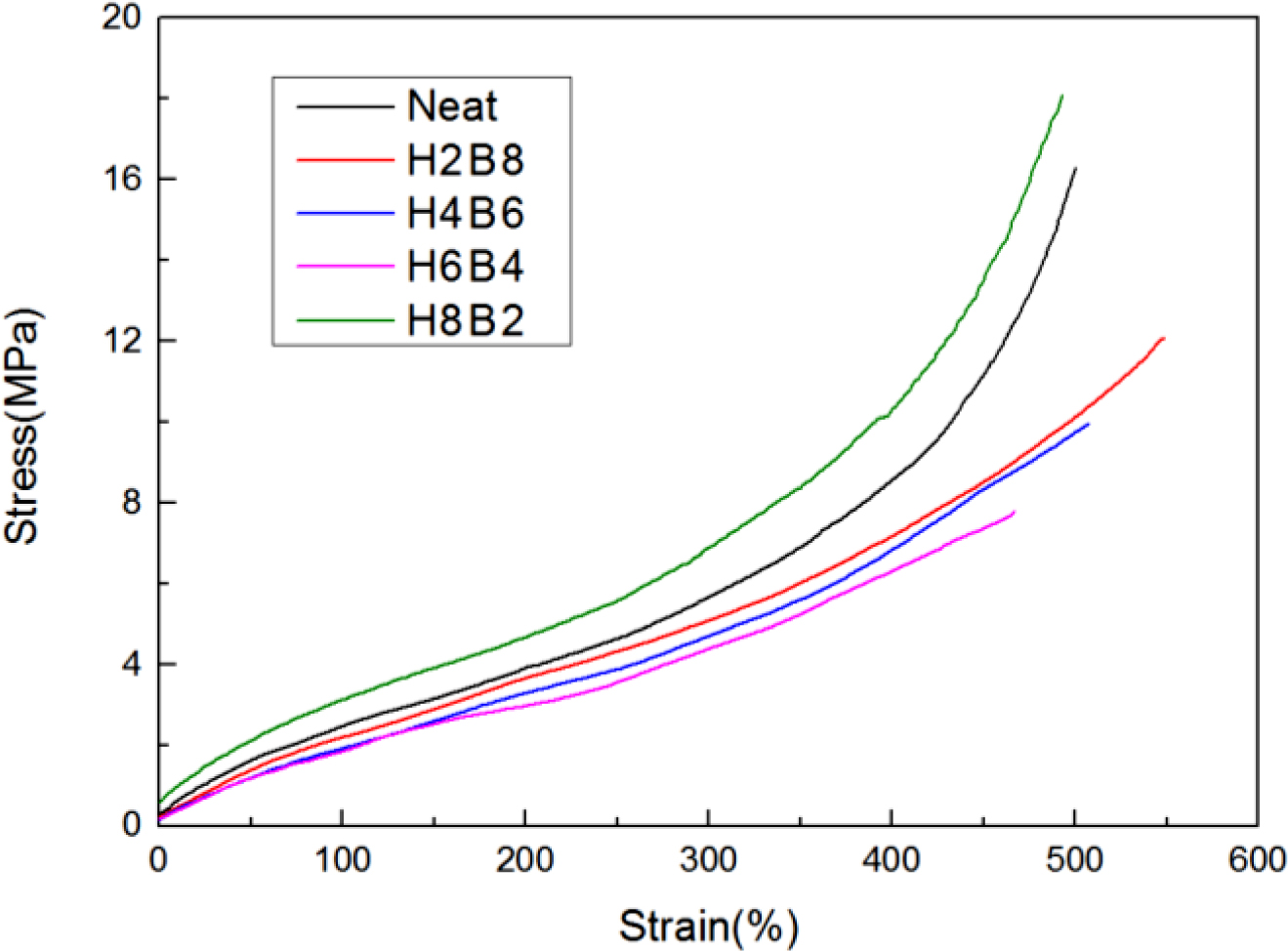
The results of tensile strength were shown in Figure 6. From these curves, natural rubber inherently possesses high strength due to strain-induced crystallization. When fillers are incorporated into NR, the regular arrangement of rubber molecules is disrupted and hence the ability for crystallization is lost.14 It is the reason why fillers reinforced natural rubber blends possess lower tensile modulus than neat NR blend. But as an interpenetrating network, which also could increase the crosslinking density, and the more ratio of HEC, the more hydrogen bonds, and the more interaction effect,15 thus H8B2 showed the best tensile stress in this research, it also could be found in Table 3, which had the largest DM value. As for H2B8, due to the small-scale hydrogel system, the formation of interpenetrating networks also showed with a small-scale, and more BC had been filled into NR matrix directly, so it showed a lower curve than neat NR and H8B2.
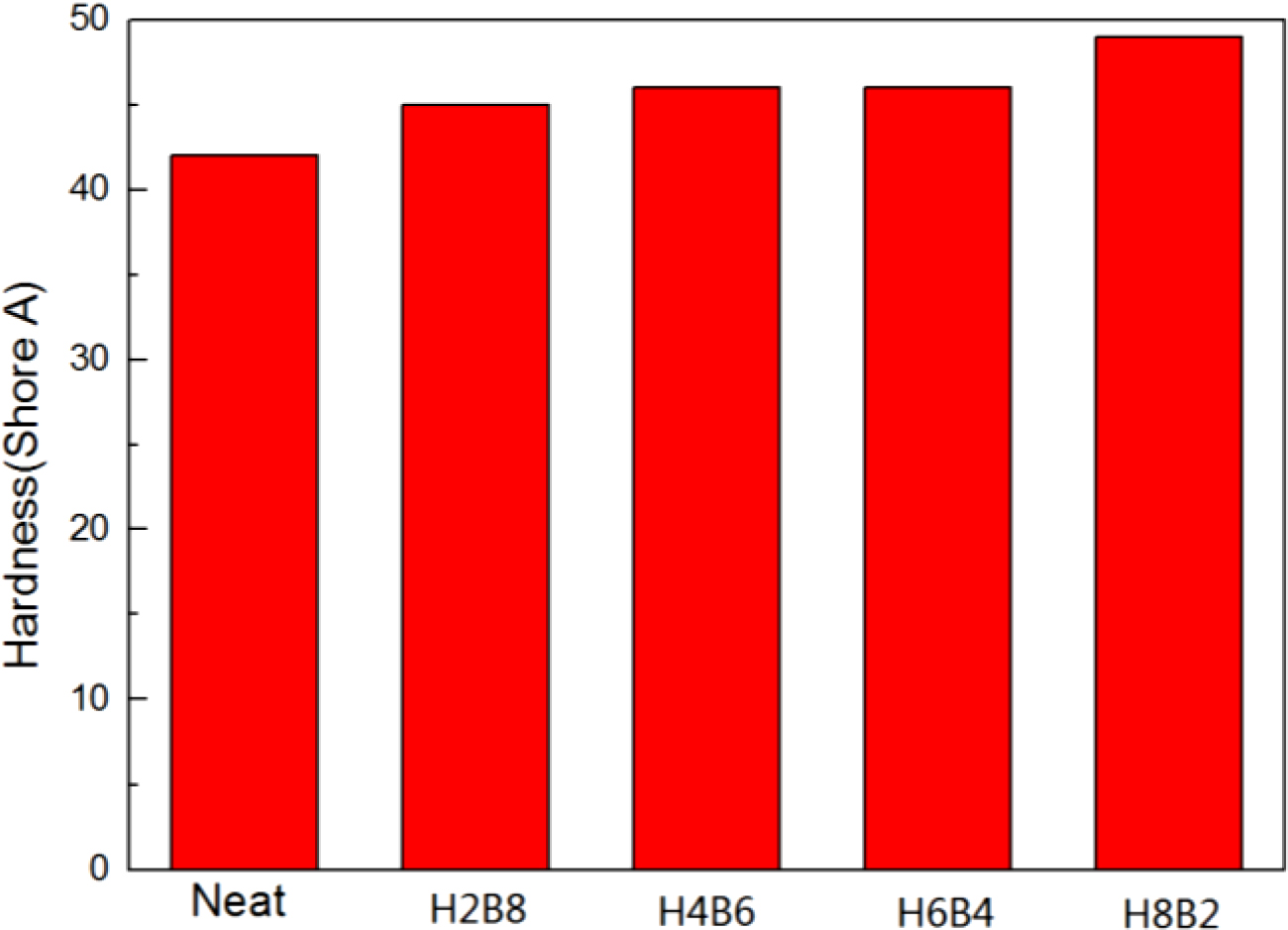
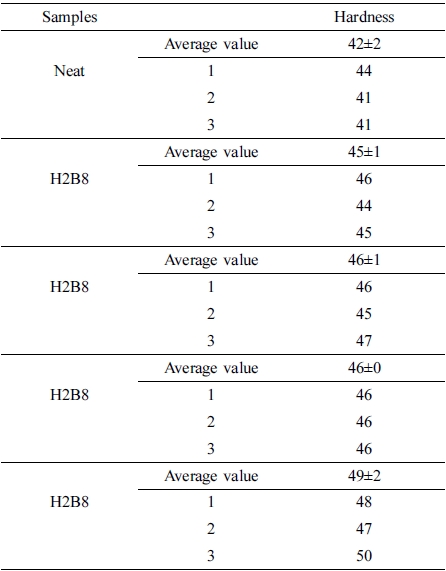
The results of hardness test were shown in Figure 7. From this figure, it can be found all the composite filled with fillers showed the higher hardness value than neat NR. The reason of this result may be the fillers could provide more crosslinking when filled into NR rubber matrix, and make the matrix compact. When they dispersed into rubber matrix, the matrix had become a whole uniform state, so the hardness value had been increased.16 (The error range is shown in Table 5.)
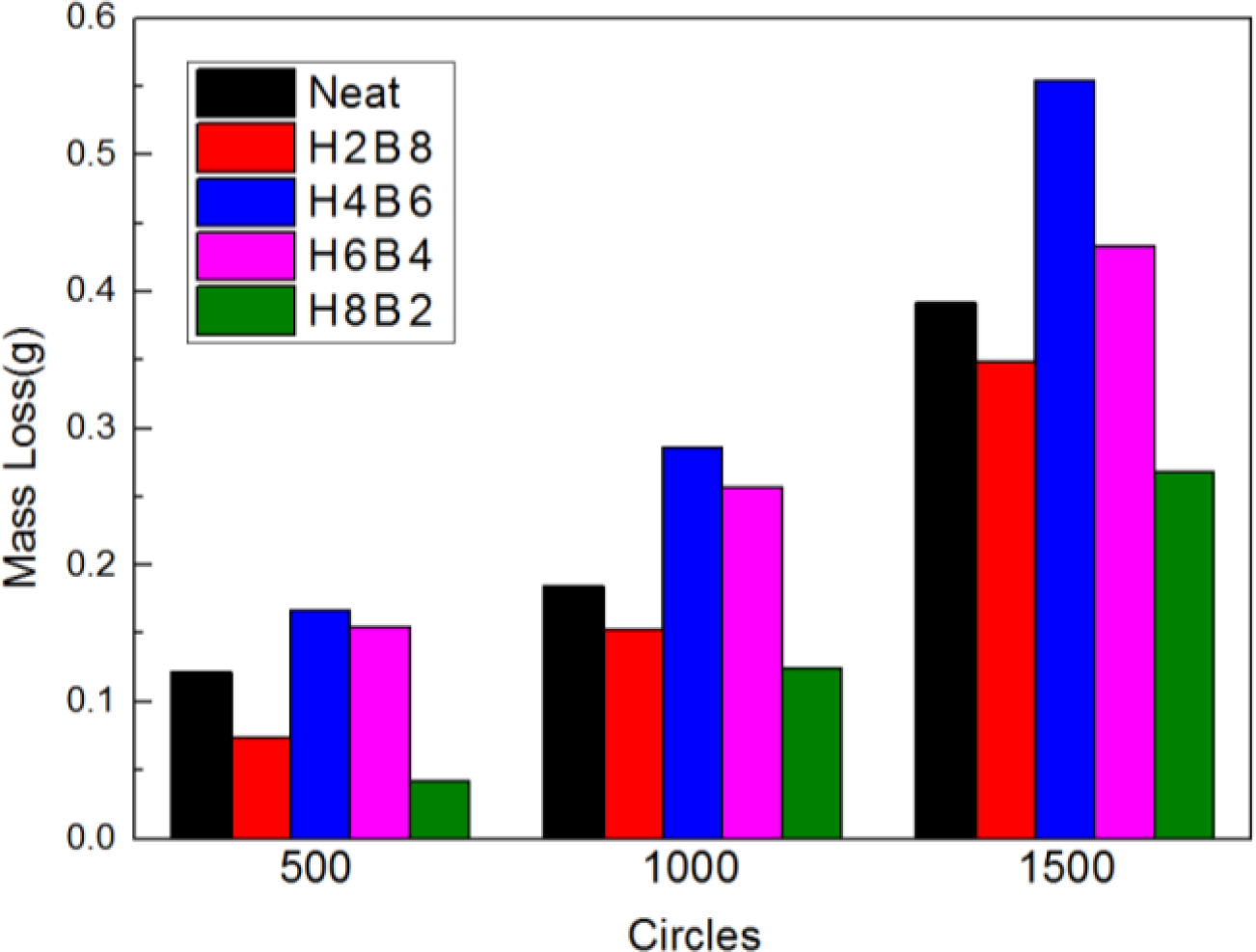
The abrasion resistance test results were shown in Figure 8. Due to the improvement of hardness for composite, H8B2 fillers showed the best abrasion resistance result in this research, compared to the neat ENR, composite filled with H8B2 presented about 1.5 times abrasion reinforcement effect than neat, which would apply in the rubber roll filled with longer service life.17 (The error range is shown in Table 6.)
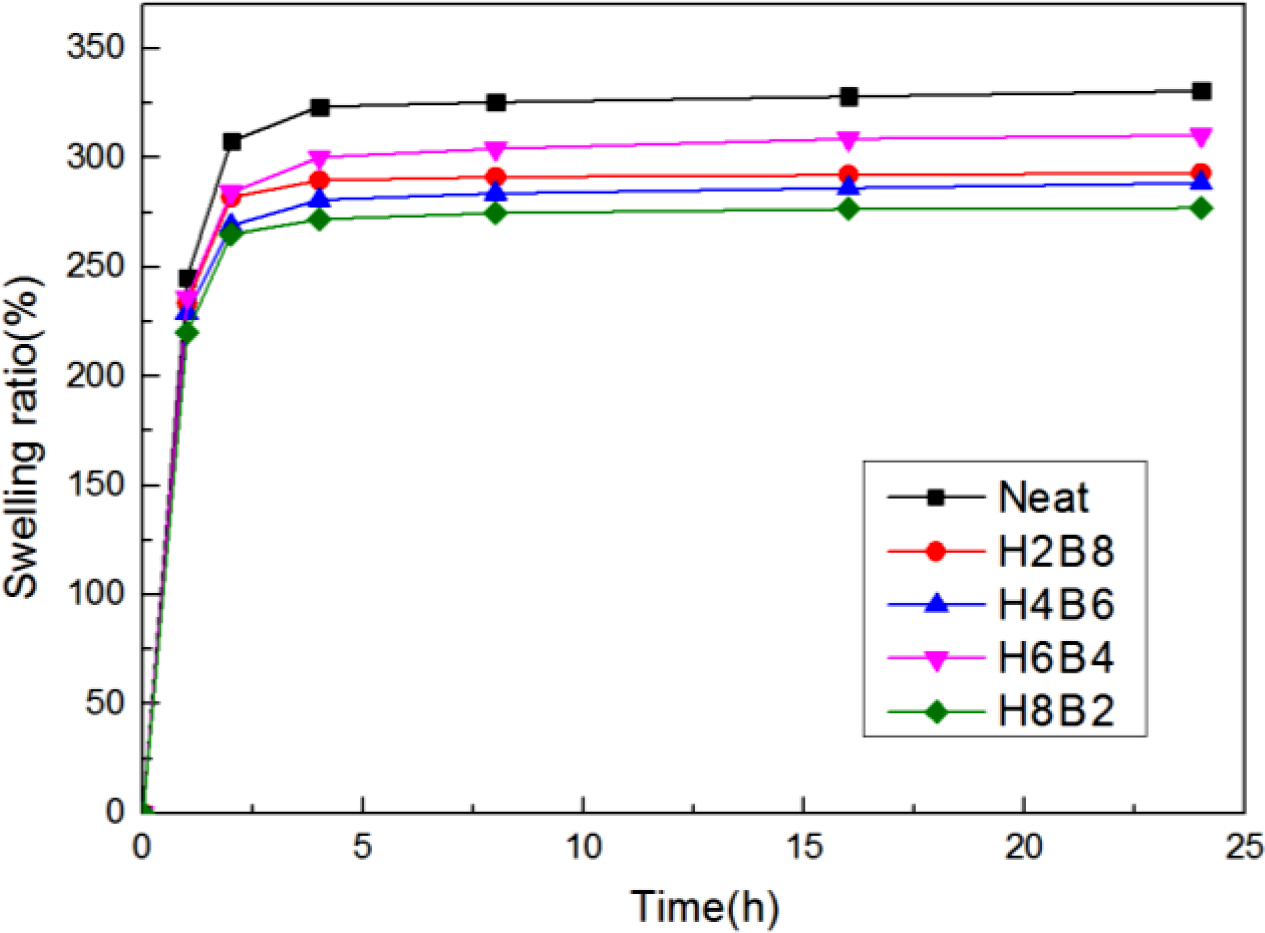
The swelling ratio test results were displayed in Figure 9. The swelling ratio (Q) was calculated by the following eq. (2):
Q(%) = 100 × (Ws − Wu)/Wu (2)
Where Ws is weight of the swollen sample and Wu is weight of the un-swollen sample (extracted sample).18 From the curves, it can be found the neat NR showed the highest swelling ratio, due to principle of similarity compatibility, the neat NR had the weakest polarity, so when swelling in toluene which also had non-polarity, it showed the largest swelling degree. But when filled with HxBy hybrid, due to the increasing of polarity, the swelling degree also decreased. Also, because H8B2 composites have the tightest interpenetrating network structure and crosslinking density, the more crosslinking density, the more compact matrix, and the better swelling resistance.19 (The error range is shown in Table 7.)
|
Figure 5 SEM picture of composites: (a) neat NR; (b) H2B8; (c) H4B6; (d) H6B4; (e) H8B2. |
|
Figure 6 Tensile strength results of the NR composites. |
|
Figure 7 Hardness results of the NR composites. |
|
Figure 8 Abrasion resistance results of the NR composites. |
|
Figure 9 Swelling ratio test results of the NR composites. |
Hydroxyethyl cellulose-bamboo charcoal (HxBy) hybrids with different content ratios were applied to develop a biochar hybrid filler for natural rubber reinforcement. The hybrids were conducted with hydroxyethyl cellulose and bamboo charcoal by a hydrogel-adsorption method, and the NR composites filled with hybrid fillers were synthesized by latex-filler dispersion mixing method.20 The curing properties, the morphology, the dispersion characterization, the tensile strength, hardness value, abrasion resistance and swelling ratio had been characterized, from the results of all the test, it could be found the NR composite filled with H8B2 showed the best mechanical properties, due to the best interpenetrating network structure has been formed during the compounding process, and also due to the more hydrogen-bond effect which could make the matrix more compact.21
- 1. S. C. Peterson, S. R. Chandrasekaran, and B. K. Sharma, J. Elastom. Plast., 48, 305 (2016).
-

- 2. M. J. John and R. D. Anandjiwala, Compos. Part A: Appl. Sci.Manuf., 40, 442 (2009).
-

- 3. A. K. Mohanty, S. Vivekanandhan, and J. M. Pin, Science, 362, 536 (2018).
-

- 4. Q. Zhao, B. Zhang, H. Quan, R. C. M. Yam, R. K. K. Yuen, and R. K. Y. Li, Compos. Sci. Technol., 69, 2675 (2009).
-

- 5. Z. M. Huang, Y. Z. Zhang, and M. Kotaki, Compos. Sci. Technol., 63, 2223 (2003).
-

- 6. Z. N. Azwa, B. F. Yousif, and A. C. Manalo, Mater. Design, 47, 424 (2013).
- 7. L. H. Sperling, Macromol. Rev., 12, 141 (1977).
-

- 8. H. R. Lin and K. C. Sung, J. Control. Release, 69, 379 (2000).
-

- 9. M. C. Li, X. Ge, and U. R. Cho, Macromol. Res., 21, 519 (2013).
-

- 10. M. S. Sobhy, D. E. El-Nashar, and N. A. Maziad, Egypt. J. Sol., 26, 241 (2003).
- 11. Y. Zimmermann, S. Anders, K. Hofmann, and S. Spange, Langmuir, 18, 9578 (2002).
-

- 12. X. X. Li and U. R. Cho, Elastom. Compos., 51, 43 (2016).
-

- 13. G. S. Banker, J. Pharmaceut. Sci., 55, 81 (1966).
-

- 14. M. Jacob, S. Thomas, and K. T. Varughese, Compos. Sci. Technol., 64, 955 (2004).
-

- 15. Berger and Jerôme, Eur. J. Pharm. Biopharm., 57, 19 (2004).
- 16. X. X. Li and U. R. Cho, Elastom. Compos., 53, 6 (2018).
- 17. X. X. Li, H. S. Jeong, and U. R. Cho, Elastom. Compos., 53, 48 (2018).
- 18. J. Rajendhran and P. Gunasekaran, J. Biosci. Bioeng., 97, 1 (2004).
-

- 19. R. P. Babu, K. O'connor, and R. Seeram, Progr. Biomater., 2, 8 (2013).
-

- 20. G. Jing, L. Wang, and H. Yu, Colloid Surface A, 416, 86 (2013).
-

- 21. S. Kitagawa, R. Kitaura, and S. Noro, Angew. Chem. Int. Ed., 43, 2334 (2004).
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2019; 43(3): 351-358
Published online May 25, 2019
- 10.7317/pk.2019.43.3.351
- Received on Dec 28, 2018
- Revised on Feb 1, 2019
- Accepted on Mar 11, 2019
 Services
Services
Shared
 Correspondence to
Correspondence to
- Ryong Cho*,**
-
*School of Energy, Materials and Chemical Engineering, Korea University of Technology and Education, Cheonan, Chungnam 31253, Korea
**Research Center of Eco-friendly & High performance Chemical Materials, Cheonan, Chungnam 31253, Korea - E-mail: urcho@koreatech.ac.kr
- ORCID:
0000-0003-4866-8109










 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.