- Transparent Epoxy Resin Toughened with In-situ Azide-alkyne Polymerized Aliphatic Toughening Agent
Nho Hoon Kwak, Jin Seo Lee, Na Yeong Ko, Hee Sang Yang, Wu Bin Ying*,†
 , and Bumjae Lee†
, and Bumjae Lee† 
Technology and Engineering, Chinese Academy of Sciences, Zhongguan West Road 1219, Ningbo 315201, China
*Department of Fine Chemical Engineering and Applied Chemistry, Chungnam National University, 220 Gung-dong, Yuseong-gu, Daejeon 34134, Korea- Azide-alkyne 반응을 이용한 In-situ 지방족 강인화제를 적용한 광투명 강인화 에폭시 수지
충남대학교 정밀공업화학과 *중국과학원, 재료기술 및 엔지니어링
To improve the toughness of the epoxy resin while maintaining their transparency, a novel polymer, which has an aliphatic structure, is synthesized via in-situ azide-alkyne polymerization as a toughening agent. Because of the dissimilar kinetics of the epoxy system and click reaction, sequential curing process was designed. First, linear aliphatic toughening agents were formed in the epoxy resin, followed by curing of the epoxy matrix as the addition of curing agent, resulting in a semi-interpenetrating polymer network (semi-IPN). The toughened epoxy with in-situ synthesized polymer exhibits significantly improved mechanical properties compared to neat and toughened epoxy with preformed polymer, and it has increased value of toughness as high as 2.62 MPa·m1/2 at 10 wt% of toughening agent contents with maintenance of their transparency and glass transition temperature.
투명성을 유지하면서 에폭시의 인성을 향상시키기 위해서, in-situ 방법을 이용한 새로운 지방족 구조의 폴리머를 강인화제로 사용하였다. 에폭시와 click 반응의 유사한 반응 속도를 연구하고, 이를 통해 연속적인 에폭시 경화단계를 확립하였다. 먼저 선형의 지방족 강인화제를 에폭시 수지에서 합성하였고 그 후 경화제를 첨가하여 에폭시를 경화하였다. 이를 통해 semi-interpenetrating polymer network(semi-IPN) 구조를 가지는 에폭시를 제작하였다. Semi-IPN 구조를 가지는 강인화 에폭시의 특성은 기존 에폭시 및 preformed 에폭시에 비해 물성이 현저히 향상되었다. 10 wt% 강인화 에폭시의 경우 투명성과 Tg의 변화를 보이지 않으면서 2.62 MPa·m1/2의 향상된 파괴인성 수치를 보였다.
Keywords: azide-alkyne click reaction, in-situ toughened epoxy, transparency
This work was supported by a Research Grant from Chungnam National University, BJ Power and the Korea Agency for Infrastructure Technology Advancement (KAIA) funded by the Ministry of Land, Infrastructure and Transport of the Korean government (Project No.: 18TBIP-C124966-02).
Epoxy resins are a thermosetting resin which used in many applications such as adhesives, coatings and construction industry because of their superior adhesion, mechanical, electrical and thermal characteristics.1-6 However, epoxy resins have a several disadvantages that are not suitable for advanced technical industries such as a yellowing, a vulnerable moisty resistance, brittle and decomposition properties.7-10 Especially, the lifetime of the epoxy is not long due to stiffness caused by high crosslinking.
To overcome the brittle disadvantage of epoxy resins, various toughening methods11-16 have been studied by adding the rubber and the engineering thermoplastic polymer to epoxy system. When rubber was used as a toughening agent such as carboxyl-terminated polybutadiene (CTBN),17 epoxy resins show improved fracture toughness via elastomer particles dispersion. However, the modulus and the glass transition temperature were significant reduced as well. Differently, when thermoplastic polymer which has a unique high Tg and toughness was added to epoxy system, a toughness of the epoxy system was improved without a significant reduction in mechanical properties and glass transition temperature. These toughened epoxy resins show an improved property of a heat resistance and have an advantage of reduction in stiffness. However, the mixing of the polymeric toughening agent with epoxy improves the viscosity of the epoxy and thus makes process difficult which causes the limitation in industrial use. Accordingly, toughening techniques by synthesizing polymers in-situ in epoxy resins have been studied to improve the mechanical properties of resins processibility.18-20
Azide-alkyne click chemical reactions are widely used as efficient methods for polymer designing.21-25 The advantage of this reaction is that there is no side reaction with high yield, solvent selection is free, and application range is wide.26,27 Recently, It has been attempted to toughen epoxy resins by azide-alkyne click polymerization as a method of in-situ polymerization based on epoxy triglycidyl p-aminophenol and 4,4'-diaminodiphenylsulfone.28 Through toughening with novel polysulfone-type polymers are synthesized via azide–alkyne polymerization for use as toughening agents, the fracture toughness and heat resistance of a cured toughened epoxy resin were simultaneously improved along with a reduction in its viscosity during the mixing process. This paper suggested this phenomenon due to the formation of semi-interpenetrating polymer networks comprising the epoxy network and the linear polysulfone-type polymers.
In toughened epoxy system, it was confirmed that the toughening agent structure is similar to the cured epoxy structure and also has a similar solubility parameter value. According to these properties, the compatibility between toughening agent and epoxy resin and transparency are maintained. For the purpose of supplement the brittleness of the epoxy resin and maintain the transparency, we used the aliphatic polymer as a toughening agent. In this study, we investigated the curing and toughening behavior of hydrogenated bisphenol A diglycidyl ether (HDGEBA). In general, due to the aromatic chromophores, bisphenol A type epoxy has a disadvantage that they are easily aged and yellowing phenomenon as a building exterior or encapsulant materials.29 However the hydrogenated DGEBA is stable to UV irradiation and has excellent weather stability and chemical resistance,30-32 so we selected HDGEBA as the epoxy system.33,34 The HDGEBA is cured with alicyclic amine and toughening agent monomers are polymerized at an elevated temperature via azide-alkyne click reaction without catalyst. In order to synthesis aliphatic polymers as in-situ toughening agent, diazide and diacetylene click monomers were synthesized. Herein, we studied changes of epoxy transparency by the content of toughening agent. The mechanical and thermal properties of toughened epoxy resin with aliphatic polymer were also investigated and the phase behavior on semi-IPNs of epoxy resins was observed.
Reagents and Materials. 1,4-Cyclohexane dimethanol (Aldrich, 99%), tosyl chloride (TCI, >99.0%), pyridine (Samchun, 99%) and sodium azide (Aldrich, 99.5%) were used for diazide monomers synthesis. Polycaprolactone diol (Aldrich, MW=2000 g/mol), Propiolic acid (Alfar aesar, >98%) p-tolunesulfonic acid monohydrate (Aldrich, ≥98%), used for diacetylene monomers synthesis. As solvents in this work dichloromethane, N,N-dimethylformamide and toluene were used and purchased from Samchun chemical. The hydrogenated diglycidyl ether of bisphenol A (HDGEBA, KUKDO chemical) and amine curing agent of isophorone diamine (TCI, >99.0%) were used without any treatment.
Synthesis of Diazide Monomers. The synthesis of 1,4-bis(azidomethyl)cyclohexane (BAC) involved two steps starting from 1,4-bis(hydroxymethyl)cyclohexane as shown in Scheme 1.29

Scheme 1. Synthesis of 1,4-bis(azidomethyl)cyclohexane.
1,4-Bis(tosylmethyl)cyclohexane:1,4-Bis(hydroxymethyl) cyclohexane (BHC, 10 g, 0.069 mol), tosyl chloride (TsCl, 52.7 g, 0.277 mol), and pyridine (21.9 g, 0.277 mol) were added in dichloromethane and stirred at room temperature for 72 h. The reaction mixture was successively extracted with NaHCO3 aqueous solution, 1 N HCl solution, NaCl solution, and water. Then the combined organic layers were dried over MgSO4 and filtered, followed by the precitation in largely excess n-hexane. The white product was dried in a vaccum oven at 60 ℃ (yield 79%).
1,4-Bis(azidomethyl)cyclohexane: 1,4-Bis(tosylmethyl) cyclohexane (BTsC, 20 g, 0.044 mol) and sodium azide (11.6 g, 0.178 mol) were added in N,N-dimethylformamide (DMF). The reaction mixture was stirred at 90 ℃ for 48 h. After completion of the reaction, sodium azide was filtered out and the reaction solvent was distilled under reduced pressure using a distillation apparatus. The yellow residue was dissolved in diethyl ether and washed by water five times to remove the generated NaOTs and trace residue NaN3. The combined organic layers were dried over MgSO4 and solvent was removed using a rotary evaporator. The yellow oily liquid was dried in a vaccum oven at 40 ℃ for 1 day and then stored in low temperature (yield 68%).
Synthesis of Diacetylene Monomers. The synthesis of polycaprolactone diacetylene ester (PCLDA) from polycaprolactone diol was shown in Scheme 2. Firstly, polycaprolactone diol (20 g, 10 mmol), propiolic acid (14.2 g, 40 mmol), and p-toluenesulfonyl acid monohydrate (0.86 g, 5 mmol) were added into toluene and stirred at 110 ℃ for 84 h. Then the solvent was removed using a distillation apparatus and the residue was precipitated in largely excess distilled water. The remaining yellow viscous liquid was dried in a vaccum oven at 60 ℃. (yield 95%).

Scheme 2. Synthesis of polycaprolactone diacetylene ester (PCLDA).
Synthesis of Poly[1,4-Bis(azidomethyl)cyclohexane-co-polycaprolactone Diacetylene Ester] (Poly(BAC/PCLDA)). Poly(BAC/PCLDA) were synthesized via azide-alkyne click reaction with equivalent azide/alkyne molar ratio. Pure BAC and PCLDA were added into dimethylformamide (monomer conc. 10 wt%) and stirred at 60, 80, and 100 ℃ until over 95% conversion. During click reaction, aliquots were taken for FTIR analysis to check the conversion. When the reaction was completed, the reaction mixture was precipitated and washed several times in methyl alcohol to remove the solvent and unreacted monomers.
Preparation of Neat Epoxy. Hydrogenated diglycidyl ether of bisphenol A (HDGEBA, Scheme 3(A)) and isophorone diamine (IPDA, Scheme 3(B)) were mixed at room temperature for 5 min by mechanical stirrer. Then, the mixture was degassed under vacuo until the bubbles were completely disappeared. After that, epoxy was cured at 80 ℃. During curing, surface hardness of epoxy resin was measured using a shore durometer(type D) and the sample was analyzed using FTIR apparatus to determinate curing conversion.
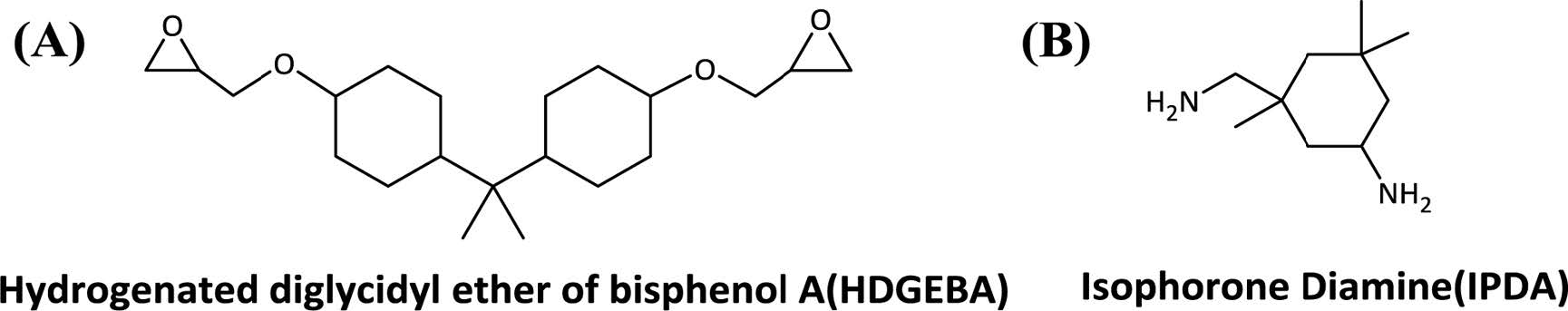
Scheme 3. Chemical structures: (A) hydrogenated diglycidyl ether of bisphenol A (HDGEBA); (B) isophorone diamine (IPDA).
Preparation of Toughened Epoxy by In-situ Epoxy Curing Reaction. Various contents of equivalent BAC and PCLDA in HDGEBA (BAC/PCLDA to HDGEBA: 5 wt%, 10 and 15 wt%) were prepared at 80 ℃ for 7 h until 70% conversion achieve excellent click conversion of more than 95% so that the curing time of epoxy species could be matched. Then, isophorone diamine (about 18% of the epoxy) was added to the epoxy mixture, followed by the stirring for 5 min and degassing under vacuo until the bubbles were completely disappeared. Subsequently, this mixture was poured into a silicone mold and cured at 80 ℃ for 4 h.
Characterization. 1H NMR analysis of the synthesized monomers and polymers was performed on a 400 MHz spectrometer (JEOL Led., Akishima, Japan; JNM-A L400), and CDCl3 was used as the solvent. Gel permeation chromatography (GPC) analyses of the poly(BAC/PCLDA) were performed on a Waters 2690 separations module with refractive index detection at a flow rate of 1.0 mL/min in THF equipped with three styragel HR 1, 2, 4 columns and a refractive index detector after calibration using polystyrene standard polymers. The conversion of the epoxy curing and azide-alkyne click reaction was measured by a FTIR spectrophotometer (RTS-175C, frequency range: 700-4000 cm–1, Bio-Rad Laboratories). The fracture toughness was measured using a universal material tester (Instron 5969, Lloyd Co.) according to the method described in the ASTM D 5045-99, at a crosshead speed of 1 mm min–1. It is determined by the average value of five specimens. The dynamic mechanical properties analysis (DMA) were measured in a temperature range of -80 to 300 ℃ under an argon atmosphere a heating rate of 2 ℃ min–1, a frequency of 1 Hz, and by using a viscoelastic analyzer (Seiko Exstar 6000; DMA/SS6100). Morphology observed using a field emission scanning microscope (SEM, JEOL, JSM-6300 or Hitachi, S-4800).
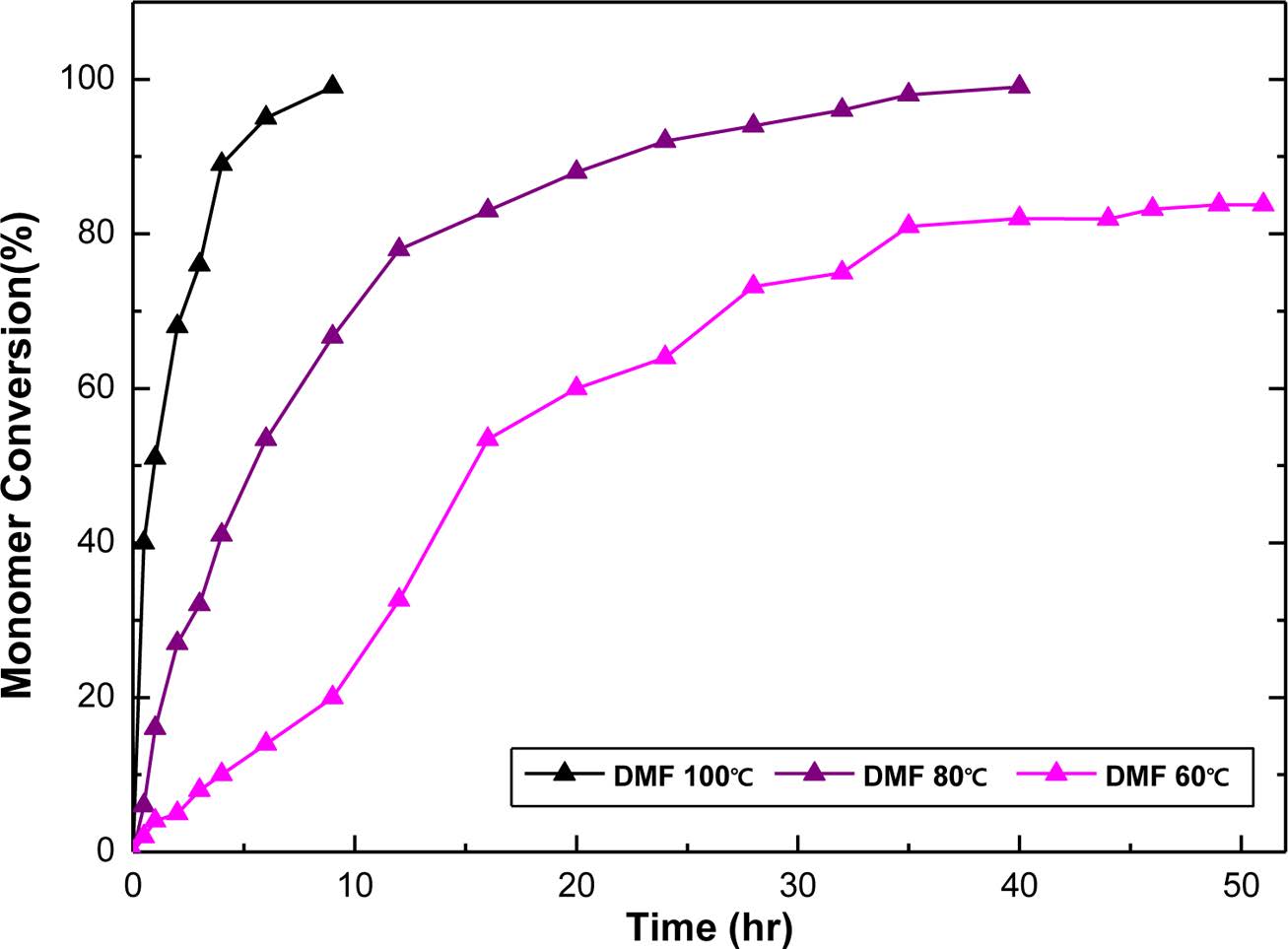
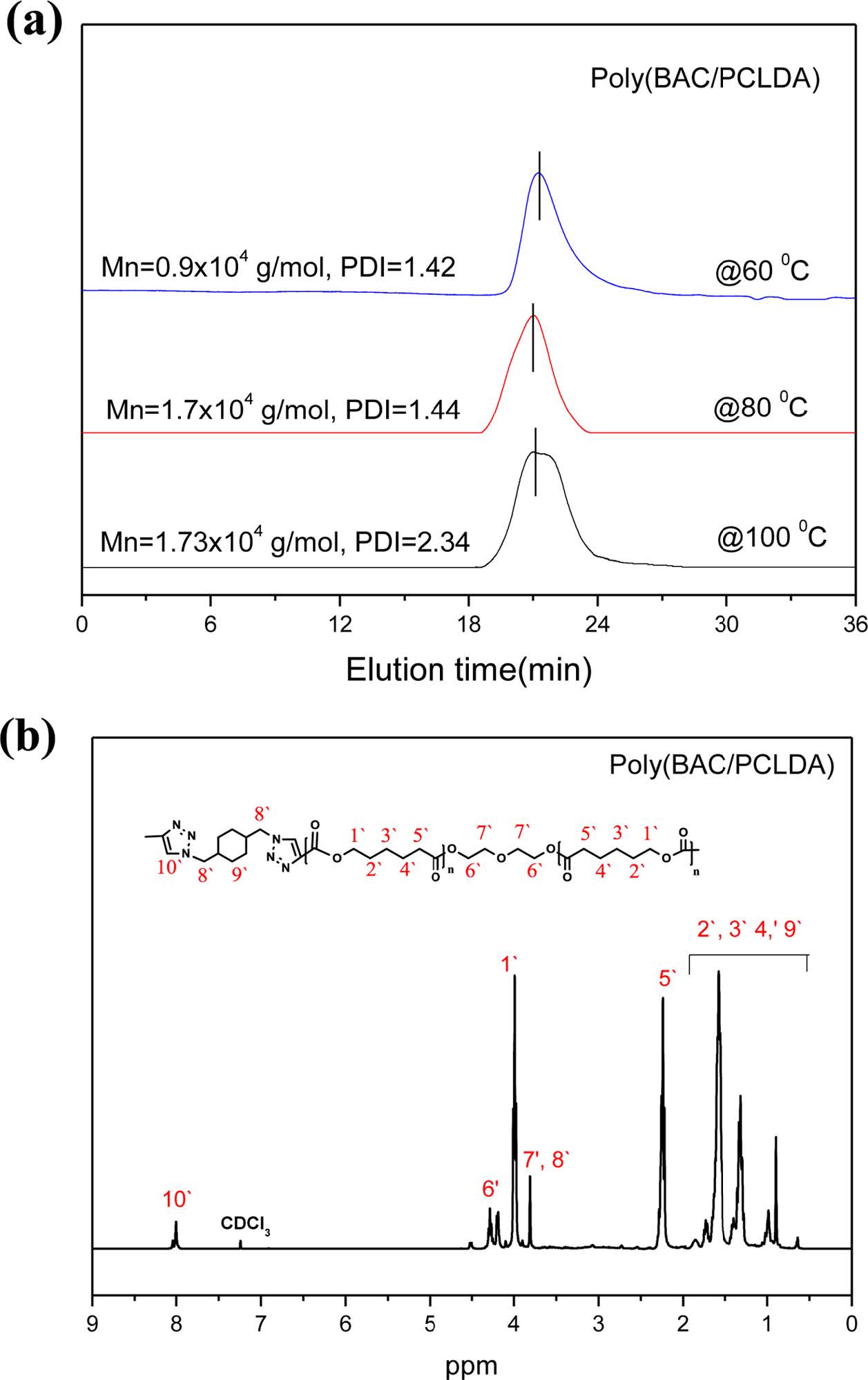
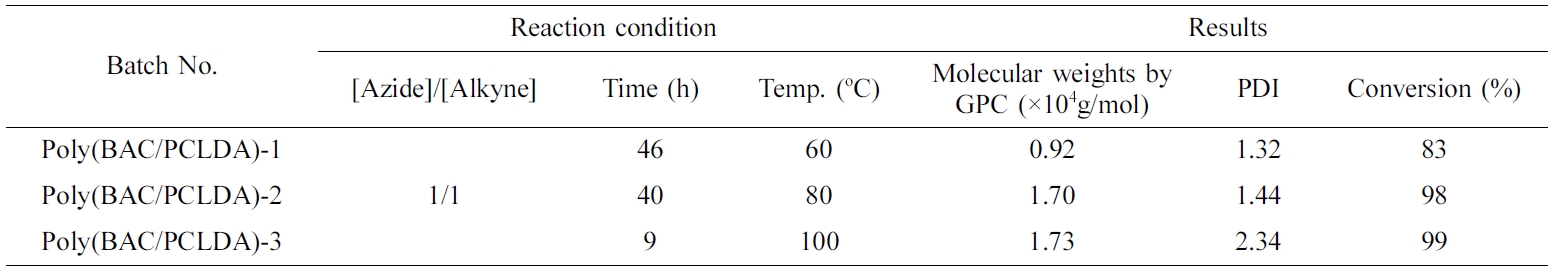
Synthesis of Poly(BAC/PCLDA). The aliphatic polymer of poly(BAC/PCLDA) was synthesized via azide-alkyne click polymerization as shown in Scheme 4. The polymerization mechanism of the poly(BAC/PCLDA) should be fully investigated before using as an epoxy toughening agent to optimize in-situ polymerization. The click polymerization rates of polymer were measured using FTIR analysis based on the reduction of the azide absorption peak at 2100 cm-1 at various temperatures (60, 80 and 100 ℃). Figure 1 shows that the conversion rate was reached 83% at 60 ℃ after 46 h. It means that poly(BAC/PCLDA) must be polymerized more than 50 h to achieve a conversion rate of 95%. However, the reaction time decreases with increasing temperature and the reaction is finished within 40 h at 80 ℃ and 9 h at 100 ℃ to reach more than 95% conversion. The GPC results showed that the poly(BAC/PCLDA) at 80 ℃ has the average molecular weight of 1.7×104 g/mol and a molecular weight distribution of 1.44, which is suitable for use as a toughening agent. Molecular weights and yield of poly(BAC/PCLDA) synthesized with different temperature in DMF was showed in Table 1. The 1H NMR structure analysis of the synthesized poly(BAC/PCLDA) is shown in Figure 2. The peak of triazole (8.0~8.1 ppm) of polymer was confirmed after the click reaction. The synthesized click polymer of poly(BAC/PCLDA) was named as a preformed polymer and used for toughening agent for comparison of morphology with in-situ toughened epoxy.

Scheme 4. Synthetic schemes of poly(BAC/PCLDA).
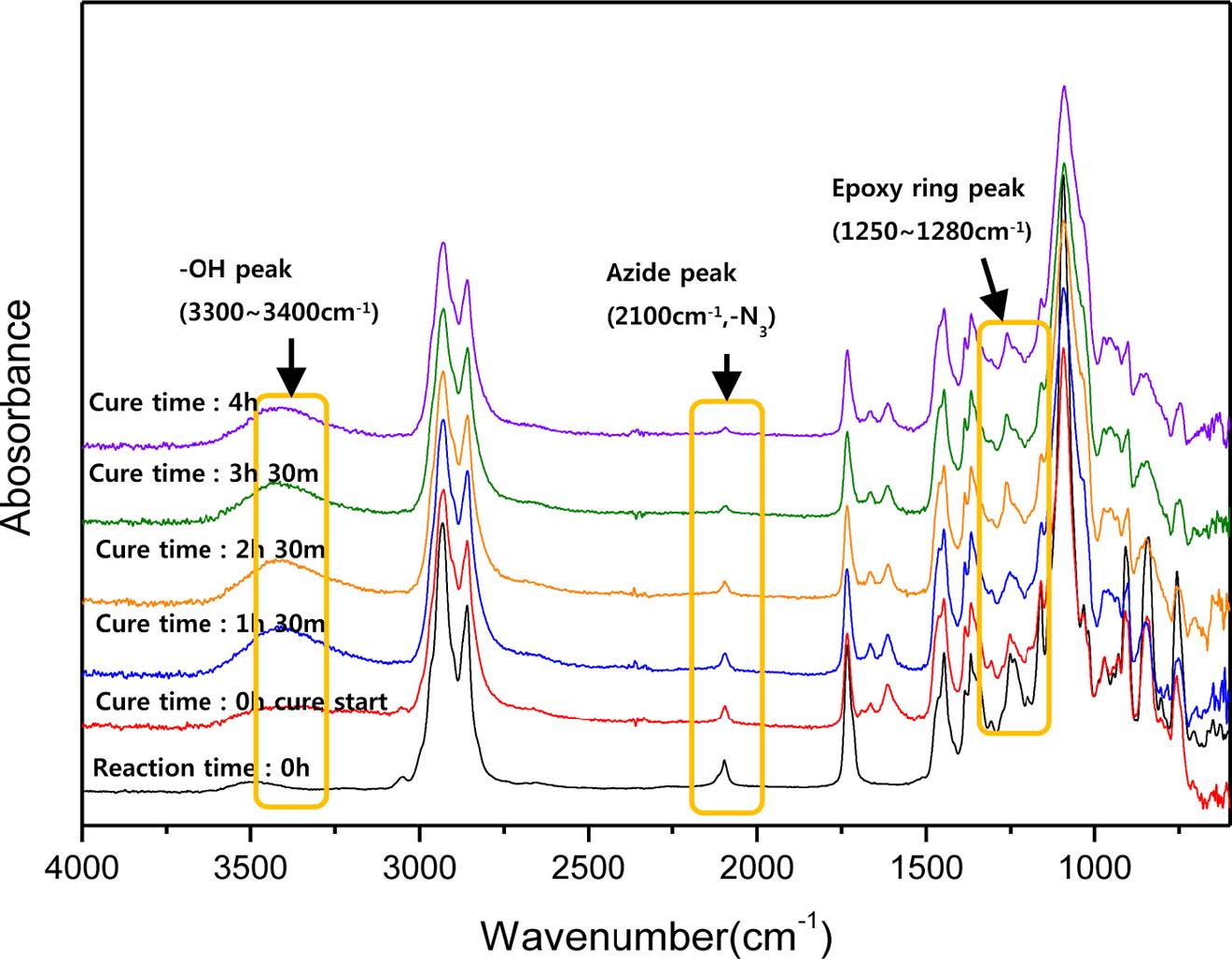
Preparation of Toughened Epoxy with In-situ Poly (BAC/PCLDA). Herein, Figure 3 shows that the conversion of the HDGEBA/IPDA curing and the click reaction of the toughening agent were monitored by FTIR at the curing temperature of 80 ℃. As the azide-alkyne reaction progressed, the stretching band at 2100 cm-1 corresponding to the azide band of BAC disappeared. It is proved not only the formation of linear poly(BAC/PCLDA) and completion of azide-alkyne click reaction. Also, the epoxy curing proceeded simultaneously and respectively. The epoxy ring of HDGEBA at 1250~1280 cm-1 decreased and the -NH stretching band of IPDA observed at 3300 cm-1 gradually changed to a -OH stretching band widely. The incremental change of the newly generated -OH peak did not change at 4 h. It means that the crosslinking structure of the epoxy resin had formed in 4 h. However, when epoxy toughening proceeded after addition of toughening agent, the conversion rate of the in-situ click reaction of poly(BAC/PCLDA) was very low within 4 h about 30% conversion. It is expected that the effect of toughening not be great because the amount of dispersed click polymer in the epoxy is not sufficient to make the strain energy and the remaining monomers may act as plasticizers. Therefore, when making toughened epoxy of in-situ poly(BAC/PCLDA), BAC and PCLDA monomers were poured into the epoxy, and in advance clicked during 7 h at 80 ℃ until reaction conversion reached to 70%. After preclick reaction of poly(BAC/PCLDA) in epoxy, curing agent was added and curing is performed at 80 ℃ for 4 h. These sequential curing process shows that the conversion of in-situ click reaction was completed more than 97%.
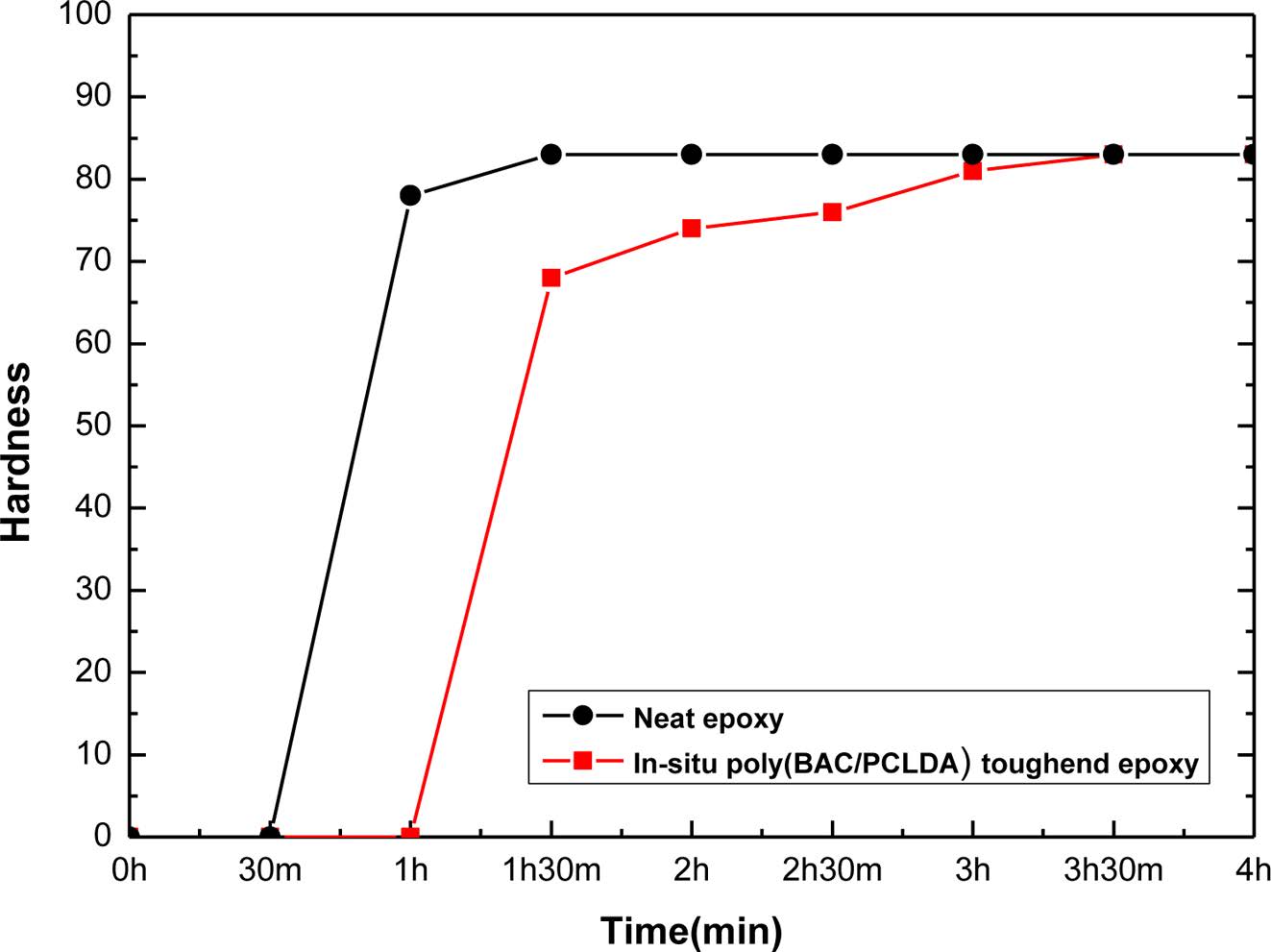
The surface hardness (shore D) shows curing behavior of neat epoxy and toughened epoxy with in-situ poly(BAC/PCLDA) species as shown in Figure 4. The surface hardness of HDGEBA/IPDA was maintained after 2 h at 83 shore D. It means that curing of neat epoxy was finished in 2 h. Meanwhile, the surface hardness of toughened epoxy with in-situ poly(BAC/PCLDA) was maintained after 4 h at 83 shore D. It was confirmed that the curing of toughened epoxy was completed that the in-situ proceeded for 2 h longer than the neat epoxy, but the surface hardness showed a similar value.
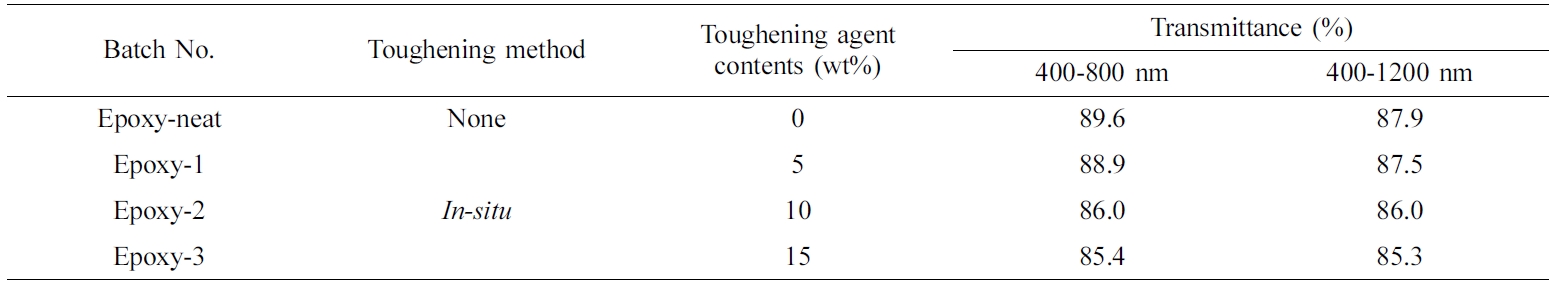
Transparency of Cured Neat Epoxy and Toughened Epoxy. The transmittance of toughened epoxy resins with the aliphatic polymer of poly(BAC/PCLDA) was measured as weight contents. It was confirmed that the epoxy specimens maintained highly transmittance, as shown in Table 2 and Figure 5. Figure 5 shows the toughened epoxy species as the toughening agent content increased (0, 5, 10, 15 wt%), turned slightly yellow because of the toughening agents color.
The light transmittance of neat epoxy was 89.6% at 400-800 nm and 87.9% at 400-1200 nm. Transmittance of toughened epoxy with in-situ poly(BAC/PCLDA) was obtained to 85.4% at 400-800 nm and 85.3% at 400-1200 nm when toughener was used as 15 wt% content. There was a slight decrease of transmittance due to the color of the specimen of toughened epoxy. However, that the transmittance was obtained above 85% even when the toughening agent was added, is considered to be a result of maintaining the transparency of the epoxy resin. These results were obtained by the polymer having good compatibility with epoxy resin .
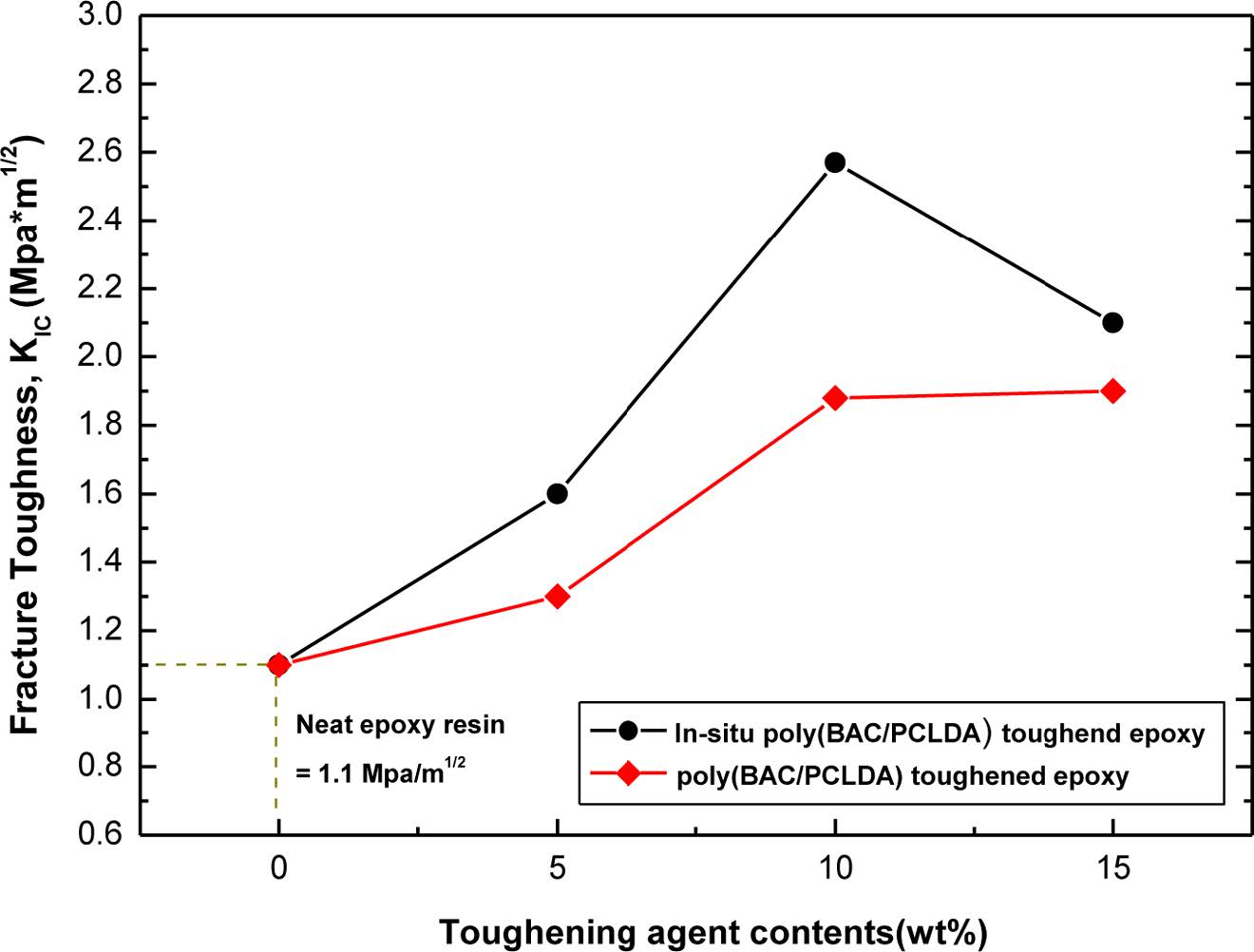
Fracture Toughness (K1C) of Cured Neat Epoxy and Toughened Epoxy. The fracture toughness (K1C) of toughened epoxy with the in-situ and preformed poly(BAC/PCLDA) is shown in Figure 6. As using a toughening agent of poly(BCA/PCLDA) in epoxy, fracture toughness was increased. The toughening polymer bridged the crack as so it propagates through the matrix and thus the crack growing could be prevented.33 In the overall trend, the results show that fracture toughness of toughened epoxy improved more superiorly with in-situ synthesized polymer than with using preformed polymer. This result might be explained based on the uniform dispersion phase by the in-situ synthesized polymers in epoxy, which morphology may be able to dissipate the applied impact energy more easily owing to their uniform dispersed phase.28 Thus, the improvement of fracture toughness was difference according to the using method of the toughening agent, and in-situ method was more effective in improving the toughness. The fracture toughness of neat epoxy species of HDGEBA/IPDA was 1.1 MPa·m1/2, and the value of in-situ toughened epoxy 1.6, 2.57, and 2.1 MPa·m1/2 as the toughening agent amounts increased from 5 to 10 wt% and 15 wt%. When the toughening agent was added in epoxy with an amount less than 10 wt%, the fracture toughness was gradually improved than neat HDGEBA/IPDA epoxy matrix and when 10 wt% in-situ toughened, the value was increased more over 2 times. However, when the amount of poly(BAC/PCLDA) used 15 wt% in epoxy, the value of K1C decreased from 2.57 to 2.1 MPa·m1/2. The same phenomenon has been observed by Wubin Ying et al. for toughened epoxy with poly(p-BAB/SPB) and poly(m-BAB/SPB) in an DDS cured TGAP epoxy.28 Fracture strength of these toughened epoxy with poly(p-BAB/SPB) and poly(m-BAB/SPB) was decreased when the polymer contents was higher than 10 wt%. Also, researchers like Tanaka et al., Mimura et al., and Park et al. have reported the similar phenomenon and description that the tendency of the fracture toughness to decrease when a over certain amount of polymer was used, despite of the difference of the used polymer in epoxy.34-36
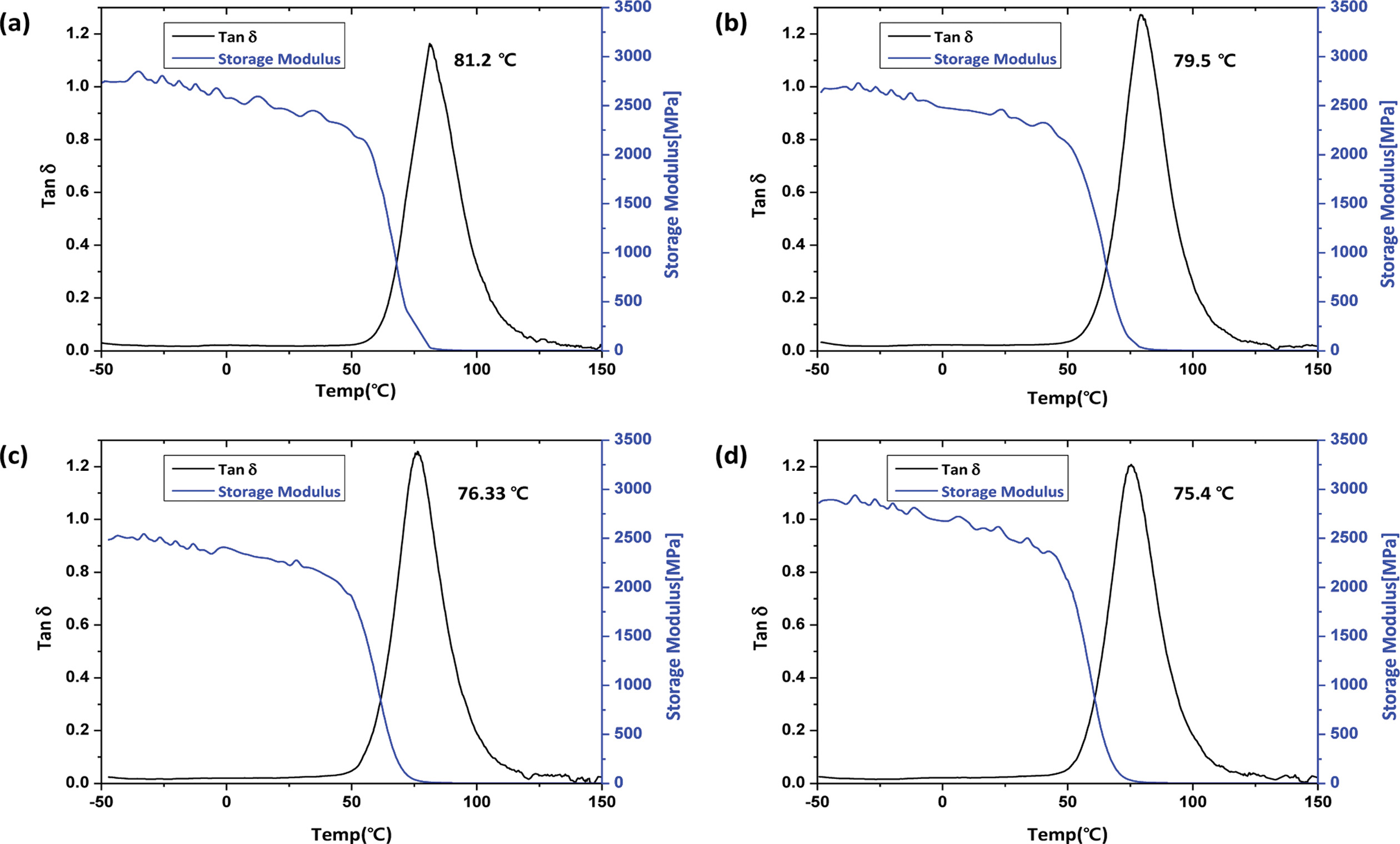
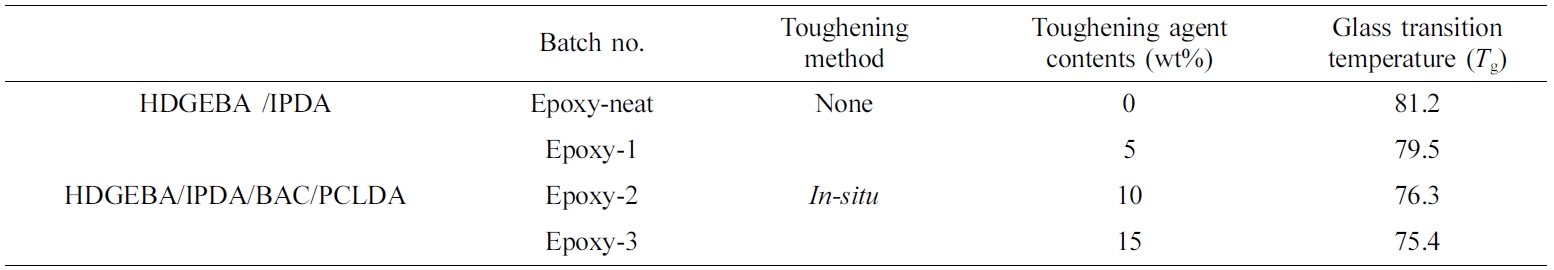
Thermal Analysis of Cured Neat Epoxy and Toughened Epoxy. The glass transition temperature (Tg) of the epoxy resin with various amounts of poly(BAC/PCLDA) was confirmed by dynamic mechanical analysis, as showed in Figure 7 and Table 3. All of which were slightly decreased with addition of poly(BAC/PCLDA). The glass transition of neat epoxy of HDGEBA/IPDA was 81.2 ℃ and the value of in-situ toughened epoxy was 79.5, 76.3 and 75.4 ℃ as the toughening agent amounts increased from 5 to 10 wt% and 15 wt%. The Tgs of toughened epoxy with in-situ poly(BAC/PCLDA) tended to decrease as the content of polymer was increased. According to Tripathi and Srivastava17 variation of Tgs of the matrix system was caused by that some of the rubber dissolved in the epoxy phase was plasticizing the glass transition.37,38 This gradual decrease of Tg suggested that the poly(BAC/PCLDA) having lower Tg than epoxy matrix resulted the difference in the Tgs of blend epoxies as the soft portion increased with the homogenous phase.
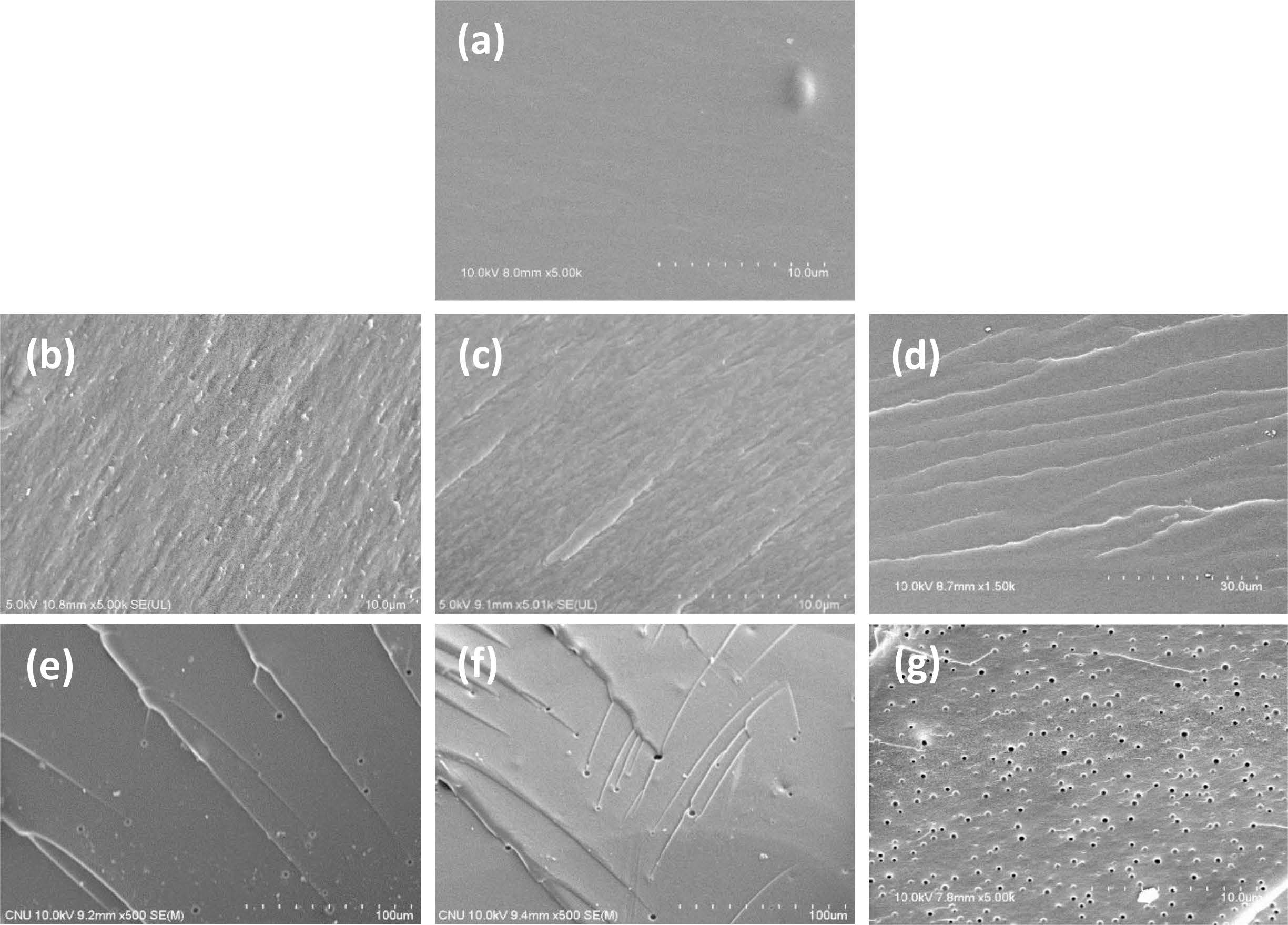
Morphology. The morphology of the cured epoxy specimens was observed by SEM to check the difference of the in-situ and the preformed toughening method, and the results were shown in Figures 8. The analysis was conducted to understand the correlation between phase separation structure and toughening effect. Neat epoxy found a mild surface without any phase separation. Toughened epoxy with in-situ 5, 10, and 15 wt% poly(BAC/PCLDA) revealed a morphology different slightly that exhibits a finely dispersed morphology forming a semi-IPN structure by linear polymer with crosslinked epoxy. It expects that the fracture due to the crack growth of the epoxy resin does not proceed forming the fracture surface by the stretching energy of the toughened polymer which induces the polymer matrix yielding to disperse the external stress. There has been a report on the results of a study of a strong toughening effect when a toughened epoxy specimen forms a semi-IPN structure.18,28 On the other hand, in the case of the preformed toughening method, 5 wt% toughened epoxy species was observed that co-continuous and spherical domain morphologies were present simultaneously. The morphology gradually becomes spherical domain as the content of the poly(BAC/PCLDA) increases, as evident in the 15 wt% preformed toughened epoxy. The size of the spherical domain was appeared randomly in 159–347 nm. It was confirmed that the preformed polymer was not dissolved in the epoxy resin but dispersed poorly in the epoxy resin (Figure 8(d)).
|
Figure 1 Conversion versus time for azide-alkyne click polymerization in DMF solvent at various temperature at 60, 80, 100 ℃. |
|
Figure 2 (a) GPC curve; (b) 1H NMR spectrum of poly(BAC/PCLDA) synthesized by azide-alkyne click reaction at 80 ℃ in DMF. |
|
Figure 3 FTIR spectroscopy of the HDGEBA/IPDA epoxy curing and BAC/PCLDA click reaction simultaneously. |
|
Figure 4 Curing behavior observed by surface hardness measurement of neat epoxy and toughened epoxy. |

|
Figure 5 Transmittance test of toughened epoxy specimens on CNU words as toughening agent contents: (a) unmodified epoxy resin; (b) 5 wt% in-situ poly(BAC/PCLDA); (c) 10 wt% in-situ poly(BAC/PCLDA); (d)15 wt% in-situ poly(BAC/PCLDA). |
|
Figure 6 Fracture toughness comparison of toughened epoxy with in-situ polymers. |
|
Figure 7 Glass transition temperature of toughened epoxy resins with various contents of poly(BAC/PCLDA): (a) unmodified resin; epoxy resins toughened with (b) 5 wt% in-situ poly(BAC/PDLCA); (c) 10 wt% in-situ poly(BAC/PDLCA); (d) 15 wt% in-situ poly(BAC/PDLCA). |
|
Figure 8 Scanning electron micrographs (SEM) of the fracture surfaces for the cured HDGEBA/IPDA epoxy resins as toughening agent contents (at 80 ℃): (a) unmodified resin; epoxy resins toughened with (b) 5 wt% in-situ poly(BAC/PDLCA); (c) 10 wt% in-situ poly(BAC/PDLCA); (d) 15 wt% in-situ poly(BAC/PDLCA); (e) 5 wt% preformed poly(BAC/PCLDA); (f) 10 wt% preformed poly(BAC/PCLDA); (g) 15 wt% preformed poly(BAC/PCLDA). |
|
Table 1 Molecular Weight and Yield of Poly(BAC/PCLDA) Synthesized with Different Temperature in DMF |
|
Table 2 Transmittance Properties of Neat and In-situ Poly(BAC/PCLDA) Toughened Epoxy Species |
|
Table 3 Glass Transition Temperature of Toughened Epoxy Resins with Various Contents of Poly(BAC/PCLDA) |
For purpose on transparently toughening of HDGEBA resin, the aliphatic linear polymers of poly(BAC/PCLDA) were synthesized by azide-alkyne click polymerization to form a well-defined semi-IPN structure and used as a toughening agent for the epoxy system. Using a in-situ method which uses a poly(BAC/PCLDA) as a toughening agent was maintained the transparency of epoxy and shows toughening effects. As a result, when the polymer added 10 wt% of the epoxy weight, fracture toughness was improved as compared with the neat HDGEBA/IPDA epoxy. Fracture toughness of toughened epoxy with in-situ 10 wt% was improved to 2.6 MPa·m1/2, which were more than twice that of the neat specimen 1.1 MPa·m1/2. In addition, dynamic mechanical thermal analysis confirmed that the Tg of the epoxy itself did not decrease significantly, and the co-continuous matrix was observed in the SEM even though the in-situ polymer was included, resulting from the formation of semi-interpenetrating polymer network (semi-IPN).
- 1. A. Sahu, D. S. Mondloe, and S. Upadhyay, Res. J. Eng. Tech., 4, 579 (2017).
- 2. T. Na, H. Jiang, X. Liu, and C. Zhao, Eur. Polym. J., 100, 96 (2018).
-

- 3. P. Mohan, Polym.-Plast. Technol. Eng., 52, 107 (2013).
-

- 4. B. Ellis, Chemistry and Technology of Epoxy Resins, Blackie Academic & Professional, Glasgow, U. K., 1993.
- 5. P. Nogueira and C. Ramírez, J. Appl. Polym. Sci., 80, 71 (2001).
-

- 6. F. L. Jin, X. Li, and S. J. Park, J. Ind. Eng. Chem., 29, 1 (2015).
-

- 7. T. Okamatsu, J. Adhes. Sci. Technol., 13, 109 (1999).
-

- 8. V. B. Gupta, Polym. Eng. Sci., 25, 812 (1985).
-

- 9. I. Shibanai and N. Kenji, U.S. Patent 4711936-A (1987).
- 10. A. D. Eselev and V. N. Stokozenko, Polym. Sci., 2, 71 (2009).
-

- 11. R. Tomas, Y. Ding, Y. He, and L. Yang, Polymer, 49, 278 (2008).
-

- 12. R. Bagheri and R. A. Pearson, J. Macromol. Sci. C: Polym. Rev., 49, 201 (2009).
-

- 13. A. Haris, A. Tadaharu, and A. Wakako, J. Mater. Sci., 43, 3289 (2008).
-

- 14. B. Qi, Compos. Struct., 75, 514 (2006).
-

- 15. S. Kumar, Ind. Eng. Chem. Res., 57, 2711 (2018).
-

- 16. R. Debdatta, J. Adhes. Sci. Technol., 17, 1655 (2003).
-

- 17. G. Tripathi and D. Srivastava, Mater. Sci. Eng. A, 443, 262 (2007).
-

- 18. K. Mimura, H. Ito, and H. Fujioka, Polymer, 41, 4451 (2000).
-

- 19. C. Bao, J. Mater. Chem., 21, 13290 (2011).
-

- 20. B. S. Kim, T. Chiba, and T. Inoue, Polymer, 36, 43 (1995).
-

- 21. R. A. Evans, Aust. J. Chem., 60, 384 (2007).
-

- 22. E. M. John and D. M. D. Adam, Chem. Soc. Rev., 36, 1249 (2007).
-

- 23. J. E. Hein and V. F. Valery, Chem. Soc. Rev., 39, 1302 (2010).
-

- 24. A. R. Katritzky, J. Polym. Sci., Part A: Polym. Chem., 46, 238 (2008).
-

- 25. P. Wu and V. F. Valery, Aldrichim. Acta, 40, 7 (2007).
-

- 26. L. Liang and D. Astruc, Coordin. Chem. Rev., 255, 2933 (2011).
-

- 27. Binder, H. Wolfgang, and C. Kluger, Curr. Org. Chem., 10, 1791 (2006).
-

- 28. W. B. Ying, H. S. Yang, D. S. Moon, M. W. Lee, N. Y. Ko, N. H. Kwak, B. Lee, J. Zhu, and R. Zhang, J. Appl. Polym. Sci., 135, 45790 (2018).
-

- 29. A. T. Narteh, M. Hosur, E. Triggs, and S. Jeelani, Polym. Degrad. Stabil., 98, 759 (2013).
-

- 30. S. A. Awad, M. F. Christopher, and S. M. Seyed, Polym. Test., 66, 70 (2018).
-

- 31. S. A. Awad, M. F. Christopher, and M. S. Saeed, J. Polym. Res., 25, 103 (2018).
-

- 32. M. Gonzalez, Mater. Chem. Phys., 132, 618 (2012).
-

- 33. C. B. Bucknall, Toughened Plastics, Applied Science Publishers, Ltd., London, 1977.
- 34. N. Tanaka, T. Lijima, W. Fukuda, and M. Tomoi, Polym. Int., 42, 95 (1997).
-

- 35. K. Mimura, H. Ito, and H. Fujikoa, Polymer, 42, 9223 (2001).
-

- 36. S. J. Park, G. D. Lyle, R. Mercier, and J. E. Mcgrath, Polymer, 34, 885 (1993).
-

- 37. I. Harismendy, T. Miner, A. Valea, R. Liano-Ponte, F. Mujika, and I. Mondragon, Polymer, 38, 5573 (1997).
-

- 38. C. W. Wise, W. D. Cook, and A. A. Goodwin, Polymer, 38, 3251 (1997).
-

- Polymer(Korea) 폴리머
- Frequency : Bimonthly(odd)
ISSN 0379-153X(Print)
ISSN 2234-8077(Online)
Abbr. Polym. Korea - 2023 Impact Factor : 0.4
- Indexed in SCIE
 This Article
This Article
-
2019; 43(2): 243-251
Published online Mar 25, 2019
- 10.7317/pk.2019.43.2.243
- Received on Oct 26, 2018
- Revised on Dec 1, 2018
- Accepted on Jan 5, 2019
 Services
Services
- Full Text PDF
- Abstract
- Acknowledgements
Introduction
Experimental
Results and Discussion
Conclusions
- References
Shared
 Correspondence to
Correspondence to
- Wu Bin Ying* , and Bumjae Lee
-
Technology and Engineering, Chinese Academy of Sciences, Zhongguan West Road 1219, Ningbo 315201, China
*Department of Fine Chemical Engineering and Applied Chemistry, Chungnam National University, 220 Gung-dong, Yuseong-gu, Daejeon 34134, Korea - E-mail: yingwubin@nimte.ac.cn, bjlee@cnu.ac.kr
- ORCID:
0000-0002-8768-7428, 0000-0002-9971-8244









 Copyright(c) The Polymer Society of Korea. All right reserved.
Copyright(c) The Polymer Society of Korea. All right reserved.